Abstract
SED1, also known as MFG-E8, is a secreted protein composed of two EGF repeats (the second of which contains an RGD motif) and two discoidin/Factor V/VIII C domains. SED1 is expressed by a wide range of cell types, where it participates in diverse cellular interactions, such as sperm binding to the egg coat and macrophage recognition of apoptotic lymphocytes. Although SED1 was originally identified as a milk protein, its function in the mammary gland remains unclear; suggested functions include inhibition of viral infection and clearance of apoptotic cells during mammary gland involution. We report here that SED1 has an unexpected obligatory role during mammary gland development. Unlike that seen in WT glands, SED1-null glands show severely reduced branching from epithelial ducts and from terminal end buds, which are thin and poorly developed. SED1 is expressed by both luminal and myoepithelial cells in the developing epithelial duct, and binds to αv integrin receptors on myoepithelial cells leading to MAPK activation and cell proliferation. The absence of SED1 leads to greatly reduced levels of activated MAPK and a concomitant reduction in cell proliferation and branching throughout the epithelial tree. These results suggest that SED1 contributes, at least partly, to the intercellular signaling between luminal and myoepithelial cells that is required for branching morphogenesis.
Keywords: cell adhesion, epithelia, integrins
The mammary gland is one of the most exhaustively studied models of branching morphogenesis (1). A host of cell surface receptors and extracellular matrix components have been identified that aid in the outgrowth and remodeling of the bilayered mammary epithelial tube into a highly branched organ with terminal alveolar units capable of secreting milk in response to lactogenic hormones. Among the many proteins identified in milk is SED1, also known as lactadherin and MFG-E8, among other names (2–5). Although the function of SED1 in milk remains obscure, SED1 secretion is markedly elevated in breast cancer (3), and serum levels of SED1 are diagnostic of tumor burden suggesting that SED1 may play a role in oncogenesis (6). SED1 is also thought to serve a protective function during lactation by inactivating rotaviruses (7) as well as facilitating the clearance of apoptotic cells during mammary gland involution (8, 9). Herein, we report that SED1 has an unexpected role during mammary gland development by participating in the intercellular signaling between luminal and myoepithelial cells that is required for branching morphogenesis of the mammary gland.
Results
Reduced Epithelial Branching During Morphogenesis of SED1-Null Glands.
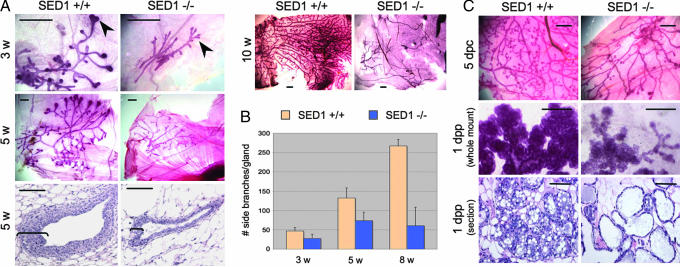
Comparison of the developing ductal tree in WT and SED1-null mammary glands illustrates a requirement for SED1 during gland development. The developing ducts are composed of a double-layered epithelial tube, with inner luminal epithelial cells surrounded by outer myoepithelial cells. During the first 2–3 weeks after birth, gland development is slow and proportional to overall body growth but progresses rapidly during puberty in response to ovarian hormones. In WT glands, the growing ends of the epithelial ducts possess a characteristic multilayered terminal end bud (TEB), which gives rise to both epithelial cell types and undergoes continual bifurcation and outgrowth (Fig. 1A, 3 weeks, arrowheads). This process continues until it reaches the limits of the mammary fat pad at ≈8–10 weeks after birth.
Fig. 1.
Mammary glands from SED1-null mice fail to undergo normal branching morphogenesis. (A) Photomicrographs of delipidated whole-mount mammary glands stained with Carmine to visualize the ductal tree. Bulbous TEBs occur at the growing ends of WT ducts (3 weeks, arrowheads) but are not evident in SED1-null glands. WT TEBs have multiple cell layers that undergo continued proliferation and bifurcation, unlike TEBs in SED1-null glands that are reduced to a few cell layers (compare 5 weeks, brackets). Paired SED1+/+ and SED1−/− images are derived from littermate females and are shown at identical magnification. Lower-magnification images (5 weeks, 10 weeks) illustrate the overall branching phenotype not evident at higher magnifications. (B) Mammary duct branching progresses throughout development of WT glands, whereas branching remains dramatically reduced in SED1-null females (+/+ vs. −/−, P < 0.004 for all ages). (C) During pregnancy (5 days postcoitum), WT glands undergo another round of side branching not seen in SED1-null glands. The residual alveolar tissue that is present in SED1-null glands is responsive to lactogenic hormones, although the acini are sparse (1 dpp, whole mount). WT acini are engorged with milk secretions, whereas SED1-null acini are distended with a thin-walled epithelium (1 dpp, section). [Scale bars, 1.0 mm in all whole-mount images and 0.1 mm for histological sections (5 weeks, 1 dpp)].
SED1-null neonates possess a rudiment of the early epithelial ductal tree, but the glands grow very slowly with truncated ducts and minimal end buds. SED1-null TEBs are reduced to a few poorly organized cell layers, unlike the prototypical multilayered cellular cap seen in WT (Fig. 1A, 5 weeks, brackets). Furthermore, there is a dramatic decrease in ductal branching in SED1-null glands, including branching that results from TEB bifurcation, as well as secondary branching along the ducts. WT glands show a 5.7-fold increase in branch number between 3 and 8 weeks of development, whereas SED1-null glands show only a 2-fold increase in branching (P < 0.004 for all ages assayed; Fig. 1B). By 8 weeks, epithelial branching in SED1-null glands is only 23% as frequent as in WT glands.
Pregnancy induces another round of epithelial proliferation and increased branching (Fig. 1C, 5 days postcoitum), and the onset of lactogenic hormones eventually leads to secretory acini [Fig. 1C, 1 day postpartum (dpp)]. Despite the greatly reduced mass of SED1-null mammary glands, they respond to hormonal stimulation during pregnancy and lactation as evidenced by a small increase in branching and duct extension; however, the resulting acini are sparse, thin-walled, and distended. Nevertheless, SED1-null females have near-normal-size litters (10) and are capable of nursing their pups to at least some degree, although we have not made a systematic study of the lactation or postweaning phenotype in this study.
SED1 Expression During Gland Morphogenesis.
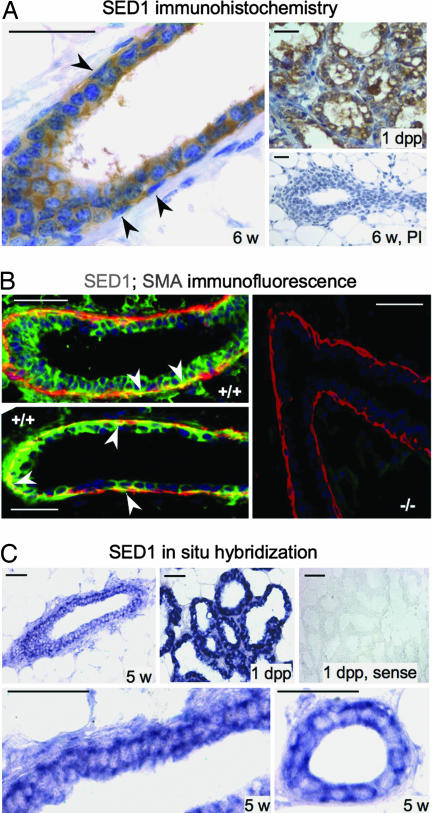
SED1 expression in milk is presumed to result from apical secretion from the acinar secretory epithelium (2–4). It was, therefore, surprising that the absence of an apically secreted milk protein resulted in greatly impaired mammary gland development. To gain insight into SED1's mode of action, its distribution during normal mammary gland development was determined by immunocytochemistry. As expected, SED1 is heavily expressed in the secretory epithelium and milk fat globules of lactating acini (Fig. 2A, 1 dpp). SED1 is also detectable in the ductal epithelium during development, being localized in association with both the luminal and myoepithelial cell populations (Fig. 2A). Double-label immunofluorescence confirms SED1 immunoreactivity associated with both luminal and myoepithelial cells, by using smooth-muscle actin (SMA) as a marker of myoepithelial cells (Fig. 2B). There is no detectable SED1 immunoreactivity in SED1-null tissues. In situ hybridization confirms the expression of SED1 during gland development, which, as expected, increases during lactation (Fig. 2C). Although it is difficult to identify cell-type-specific expression, the double layer of hybridization in the ductal epithelium suggests that both luminal and myoepithelial cells synthesize SED1.
Fig. 2.
SED1 is expressed in the mammary epithelium during development. (A) SED1 immunoreactivity is seen at the earliest stages of ductal development associated with both luminal and myoepithelial cells. Immunoreactivity increases during development and reaches peak levels at ≈5–6 weeks, when both luminal and myoepithelial cells are embedded in SED1 immunoreactivity (arrowheads denote myoepithelial cells based on nuclear architecture, apposition to the basal lamina, and SMA reactivity). SED1 expression remains stable or decreases slightly until the onset of lactation, when it is abundantly expressed in the secretory acini (1 dpp). No immunoreactivity is seen with preimmune sera (PI). (B) Double-label immunofluorescence in WT glands (+/+) illustrates SED1 immunoreactivity (Alexa Fluor 488, green) associated with both luminal and myoepithelial cells, as seen by colocalization with SMA (Alexa Fluor 594, red) (arrowheads). (C) In situ hybridization confirms SED1 transcription during gland development (5 weeks) and is up-regulated during lactation (1 dpp). Sense probes produced no hybridization. The double layer of hybridization is consistent with SED1 being synthesized by both epithelial cell types. (Scale bars, 0.05 mm in all images.)
Defective Branching Morphogenesis Results from Reduced Proliferation Rather Than Increased Apoptosis.
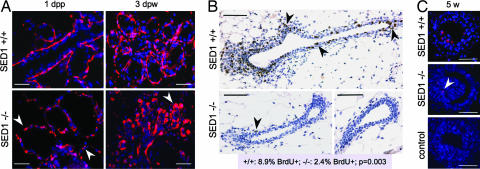
The reduced development of SED1-null glands is not a consequence of a selective loss of myoepithelial cells, as seen by the expression of SMA in SED1-null glands (Fig. 2B). However, myoepithelial cells acquire a characteristically abnormal morphology as SED1-null glands mature. Unlike WT cells, which maintain a uniform squamous phenotype surrounding the luminal epithelium and alveoli, myoepithelial cells in SED1-null glands appear rounded, producing a noncontiguous, dispersed cell layer (Fig. 3A). Differences in myoepithelial architecture become most evident when WT acini become secretory, at which time SED1-null myoepithelial cells are highly dysplastic and show no evidence of a traditional squamous phenotype (3 days postweaning).
Fig. 3.
SED1-null mammary glands show abnormal cellular architecture and greatly reduced cell proliferation. (A) Myoepithelial cells in WT glands have a characteristic squamous morphology as they surround the ductal epithelium and acini (1 dpp, 3 days postweaning). However, myoepithelial cells in SED1-null glands are bulbous and discontinuous around the ductal epithelium (arrowheads). Myoepithelial cells are identified by SMA immunocytochemistry; nuclei are visualized with DAPI. (Scale bars, 0.05 mm in all images.) (B) Cell proliferation, as judged by BrdU incorporation (brown nuclei, arrowheads), is abundant in the epithelial and mesenchymal compartments of WT ducts (5 weeks shown) but is greatly reduced in SED1-null glands (P = 0.003). (Scale bars, 0.1 mm in all images.) (C) The degree of apoptosis, as assessed by TUNEL staining, is low and similar during early development (5 weeks) of both gland genotypes (red nuclei, arrowhead). No TUNEL-positive nuclei are seen in control incubations. (Scale bars, 0.05 mm in all images.)
We therefore determined whether reduced development could be attributed to alterations in the extent of cell proliferation or apoptosis that normally accompany mammary gland development (11). Cell proliferation, as judged by BrdU incorporation, is present throughout the epithelial compartment of developing WT ducts, as well as in the multilayered TEB and adjacent mesenchyme (Fig. 3B, 8.9% BrdU+ cells of 2,310 counted), similar to that previously reported (11). In contrast, BrdU incorporation is greatly reduced in SED1-null glands (2.4% BrdU+ cells of 1,551 counted; P = 0.003). Decreased BrdU incorporation is evident in both TEBs (−/−, 4.5%; +/+, 9.1%) and ducts (−/−, 0.78%; +/+, 7.3%), although the ducts show a more dramatic decrease in proliferation. Unlike that seen with BrdU incorporation, the low level of apoptosis characteristic of early gland development appears relatively normal in SED1-null glands (11) (Fig. 3C). These results suggest that the loss of SED1 leads to a dramatic reduction in cell proliferation during mammary gland morphogenesis, resulting in a greatly diminished ductal tree. The residual mammary epithelium that remains at sexual maturity is responsive to placental and lactogenic hormones, as evidenced by increased ductal branching and TEB-like protrusions. However, the resulting acini are distended and disorganized.
SED1-Null Mammary Gland Organoids Show Reduced Branching Morphogenesis in Vitro.
The behavior of epithelial organoids was analyzed in 3D collagen gels, in which branching morphogenesis occurs in the absence of systemic influences (12). WT organoids elaborate a highly branched ductal tree after 1 week in culture, with a characteristic multilayered epithelium (Fig. 4A). In contrast, organoids from SED1-null females remain small and undergo very limited branching during the same time period. Those branches that do occur are very different from WT, in that their epithelium is thin, composed of the minimal bilayered cellular architecture. In WT organoids, SED1 immunoreactivity is localized to both the luminal compartment (Fig. 4A, red arrowhead), indicative of apically secreted protein, as well as within the adjacent collagenous matrix (Fig. 4A, black arrowheads), which likely reflects SED1 that is secreted basally from luminal epithelial cells and/or by myoepithelial cells. No SED1 immunoreactivity is seen in SED1-null organoids. Although in vitro assays of branching morphogenesis do not replicate all of the conditions in the developing mammary fat pad, most notably the absence of TEBs normally seen in vivo, organoids cultured in vitro replicate the SED1-dependent phenotype of reduced proliferation and branching. Thus, the branching defect seen in SED1-null glands is autonomous to the developing epithelial duct and does not depend upon systemic factors that could contribute to the phenotype (13).
Fig. 4.
SED1 participates in RGD-dependent adhesion required for branching morphogenesis. (A) WT organoids cultured in 3D collagen gels produce highly branched ductal trees (arrowheads, Upper) and are characterized by a multilayered epithelia invading the collagenous matrix (Lower). In contrast, organoids from age-matched SED1-null females remain viable during the same culture period but fail to extend branches and possess thin-walled epithelia. SED1 immunoreactivity is detected within the lumen (red arrowhead) of WT organoids and in the adjacent extracellular matrix (black arrowheads); no SED1 immunoreactivity is detectable in SED1-null organoids. [Scale bars, 0.1 mm for images (Upper), 0.05 mm for Lower.] (B) WT organoids adhere and grow out on 2D Matrigel substrates, whereas SED1-null cells adhere but grow out poorly, consistent with SED1 contributing to a normal adhesive phenotype. Heat-inactivated anti-SED1 antiserum (1:100) prevents outgrowth and spreading of WT cells, thus recapitulating the SED1-null phenotype. The addition of preimmune (PI) reagents had no effect on epithelial cell outgrowth. Nuclei stained with Syto24 (green). (Scale bars, 0.2 mm for all images.) (C) Recombinant SED1 supports mammary epithelial cell adhesion and is inhibited by competitive RGD containing peptides (0.5 mM), whereas RAD peptides have no effect. (D) RGD-dependent inhibition of single cell adhesion to SED1 matrices is dose-dependent, whereas RAD peptides fail to inhibit adhesion (10-min adhesion assay shown). (Error bars, SD.) (E) The presence of the 120-kDa αv integrin subunit was confirmed by immunoblotting lysates of WT organoids; nonimmune (ni) serum produced no reactivity. The antiserum localizes αv to the myoepithelial cells (arrowheads) surrounding the developing luminal cells. (F) The inclusion of anti-αv antiserum produced a dose-dependent inhibition of single-cell adhesion to SED1 matrices (10- and 20-min assays shown); ni serum had no effect. (Error bars, SD.)
SED1-Null Epithelial Cells Show Defective Adhesion.
Studies of MFG-E8 purified from milk suggest that it may function as a cell adhesion molecule by virtue of its two binding motifs: recognition of the RGD motif within the second EGF domain by αvβ5/3 integrins and binding of the discoidin/C domains to membrane phospholipids of mammary epithelial cells (5, 14). Consequently, the adhesive behavior of SED1-null mammary epithelial cells was examined by using organoid cultures as well as quantitative single-cell adhesion assays.
Initially, we examined the ability of organoids to adhere and grow out on 2D substrates, on which epithelial–matrix adhesions can be isolated from other complex morphogenetic events that occur in 3D cultures (12). SED1-null organoids were unable to adhere to glass substrates as did WT organoids (not shown), and consequently, organoids of both genotypes were plated on Matrigel substrates. After 3 days, WT organoids readily grow out and spread on Matrigel substrates, whereas SED1-null organoids survive but fail to grow out to the same degree (Fig. 4B Upper). To assess the involvement of SED1 in promoting WT outgrowth, organoids were allowed to adhere for 6 hr, after which cultures were supplemented with heat-inactivated anti-SED1 antiserum, purified IgG, or preimmune reagents. Anti-SED1 antiserum inhibits the outgrowth of WT organoids, thus phenocopying the poorly adhesive behavior of SED1-null cells (Fig. 4B Lower). Purified IgG produces similar results (not shown). As expected, SED1-null cells are refractory to the addition of anti-SED1 reagents. Thus, SED1 promotes the adhesion and outgrowth of mammary gland organoids, but in its absence, organoids fail to spread and ducts fail to branch and elongate, either in vitro or in vivo.
SED1-Mediated Adhesion Is RGD-Dependent.
SED1-mediated adhesion depends on the RGD motif present within the second EGF repeat, as shown by the ability of RGD-containing peptides to produce a dose-dependent inhibition of mammary epithelial cell adhesion to substrates coated with recombinant SED1 (rSED1). Equal concentrations of the control peptide, RAD, have no effect (Fig. 4C, representative images; Fig. 4D, quantitative assay). The RGD-dependent adhesion is consistent with SED1 binding to αvβ5/3 integrin receptors, which serve as SED1 receptors in other systems (5, 14). The presence of the αv integrin subunit in mammary gland organoids was confirmed by immunoblotting and is primarily associated with myoepithelial cells (Fig. 4E), consistent with earlier studies indicating that the αv subunit is restricted to the myoepithelium (15). The involvement of αv integrin in SED1-dependent adhesion was confirmed by the ability of anti-αv antiserum to inhibit cell adhesion (Fig. 4F). The level of inhibition produced by anti-αv integrin antibodies is consistent with the expected contribution of myoepithelial cells in the dissociated organoid. However, it is unclear why RGD produced near total inhibition of adhesion, although similar results have been reported by others (16).
Loss of SED1 Leads to Greatly Reduced MAPK Activation.
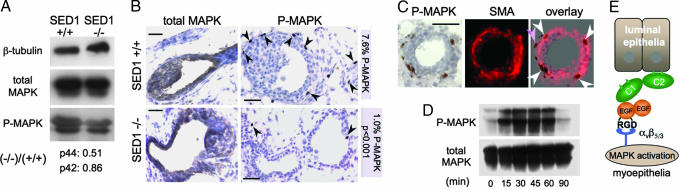
Ligation of αvβ5/3 integrins by the SED1-related protein, Del1, leads to MAPK activation and subsequent cell proliferation (17). Consequently, we determined whether the loss of SED1 from mammary epithelium correlated with altered MAPK activation. Immunoblot analysis indicates that total MAPK protein levels are similar in WT and SED1-null organoids, whereas the level of activated MAPK is reduced in SED1-null, relative to WT (Fig. 5A). The decrease in MAPK activation is largely confined to the 44-kDa MAPK isoform (51% of WT levels); the 42-kDa isoform is more similar to WT levels (Fig. 5A). Nevertheless, immunoblots of organoid lysates are unable to identify the relevant cell types, and therefore differences in MAPK activation could be influenced by the presence of nonepithelial cells. Consequently, we turned to immunocytochemistry to identify and quantify the extent of MAPK activation specifically in mammary epithelial cells.
Fig. 5.
The loss of SED1 is associated with reduced MAPK activation in myoepithelial cells. (A) The level of total MAPK protein is similar between WT and SED1-null organoids as assessed by immunoblotting, whereas the activated form of MAPK is greatly diminished in SED1-null organoids. Immunoblotting α-tubulin served as a protein-loading control. Densitometry analysis indicates that activation of the 44-kDa MAPK is reduced by nearly 50%, whereas activation of the 42-kDa isoform is closer to normal levels. (B) Immunocytochemistry reveals total MAPK (brown stain) in both the luminal and myoepithelial compartments in both WT and SED1-null organoids, but activated MAPK (black arrowheads) is confined primarily to the myoepithelial compartment, consistent with the expression of the αv integrin on myoepithelial cells. In contrast, MAPK activation is greatly reduced in the epithelial compartment of SED1-null organoids (P < 0.001). (Scale bars, 0.05 mm in all images.) (C) The identity of cells showing activated MAPK was assessed by overlaying images of phospho-MAPK-positive cells (immunocytochemistry) and SMA (immunofluorescence). Most cells with activated MAPK colocalize with SMA, indicative of myoepithelial cells (white arrowheads), whereas others lie adjacent to or outside of the SMA reactivity (pink arrowheads), suggesting they may be nonepithelial cells, epithelial precursors, or myoepithelial cell fragments from adjacent sections. (Scale bars, 0.05 mm in all images.) (D) The addition of rSED1 to primary epithelial cell cultures leads to a transient activation of MAPK. It is unknown why SED1 leads to activation of both MAPK isoforms in vitro, whereas the 44-kDa isoform is more sensitive to SED1 in vivo (A). (E) Hypothetical model to account for SED1 function during mammary gland morphogenesis. SED1 is secreted by myoepithelial and/or basally from luminal epithelial cells. The RGD motif within the second EGF repeat anchors SED1 to myoepithelial cells by the αvβ5/3 integrin, whereas adhesion to luminal epithelial cells is thought to be mediated by intercalation of the discoidin/C domains into the phospholipid bilayers (5, 14). Ligation of αvβ5/3 integrin induces MAPK activation in myoepithelial cells, leading to proliferation of the epithelial compartment, duct elongation, and branching.
As expected, MAPK immunoreactivity is present in both the luminal and myoepithelial cell compartments in both WT and SED1-null organoids (Fig. 5B). In marked contrast, activated MAPK is confined predominantly to the region of myoepithelial cells (Fig. 5B, P-MAPK, arrowheads). A small percentage of cells positive for activated MAPK are not clearly associated with the epithelial duct and may reflect fibroblastic or adipose cells.
We further investigated the identity of cells displaying activated MAPK by double-label immunocytochemistry by using SMA as a marker for myoepithelial cells. Virtually all cells with activated MAPK occur in the outer layers of the ductal epithelium (Fig. 5C), similar to that seen in Fig. 5B. Overlay of the activated MAPK and SMA images shows that most of the cells with activated MAPK lie adjacent to or overlie the SMA label (white arrowheads), consistent with their being myoepithelial cells. The few remaining cells with activated MAPK appear to lie outside of the SMA immunoreactivity (pink arrowhead) and may reflect fragments of myoepithelial cells from adjacent sections, nonepithelial cells, and/or progenitors of myoepithelial and/or luminal epithelia cells. In any event, MAPK activation is readily apparent throughout the myoepithelial cell layer in WT glands (≥7.6% of cells of 5,300 counted), whereas MAPK activation is markedly reduced in SED1-null glands (≥1.0% of cells of 3,640 counted, P < 0.001).
These studies predict that SED1 should be able to directly activate MAPK when added to isolated mammary epithelial cells. This was shown to be the case, as seen by the time-dependent, transient activation of MAPK after addition of rSED1 to primary mammary epithelial cell cultures (Fig. 5D).
Discussion
Mammary gland morphogenesis is orchestrated by a wide range of cell surface receptors and extracellular matrix components, including, but not limited to, integrin (15) and nonintegrin receptors (18–20), their laminin and collagen ligands (20), metalloproteinases (20, 21), epimorphin (22), and other extracellular effectors that aid in the remodeling of the epithelial–mesenchymal interface. The results presented here indicate that SED1, a protein previously implicated in sperm–egg adhesion (10, 23) and macrophage–lymphocyte interactions (24, 25), plays an obligatory role in branching morphogenesis as well.
The evidence suggests that SED1 functions as a signaling adhesive molecule, influencing interactions between myoepithelial and luminal epithelial cells that are known to be critical for mammary gland development (21, 22). A simple working model for SED1 function can be formulated that is consistent with results presented here, as well as with earlier biochemical studies of SED1's binding motifs (5, 14–16). The model suggests that SED1, secreted by either myoepithelial or luminal epithelial cells, participates in branching morphogenesis by virtue of its N-terminal RGD motif binding to αv-containing integrins on myoepithelial cells, and intercalation of the C-terminal discoidin/C domains into the phospholipid bilayer of luminal epithelial cells, as shown by others (5, 14) (Fig. 5E). Ligation of αvβ5/3 integrin leads to MAPK activation in myoepithelial cells, which directly or indirectly, maintains cell proliferation throughout the developing ductal tree. In the absence of SED1, MAPK activation fails to occur with a concomitant reduction in cell proliferation, leading to grossly reduced mammary gland morphogenesis. In this scenario, the adhesive function of SED1 could be modulated by metalloproteinases known to regulate the activities of other extracellular morphogens and adhesive molecules (20, 21). Whether the increase in SED1 characteristic of breast cancer is instructive or incidental to breast metastasis is unknown, but it is intriguing that tumorigenesis often results from unrestrained side branching (26), a process that is regulated, at least to some degree, by SED1.
Two recent studies report that SED1-null females show abnormalities in mammary gland involution (8, 9). We have not made a systematic study of the involution phenotype in this study, because it focuses on SED1 function during mammary gland development. Nevertheless, one study analyzed SED1 function during involution by using animals backcrossed onto the C57/B6 background (9). In our laboratory, backcrossing the original C57/B6:129 SED1 line onto the C57/B6 background resulted in a weak developmental phenotype, whereas backcrossing to the 129 line produced a highly penetrant developmental phenotype (not shown). However, SED1-null C57/B6 females retain the dilated duct phenotype observed after repeated pregnancies, as reported by others (9). The second study (8) used females maintained on the hybrid C57/B6:129 background, as in this study, and reported a variable developmental defect similar to that reported here, particularly with regard to reduced branching and underdeveloped TEBs (see figure 3 a and b, ref. 8). The low penetrance of the developmental phenotype may likely reflect the targeting construct used, which results in the retention of a truncated SED1 protein (devoid of one of the two discoidin/C domains) anchored to the cell surface, rather than secreted into the lumen. Thus, the construct developed by Atabai et al. (8) may distinguish between those cell interactions influenced by secreted SED1 (clearance of apoptotic cells at involution), as opposed to those that involve cell-associated SED1 (signaling between luminal and myoepithelial cells).
In summary, SED1 appears to facilitate a diverse range of cellular interactions as varied as sperm-egg binding (10, 23), macrophage clearance of apoptotic lymphocytes (24, 25) and mammary epithelial cells (8, 9), and mammary gland branching morphogenesis (current study). That SED1 may represent one member of a family of similar bi-motif signaling proteins is illustrated by the homologous protein Del1, which participates in endothelial adhesion to basal lamina during vascularization (17). It is not unreasonable to expect other EGF-discoidin/C domain proteins will be identified that contribute to distinct cellular interactions.
Materials and Methods
Mammary Gland Whole Mounts and Organoid Cultures.
Whole-mount mammary tissue was prepared (6) from WT and SED1-null mice (10), and duct branching was quantified from photographs of delipidated Carmine-stained glands by counting each branch point in the entire gland. Fifty-four glands were analyzed: two (gland nos. 2 and 4) from each of 27 females; three to six females of each age and genotype. Significance was determined by using Student's t test. Primary mammary gland organoids from 5- to 6-week-old mice were prepared (21) and cultured in 3D collagen gels or on Matrigel substrates in defined medium [DMEM/F12 containing Nutridoma NS (Roche, Indianapolis, IN), ITS (Sigma, St. Louis, MO), and EGF (20 ng/ml; BD Biosciences, Franklin Lakes, NJ)].
Histology and Immunocytochemistry.
Paraffin-embedded sections were rehydrated, subjected to microwave antigen retrieval, and stained for SED1, as described (10). SMA was detected by using mouse monoclonal anti-SMA (1:200; Sigma), followed by anti-mouse Alexa Fluor 594 (Molecular Probes, Eugene, OR). DNA was stained with DAPI.
To assess cell proliferation, 5-week-old mice were injected with BrdU (100 μg/g body weight; Sigma) 4 h before death. Incorporated BrdU was detected with a BrdU staining kit (Zymed, Carlsbad, CA). Total and BrdU-positive cells were counted in random microscope fields. Significance was determined by using Student's t test. TUNEL staining was conducted on sections after microwave antigen retrieval, and apoptotic cells were detected with the In Situ Cell Death Detection kit (Roche). Ten-micrometer cryostat sections were used for immunolocalization of αv integrin (1:100; Chemicon, Hampshire, U.K.), MAPK (1:100; Upstate Biotechnology, Lake Placid, NY), and p42/44 MAPK (1:100; Cell Signaling Technology, Beverly, MA), and antibodies were detected by using HRP-conjugated secondary antibodies (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). The percentage of cells with activated (i.e., phospho) MAPK was determined by counting the total and DAB-positive cells in 19 (SED1-null) or 20 (WT) random sections. Significance was determined by using Student's t test.
In Situ Hybridization.
Digoxigenin (DIG)-labeled RNA probes were generated from a 160-bp DNA fragment of SED1 cDNA, spanning exons 2 and 3, cloned into Bluescript vector KS+. Sense and antisense RNA was produced by using T7 or T3 polymerase (Roche). Mammary gland sections were permeabilized with Proteinase K (10 μg/ml, 30 min, 25°C). Hybridization was carried out overnight at 41°C, and unbound probe was removed by washing in graded dilutions of SSC. DIG-labeled RNA was detected with anti-DIG alkaline phosphatase conjugate (1:200; Roche) and developed by using the BCIP/NBT staining kit (Vector Laboratories, Burlingame, CA).
Cell Adhesion Assays.
Organoids were treated with 0.2% collagenase/2.5 units/ml dispase (Invitrogen, Carlsbad, CA). Single cells were separated from undigested clumps and washed repeatedly with DMEM/F12. Epithelial cells were plated on bicameral filter dishes (1.1 cm2), previously coated with 10 μg of rSED1, in DMEM/F12 containing 0.5 mM GRGDNP (Biomol, Plymouth, PA), GRADSP, or no peptide. After 20 min, cells and medium were replaced with fresh DMEM/F12 and cultured for 5 h to allow the attached cells to become adherent. The adherent cells were fixed, washed, and photographed.
To quantify cell adhesion, single cells were prepared from organoids as above and cultured on plastic dishes for 3–4 days to increase cell yield. Contaminating fibroblasts were removed by incubating 1–2 min in trypsin/EDTA (Invitrogen), followed by 5 min in trypsin/EDTA to remove epithelial cells. Cells were washed in DMEM/F12/5% FBS and incubated 45 min to recover from trypsinization, the medium was replaced with DMEM/F12/1% BSA, and cells were incubated 10–30 min with or without inhibitors. Ten thousand cells in 100 μl of DMEM/F12/1% BSA were added to 96-well plates previously coated with rSED1. After 10–20 min, unbound cells were aspirated, and the wells were washed with PBS. The adherent cells were fixed, rinsed with water, and stained with 0.1% Crystal violet. Cells were washed in water, stain was released into the supernatant with 100 μl of 10% acetic acid, and absorbance was read at 595 nm.
MAPK Activation in Cultured Mammary Epithelial Cells.
WT mammary epithelial cells were cultured in six-well plates containing DMEM/F12, 5% FBS, insulin transferin selenium, and 10 ng/ml EGF. At confluency, the medium was replaced with serum-free medium, and cells were serum-starved overnight. rSED1 (50 μg/ml) was added in serum-free medium, after which cells were lysed at the indicated times and subjected to immunoblotting for MAPK and phospho-MAPK.
Acknowledgments
We are grateful to Drs. Karen Schmeichel, Win Sale, and Victor Faundez for helpful suggestions regarding the manuscript. This work was supported by National Institutes of Health Grant HD23479 (to B.D.S.).
Abbreviations
- TEB
terminal end bud
- dpp
days postpartum
- SMA
smooth-muscle actin
- rSED1
recombinant SED1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Bissell MJ, Rizki A, Mian IS. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, Parry G. Proc Natl Acad Sci USA. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larocca D, Peterson JA, Urrea R, Kuniyoshi J, Bistrain AM, Ceriani RL. Cancer Res. 1991;51:4994–4998. [PubMed] [Google Scholar]
- 4.Couto JR, Taylor MR, Godwin SG, Ceriani RL, Peterson JA. DNA Cell Biol. 1996;15:281–286. doi: 10.1089/dna.1996.15.281. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Biochemistry. 1997;36:5441–5446. doi: 10.1021/bi963119m. [DOI] [PubMed] [Google Scholar]
- 6.Couto JR, Blank EW, Peterson JA, Ceriani RL. Cancer Res. 1995;55:1717–1722. [PubMed] [Google Scholar]
- 7.Newburg DS, Peterson JA, Ruiz-Palacios GM, Matson DO, Morrow AL, Shults J, Guerrero ML, Chaturvedi P, Newburg SO, Scallan CD, et al. Lancet. 1998;351:1160–1164. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- 8.Atabai K, Fernandez R, Huang X, Ueki I, Kline A, Li Y, Sadatmansoori S, Smith-Steinhart C, Zhu W, Pytela R, et al. Mol Biol Cell. 2005;16:5528–5537. doi: 10.1091/mbc.E05-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanayama R, Nagata S. Proc Natl Acad Sci USA. 2005;102:16886–16891. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensslin MA, Shur BD. Cell. 2003;114:405–417. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 11.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC, Rosen JM. Development (Cambridge, UK) 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 12.Schmeichel KL, Bissell MJ. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouon-Evans V, Rothenberg ME, Pollard JW. Development (Cambridge, UK) 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 14.Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 15.Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA. J Mammary Gland Biol Neoplasia. 2004;8:383–394. doi: 10.1023/B:JOMG.0000017426.74915.b9. [DOI] [PubMed] [Google Scholar]
- 16.Taylor MR, Couto JR, Scallan CD, Ceriani R, Peterson JA. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 17.Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T. J Biol Chem. 1999;274:11101–11109. doi: 10.1074/jbc.274.16.11101. [DOI] [PubMed] [Google Scholar]
- 18.Steffgen K, Dufraux K, Hathaway H. Dev Biol. 2002;244:114–133. doi: 10.1006/dbio.2002.0599. [DOI] [PubMed] [Google Scholar]
- 19.Weir ML, Muschler J. J Mammary Gland Biol Neoplasia. 2004;8:409–419. doi: 10.1023/B:JOMG.0000017428.38034.a7. [DOI] [PubMed] [Google Scholar]
- 20.Fata JE, Werb Z, Bissell MJ. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. Development (Cambridge, UK) 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radisky DC, Hirai Y, Bissell MJ. Trends Cell Biol. 2003;13:426–434. doi: 10.1016/s0962-8924(03)00146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensslin M, Vogel T, Calvete JJ, Thole HH, Schmidtke J, Matsuda T, Topfer-Petersen E. Biol Reprod. 1998;58:1057–1064. doi: 10.1095/biolreprod58.4.1057. [DOI] [PubMed] [Google Scholar]
- 24.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 25.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 26.Wiseman BS, Werb Z. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]