Abstract
Predicting the magnitude of enemy release in host–pathogen systems after introduction of novel disease resistance genes has become a central problem in ecology. Here, we develop a general quantitative framework for predicting changes in realized niche size and intrinsic population growth rate after introgression of disease resistance genes into wild host populations. We then apply this framework to a model host–pathogen system targeted by genetically modified and conventionally bred disease-resistant host lines (Trifolium repens lines expressing resistance to Clover yellow vein potyvirus) and show that, under a range of ecologically realistic conditions, the introduction of novel pathogen resistance genes into host populations can pose a quantifiable risk to associated nontarget native plant communities. In the host–pathogen system studied, we predict that pathogen release could result in an increase in the intrinsic rate of population growth of up to 15% and the expansion of host populations into some marginal environments. This approach has general applicability to the ecological risk assessment of all novel disease-resistant plant genotypes that target coevolutionary host–pathogen systems for improvement of agricultural productivity.
Keywords: enemy release hypothesis, genetically modified plants, nontarget ecosystems, risk assessment, Trifolium repens
Until relatively recently, it was thought that diseases were of little importance in most natural plant communities (1, 2), but now it is known that diseases can influence the genetic structure of host populations, the dynamics of host metapopulations, and even the physiognomic and floristic structure of entire natural plant assemblages (1, 3–5). Recent evidence also suggests that release from pathogen attack is at least partly responsible for the success of many invasive species worldwide (the enemy release hypothesis) (6, 7), and indeed several biological control programs owe their success to the suppressive effects of pathogens in weedy host populations (8). For these reasons, it has been suggested that novel pathogen-resistant plants may pose a major risk to the environment, because genes coding for pathogen resistance could result in population expansion and increased invasiveness of either the resistant plant itself or of sexually compatible nontarget species (9, 10). Certainly, the literature is replete with examples of crop protection by means of the introduction of disease resistance genes (11), but is this situation likely in natural systems, and under what conditions could it occur?
Although it is clear that newly introduced pathogens can have devastating impacts on naïve host populations (1), surprisingly little information is available about the role of pathogens in shaping the demography of long-term coevolved host–pathogen systems, although effects are likely to be highly variable (1, 2). Moreover, detailed experimental case studies are few, particularly for understudied but numerically important pathogen groups such as viruses (9). Because a number of plant species being targeted for genetic modification for pathogen resistance (12, 13) are also significant components of natural plant communities, this lack of information makes accurate prediction of enemy release in these systems extremely difficult. In this article, we develop a conceptual and experimental framework for evaluating host–pathogen interactions across the realized niche of the host plant and then assess its utility by experimentally quantifying the magnitude and pattern of host population changes expected in a model nonagricultural host–pathogen system after pathogen release.
Development of Host–Pathogen Enemy Release Model.
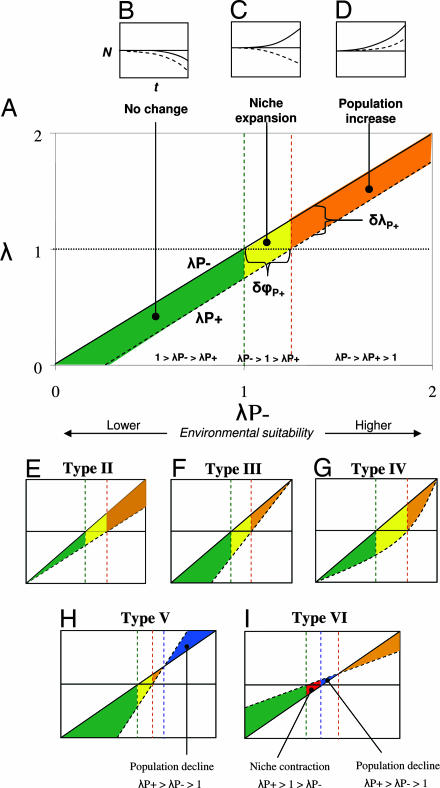
The performance of a host plant species in the environment can be framed in terms of the intrinsic rate of population increase, λ, which may be defined as λ = N(t + 1)/N(t), where N is the host plant population size and t is time. Expanding populations are characterized by λ > 1, whereas for contracting populations, λ < 1. The impact of a pathogen on host performance in an environmentally heterogeneous system can be quantified by comparing λ of genetically identical infected (λP+) and pathogen-free (λP−) host plant populations in suitable environments (λP− > 1) with those in unsuitable environments (λP− < 1) (Fig. 1A), given that the growth rate of pathogen-free host populations reflects environmental quality. In this model, the relative performance of infected populations is determined by plotting λP+ against λP− and comparing the resulting relationship with the null model y = x (Fig. 1A). If pathogen release results in a constant mean increase in λ irrespective of the value of λP+, three ecologically distinct situations are possible: (i) 1 > λP− > λP+, and populations remain nonviable irrespective of pathogen release (Fig. 1B); (ii) λP− > 1 > λP+, which leads to expansion of the realized niche (i.e., invasion of new habitats) (Fig. 1C); and (iii) λP− > λP+ > 1, where an increase in λ and size of the extant host population occurs (Fig. 1D). Here, the best estimate of the impact of a pathogen on performance of its host (δλP+) in a given habitat is δλP+ = λP− − λP+, with positive values indicating a reduction in λ associated with infection (Fig. 1A).
Fig. 1.
Potential relationships between pathogen impact on host populations and habitat suitability. (A) Schematic showing a type I scenario in which pathogen release changes the intrinsic growth rate (λ) and niche size (ϕ) of a host plant. Here λP− = λ of pathogen-free populations, and λP+ = λ of diseased populations. (B–D) Based on hypothetical exponential growth curves, an increase in λ results in three situations: no change in population or niche size (B), niche expansion (C), or population increase (D). In B–D, Nt = N0ert, where Nt is the population size after time t, and r = ln(λ). Shading in A indicates the difference between P− and P+ populations under conditions where λP− > λP+ > 1 (orange shading), λP− > 1 > λP+ (yellow shading), and 1 > λP− > λP+ (green shading). (E–I) Five further ways in which pathogens may influence host plant demographics. (E) Pathogen impact increases with performance of host populations, leading to population increase and realized niche expansion. (F) Pathogen impact is highest in the least favorable sites. (G) Pathogen impact is highest in intermediate environments, leading to greater niche expansion. (H) In the most favorable habitats, the pathogen increases host growth rate. If release were to occur, host populations would fall (λP+ > λP− > 1; blue shading). (I) In marginal environments, pathogen release would lead to population decline and host niche contraction (λP+ > 1 > λP−; red shading).
Given the above situations, there are six general scenarios in which a pathogen may influence the population growth rate of its host (Fig. 1 A and E–I). First, pathogenicity may be invariant with respect to host performance, with λP− and λP+ lines being parallel (Fig. 1A). In this scenario, mP− = mP+, but bP− > bP+, where m is the slope and b is the line intercept in the standard regression model y = mx + b. In the null model, mP− = 1, and bP− = 0. In the remaining scenarios, the impact of the pathogen depends on how the host–pathogen interaction is influenced by environmental quality. Pathogens may have a greater effect on λ in the most suitable environments (mP+ < mP−, and bP+ = 0) (Fig. 1E), in the most unfavorable environments (mP+ > mP−, and bP+ < 0) (Fig. 1F), or even in intermediate environments in a nonlinear manner (Fig. 1G). More complex situations could arise when pathogen-free populations perform better in some environments and worse in others (Fig. 1 H and I). This enemy release model therefore distinguishes among a range of host–pathogen scenarios on the basis of a small number of easily derived parameters.
Although there is little information available on the impact of environmental variation on host resistance or tolerance to pathogens in natural systems (14), some theoretical and experimental evidence for scenario types II (15) and III (14) exists. There is also evidence of benefits to infection in some host–pathogen systems (types V and VI) (16). In fact, these more complex interactions may occur frequently in nature. The endophytic fungi that infect many different grass hosts can decrease host reproductive success and alter plant growth but may confer benefits (e.g., drought tolerance, protection against herbivory), the magnitude of which will partly depend on community structure (17), thus generating a mosaic of positive and negative interactions. Similarly, interactions between plants and soil symbionts (e.g., mycorrhizal fungi, rhizobia) vary from mutualism to parasitism (18). Theoretical predictions (19) also suggest that evolutionary shifts between mutualism and parasitism as a function of environmental quality and host performance scenarios may be a general phenomenon.
Application of Enemy Release Model to a Model Host–Pathogen System.
To illustrate the utility of this framework for risk assessment, a 3-year study was conducted to assess the risk posed by transgenic and conventionally bred Trifolium repens L. lines expressing resistance to Clover yellow vein potyvirus (ClYVV) to a range of native nontarget grassland and woodland communities in southeastern Australia. T. repens (white clover) is a nitrogen-fixing forb native to the Mediterranean region of Europe that has now become the most economically important perennial pasture legume in temperate environments worldwide (20). In southern Australia, T. repens is grown over ≈15 million ha of grazing land (21) where annual precipitation exceeds 750 mm/year and crucially underpins the dairy, meat, and wool industries of this region. T. repens reproduces both clonally by stolon fragmentation and by seed; generation of the latter is determined by a well defined gametophytic self-incompatibility complex (22). ClYVV is a single-stranded RNA virus that infects a wide variety of host species, including T. repens. It is transmitted by aphids (e.g., Myzus persicae and Acyrthosiphon pisum) in a nonpersistent manner (23) but is not transmitted by white clover seed. Because of its strong impact on host growth, stolon production, nodulation rate, and yield (24, 25), ClYVV can have a multimillion-dollar economic impact on industries reliant on T. repens pastures for production (12).
Over the past decade, several commercial genotypes of T. repens have been genetically modified for virus resistance and field tested (12–13, 26) and, together with other lines expressing natural virus resistance, are intended for future agricultural release. However, T. repens is also a globally significant environmental weed, and in some high conservation value ecosystems in Australia, it has become an important invader of native plant communities (27, 28). Because ClYVV is widely distributed in Australia both in agricultural and high-quality remnant montane ecosystems (27, 29), one risk associated with release of virus-resistant lines is that wild populations in sensitive plant communities may acquire virus-resistance (trans)genes and expand in size (27). Because commercial use of T. repens is conducted on a very broad scale in Australia (30), numerous native plant communities could be at risk (27).
The strategy underpinning this study was to compare the performance of infected and virus-free genetically identical experimental T. repens populations across a range of ecologically threatened habitats varying in suitability for T. repens persistence and, based on application of our general enemy release model (Fig. 1), to determine the likely impact of pathogen release on the dynamics of wild T. repens populations. Accordingly, we selected invasive populations of T. repens from montane grassland and woodland communities in a representative study area in southeastern Australia (28) that are known to contain ClYVV (27), and we used plants from these populations to develop two host–pathogen arrays (one woodland and one grassland) that contained both virus-free and ClYVV-infected clonal lines. Each host–pathogen clonal array consisted of clonal cuttings from 10 randomly selected T. repens plants inoculated with each of seven randomly selected ClYVV lines and a virus-free control, thus containing a total of 10 ClYVV-free and 70 ClYVV-infected T. repens clone lines (80 ClYVV–T. repens combinations total).
Replicated field studies using both ClYVV-free and ClYVV-infected transplanted clone lines were then performed in 11 woodland and 11 grassland sites (22 study sites total), with T. repens clones and ClYVV lines separately collected from woodland and grassland environments being used for experiments conducted in each of these communities. Each study site contained 12–80 ClYVV–T. repens combinations, including genetically identical ClYVV-free and ClYVV-infected clone lines, thus allowing for a controlled assessment of the partial effects of ClYVV on site-level T. repens population dynamics. Data on plant survival, vegetative fragmentation, and seed production were collected over a 2-year period from these experimental plants, while seedbank dynamics were determined by performing a series of supplementary seed burial/recovery experiments in each study site. These data were used to determine the intrinsic population growth rates (λ) of all virus-free and ClYVV-infected experimental T. repens populations by using matrix-based population modeling of associated life cycle graphs (31). The final data set consisted of λ estimates for two populations of T. repens (one infected and one virus-free) at each of 21 study sites (one site was destroyed by feral animals).
Results and Discussion
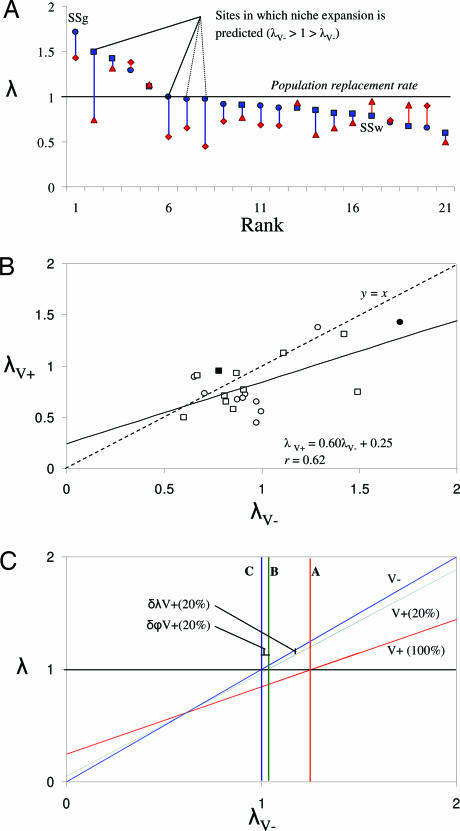
Population growth rate (λ) estimates for all 42 infected and virus-free T. repens populations are shown in Fig. 2A. Two-way ANOVA on combined grassland and woodland data, with site and ClYVV presence/absence as blocking variables, showed that λ of virus-free T. repens populations [mean λV− = 0.97, 95% confidence interval (C.I.) = 1.09, 0.84] significantly exceeded that of infected plants (mean λV+ =0.83, 95% C.I. = 0.95, 0.71) overall (F1,20 = 4.4, P = 0.02), representing a shift in the system from one of stability (λ ≈ 1) to one of population decline. Similar reductions in λ occurred in both grasslands (18%) and woodlands (11%). This result demonstrates that ClYVV presence can significantly alter the growth rate of wild T. repens populations. λV− was larger than λV+ in 14 of 21 sites, and in four sites, ClYVV-free populations were stable or expanding (λV− = 0.97–1.49), whereas infected populations were contracting rapidly (λV+ = 0.45–0.75); three of these populations were grassland (two mesic, one dry), whereas one was in a dry woodland site (Fig. 2A). No sites were characterized by populations where λV+ > 1 > λV−, and so collectively the data indicate that ClYVV may be responsible for elimination of T. repens populations from 4/21 = 19% of study sites when it is present in 100% of plants.
Fig. 2.
Impact of ClYVV on intrinsic rate of population growth (λ) of T. repens, based on experimental data. (A) λ of virus-free (blue shading) and virus-infected (red shading) populations of T. repens grown in 10 grassland (blue circles and red diamonds, respectively) and 11 woodland (blue squares and red triangles, respectively) sites ranked according to λ of virus-free populations (λV−). Solid blue bars indicate differences where λ of virus-free populations was greater; red bars indicate differences where λ of infected populations was greater. SSg, data from single-site grassland study; SSw, data from single-site woodland study, each containing 80 T. repens–ClYVV combinations. All other data are based on multiple-site studies (12 combinations each; see Methods). Niche expansion is predicted in two plots (where λV− > 1 > λV+(100%), see text for details); a further two plots in which niche expansion is predicted to be marginal (λV− = 0.97) are indicated by dashed lines. (B) The relationship between λ of virus-free (λV−) and infected populations (λV+). The null model, y = x, is shown as the dashed line. Grassland and woodland sites are shown as circles and squares, respectively; sites used in single-site studies are shown as filled symbols. (C) Predicted performance of ClYVV-free (V−) and ClYVV-infected (V+(100%) = 100% infection rate, V+(20%) = 20% infection rate) T. repens populations, based on modeled λV+ values. At line A, λV− = 1.25, at line B λV− = 1.03, and at line C, λV− = 1.0. For theoretical populations where the virus infection frequency is 20%, niche expansion (δϕV+(20%)) occurs between lines B and C (1.03 > λV− > 1.00), whereas population expansion (δλV+(20%)) occurs where λV+(20%) > B. The same model fit to experimental data (V+(100%)) is also provided. Additional details for C are provided in http://www.pnas.org/cgi/content/full/0608356104/DC1SI Text.
That λV+ was positively correlated with λV− (r = 0.62, P < 0.05; Fig. 2B) across all study sites indicates that environments most suitable for performance of ClYVV-free T. repens populations are also most favorable for infected populations; these populations occurred in a range of grassland and woodland communities (Fig. 2A). However, the slope of the relationship between λV+ and λV− was <1 (m = 0.60, 95% C.I. = 0.94, 0.26) (Fig. 2B), which indicates that the impact of ClYVV on T. repens populations is largest in sites where virus-free plants perform best. The associated regression equation also suggests that at very low values of λV−, virus presence may increase λ (Fig. 2B); but because the condition λV+ > 1 > λV− does not occur, the T. repens–ClYVV system appears to conform to a type II scenario (Fig. 1E). Clearly then, if wild T. repens populations were to be released from the effects of ClYVV, population growth and expansion of the realized niche would both be possible.
Given that the data presented in Fig. 2 A and B are based on experimental T. repens populations that are characterized by a mean ClYVV prevalence of 100%, it is important to predict the magnitude of these changes in more typical wild populations. We have shown (27) that ClYVV prevalence in wild T. repens populations in similar plant communities in Australia ranges from 0% to 60%, with a mean of ≈20%. Experimentally, the suppressive effect of ClYVV on λ where ClYVV prevalence is 100% is 11–18% (δλV+(100%); mean 15%). If we assume that the impact of ClYVV on λ of a given T. repens population is directly proportional to virus prevalence, then for a population in which mean prevalence is 20%, the mean effect of ClYVV on λ is δλV+(20%) = 0.2 × δλV+(100%) = 3%. Thus, it follows that if all plants were released from pathogenic effects, then the mean population growth rate would increase by ≈2% in woodlands and ≈4% in grasslands. Fig. 2C shows the predicted impact of ClYVV on wild T. repens populations growing in a range of environments with a virus prevalence of 20%. Because it is a type II scenario, the magnitude of enemy release depends on habitat suitability, with λ values increasing by up to 5% in the most favorable environments. Niche expansion (δϕP+, Fig. 1A) represents 3% of the experimental range in λV− values (1.7–0.6) observed in the study area (Fig. 2C). Using the same rationale, maximal observed values of ClYVV prevalence in wild T. repens populations of 60% (21) and a predicted value δλV+(100%) of 26% in the most favorable environments (i.e., where λV− = 1.7; Fig. 2B) indicate that the upper limits for site-level increases in λ and δϕP+ are 15% and 14%, respectively. The greatest increases are likely to occur in favorable grassland or woodland environments where population growth rates are high and where a majority of plants are infected with ClYVV.
These are probably worst-case estimates, because several factors in this system are likely to reduce the frequency of introgression and release of host populations from the effects of ClYVV. These factors include the variable level of resistance and/or tolerance to ClYVV exhibited by T. repens [supporting information (SI) Fig. 3], the largely clonal nature of many wild T. repens populations (32), the possibility of coimmigration of disadvantageous alleles from less persistent commercial lines of T. repens (30) from which pathogen-resistance transgenes are likely to be sourced, potential compensation for poor performance of infected plants by pathogen-free plants, pleiotropic effects of transgene insertion, and the possibility that introgression of genes coding for virus resistance will drive selection of more virulent strains of ClYVV. It is also possible that demographic data collected over a longer time frame could reveal conditions in which ClYVV has a different impact on host plants. Nevertheless, there appears to be a significant fitness cost associated with ClYVV infection; wild and cultivar populations of ClYVV are interfertile (the same species), and performance of wild–cultivar F1 hybrids has been shown to sometimes exceed that of some parental cultivars under grazed conditions (33), so long-term introgression of virus-resistance transgenes into wild populations of T. repens must be considered probable (34). If such introgression occurs, and ecological release results, then a small expansion of recipient populations in more suitable environments and niche expansion in a small subset of marginal habitats is likely. Given the number of communities invaded by T. repens (27) and the large commercial scale at which it is grown, we cannot rule out the possibility of ecological damage occurring in some situations. We know of no other case in which increased invasiveness and niche breadth of a weedy plant in endangered natural plant communities, introduced by means of resistance gene-mediated pathogen release, has been experimentally demonstrated to be an ecologically realistic scenario.
In the host–pathogen system investigated here, pathogenicity was much lower than that observed in many heavily cited cases where novel pathogens have devastated new host populations (3). Nonetheless, the data reported support the conclusion that plant pathogens can significantly reduce the fitness and growth rate of nonagricultural host populations and that novel pathogen-resistance genes that target these systems may pose a quantifiable risk to nontarget ecosystems. These data thus provide further evidence of the potential for variation in host resistance to influence patterns of disease incidence and prevalence and underscore the need for studies that explicitly link life history, epidemiology, and genetics in natural systems (35). Here, we have presented a general framework for assessing environmental risks associated with host–pathogen interactions, which uses plant performance as an indicator of environmental quality. Rigorous evaluation of plant performance and pathogen behavior requires consideration of a range of biophysical variables (e.g., community structure, vector abundance, disturbance regimes, soil moisture, and nutrient levels), the relative importance of which may vary for different systems. Thus, from both applied and theoretical perspectives, the results presented emphasize the importance of embedding studies of host–pathogen interactions within a community ecology context.
Methods
Establishment of Study Area and T. repens–ClYVV Treatment Arrays.
All experimental trials were conducted by using a series of T. repens and ClYVV lines that were collected from a 9-km2 study area located at Grassy Creek (lat 36°52′S, long 148°57′E) in southern Namadgi National Park, Australian Capital Territory, Australia. The study area contains endangered natural temperate grassland communities that have been invaded by extensive wild populations of T. repens infected with ClYVV (27, 28). The vegetation at the study site consists of frost hollows that are dominated by tussock grasses (Poa spp.) grading into a Eucalyptus forest complex that occurs on shallower soils at higher elevations (28). In August 2002, 100 T. repens stolons from different white clover plants were collected from each of two sites (one woodland and one grassland) in the Grassy Creek catchment. Stolons were established in a cold room and subsequently moved to a greenhouse for further growth. Leaf samples were removed from all plants and screened for the presence of a range of viruses and other pathogens by using indicator plant bioassays (27) and PCR of viral coat protein segments based on Bariana et al. (36) and Sasaya et al. (37). Based on these data, seven white clover plants from both the woodland and grassland collection were identified as being infected with ClYVV (ClYVV+); 20 ClYVV− lines (10 woodland, 10 grassland) were also randomly selected from the pool of virus-free wild lines. Clones of each of the 10 ClYVV− lines from each vegetation community were inoculated with ClYVV inoculum from each of the 7 related ClYVV+ lines, and a virus-free control line was retained for all ClYVV− lines. This inoculation regime yielded two 8 × 10 T. repens-ClYVV treatment arrays (SI Fig. 4). Positive infections were recorded for all combinations except ClYVV− controls.
Experimental Site Selection and Establishment.
Four experiments were conducted in the study area: (i) two single-site (SS) studies that were conducted with 80 virus–clover treatment combinations from grassland and woodland collection series but in only one study site each (n = two sites total); and (ii) two multiple-site (MS) studies that were conducted with 12 clover–virus treatment combinations but replicated over 10 sites in each of a range of grassland and woodland plant communities (n = 20 sites total). These experiments were designed to capture maximal genetic and environmental variability, respectively. Individual sites used in all SS and MS studies were selected from a larger sample of 40 27-m2 sites located across the Grassy Creek catchment (28), with MS study sites selected to represent the full range of floristic variability in these vegetation types and SS study sites that were selected from near the center of associated community ordination diagrams. Cuttings from each T. repens–ClYVV combination were taken from parental source lines, established, and then randomly planted into each of the study sites in November 2003.
After seed collection in summer 2005, two experiments were used to determine seedbank dynamics of T. repens in all study sites: (i) a seed burial experiment, aimed at determining the rate of breakdown of seed dormancy under field conditions; (ii) a sown-seed experiment, aimed at determining seed germination rates and survivorship at different times of year. In the seed burial experiment, plastic mesh packets containing 20–50 seeds collected from wild T. repens were lightly covered with litter in each of the 22 site locations described above. Over the course of 1 year packets were removed at 3-month intervals and seedbank dormancy and persistence were determined. In this experiment, seed from wild plants were used because experimental plants produced too few seed to enable accurate description of seedbank dynamics. However, seed viability and dormancy testing conducted by using seed collected from experimental plants in January 2005 showed that ClYVV− and ClYVV+ plants produced seed with similar dormancy (81% vs. 82%), viability (98% vs. 96%), and 1,000-seed weight (0.65 vs. 0.64 g). Dormancy and viability of seed collected from wild T. repens plants were also similar (86% and 96.9%, respectively). Packets containing 21–50 seeds collected from experimental ClYVV− and ClYVV+ plants that were buried alongside seed collected from wild plants in four sites also showed similar dormancy after 12 months (60% vs. 63%, respectively), which further indicates that the effects of ClYVV infection on parent plants have no impact on seed dormancy or viability or on seedbank dynamics. The sown-seed experiment was conducted by hand, with 25 seed from T. repens sown into two plots at each of 22 locations (n = 44 plots) in grassland and woodland environments and then recording germination and seedling survival rates at monthly intervals over the following year.
Data Collection.
Survival was recorded for all plants in January 2004, April 2004, August 2004, October 2004, April 2005, October 2005, and April 2006. ClYVV− plants were also periodically tested for infection by ClYVV; newly infected plants were removed from the data set. Seed production data were obtained for the December 2004–April 2005 reproductive period. In the seed burial experiment, seed packets were buried in March 2005, and one seed packet containing 50 seed was then removed from each site in June 2005, September 2005, and December 2005. All remaining packets were removed in March 2006. Seed dormancy and persistence were obtained for seed collected at all census dates. In the sown-seed experiment, plots were surveyed for the presence of T. repens seedlings monthly, beginning in April 2005 and ending in March 2006.
Stage-Class Population Analysis.
Life cycle graphs were developed for ClYVV-infected and ClYVV-free populations of T. repens in all sites, except one where disturbance destroyed all experimental plants. The overall life cycle graph for T. repens (SI Fig. 5) was applied to seasonal data, resulting in spring, summer, autumn, and winter diagrams, each containing a subset of all population transitions. Mean transition probabilities were determined based on survival data collected between April 2004 and October 2005, and fecundity rates were determined based on flower head and seed production data collected between November 2004 and April 2005. Seedbank dynamics, seedling emergence, and survival rates were determined based on data collected between April 2005 and March 2006. The intrinsic rate of population increase (λ) was determined for ClYVV-infected and ClYVV-free populations in all 21 sites for which data were available (one site was destroyed by feral animals) by using temporal projection of a hypothetical starting population vector n(t) that contained 100 vegetative adults, with four seasonal projection matrices A1–4 according to the equation n(t + 1) = Ann(t) (31). The projection interval for each iteration was 3 months, such that 1 year of demographic changes is represented by n(t + 4). The four seasonal projection matrices A1–4 were determined based on the seasonal life cycle graphs shown in SI Fig. 5. λ was determined by comparing numbers of vegetative adults at t + 4 with numbers present at time t after stabilization of the stage-class vector. Final data consisted of λ values for ClYVV− and ClYVV+ populations at each of the 21 final study sites (total n = 42). The overall impact of ClYVV on population-level λ was determined by performing two-way ANOVA with ClYVV presence/absence and site (n = 21) as blocking variables. The relationship between λV+ and λV− was determined by using standard linear regression with λV− and λV+ as predictor and dependent variables, respectively. All methods pertaining to site selection, development of host–pathogen arrays, experimental protocols, and population modeling are described in greater detail in http://www.pnas.org/cgi/content/full/0608356104/DC1SI Text.
Supplementary Material
Acknowledgments
We thank Matthew Woods for field work assistance, Augusto Becerra Lopez-Lavalle and Linda Broadhurst for expertise in PCR protocols, Paul Chu for assistance with virological work, and Jeremy Burdon for manuscript feedback. This work was supported by Dairy Australia. Environment ACT provided access to study sites.
Abbreviations
- ClYVV
Clover yellow vein potyvirus
- SS
single-site.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608356104/DC1.
References
- 1.Burdon JJ. Diseases and Plant Population Biology. Cambridge, UK: Cambridge Univ Press; 1987. [Google Scholar]
- 2.Roy BA, Kirchner JW, Christian CE, Rose LE. Evol Ecol. 2000;14:421–438. [Google Scholar]
- Dickman A. In: The Fungal Community: Its Organization and Role in the Ecosystem. 2nd Ed. Carroll G, Wicklow D, editors. New York: Dekker; 1992. pp. 499–520. [Google Scholar]
- 4.Dobson A, Crawley M. Trends Ecol Evol. 1994;9:393–398. doi: 10.1016/0169-5347(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 5.Thrall PH, Burdon JJ. J Ecol. 1997;85:743–753. [Google Scholar]
- 6.Mitchell CE, Power AG. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- 7.Keane RM, Crawley MJ. Trends Ecol Evol. 2002;17:164–169. [Google Scholar]
- Rosskopf EN, Charudattan R, Kadir JB. In: Handbook of Biological Control. Bellows T, Fisher T, editors. San Diego: Academic; 1999. pp. 894–918. [Google Scholar]
- 9.Tepfer M. Annu Rev Phytopathol. 2002;40:467–491. doi: 10.1146/annurev.phyto.40.120301.093728. [DOI] [PubMed] [Google Scholar]
- Power AG. In: Genetically Engineered Organisms: Assessing Environmental and Human Health Effects. Letourneau D, Burrows B, editors. Boca Raton, FL: CRC; 2002. pp. 125–142. [Google Scholar]
- Russell GE. Plant Breeding for Pest and Disease Resistance. London: Butterworths; 1978. [Google Scholar]
- Kalla R, Chu P, Spangenberg G. In: Molecular Breeding of Forage Crops. Spangenberg G, editor. Dordrecht, The Netherlands: Kluwer; 2001. pp. 219–237. [Google Scholar]
- 13.Dudas B, Woodfield DR, Tong PM, Nicholls MF, Cousins GR, Bugess R, White DWR, Beck DL, Lough TJ, Forster RLS. N Z J Agric Res. 1998;41:171–178. [Google Scholar]
- 14.Kniskern JM, Rausher MD. Ecology. 2006;87:675–685. doi: 10.1890/05-1327. [DOI] [PubMed] [Google Scholar]
- 15.Thrall PH, Jarosz AM. J Ecol. 1994;82:549–559. [Google Scholar]
- 16.Gibbs A. Intervirology. 1980;13:42–47. doi: 10.1159/000149105. [DOI] [PubMed] [Google Scholar]
- 17.Clay K. Res Popul Ecol. 1996;38:191–201. [Google Scholar]
- 18.Johnson NC, Graham JH, Smith FA. New Phytol. 1997;135:575–585. [Google Scholar]
- 19.Hochberg ME, van Baalen M. In: Evolutionary Biology of Host-Parasite Relationships: Theory Meets Reality. Poulin R, Morand S, Skorping A, editors. Amsterdam: Elsevier; 2000. pp. 81–96. [Google Scholar]
- 20.Jahufer MZZ, Cooper M, Ayres JF, Bray RA. Aust J Agric Res. 2002;53:239–257. [Google Scholar]
- 21.Abberton MT, Marshall AH. J Agric Sci. 2005;143:117–135. [Google Scholar]
- Thomas RG. In: White Clover. Baker M, Williams M, editors. Wallingford, UK: CAB International; 1987. pp. 1–29. [Google Scholar]
- 23.Hollings M, Nariani TK. Ann Appl Biol. 1965;56:99–109. [Google Scholar]
- 24.Gibson PB, Barnett OW, Skipper HD, McLaughlin MR. Plant Disease. 1981;65:50–51. [Google Scholar]
- 25.Gibson PB, Barnett OW, Burrows PM, King FD. Plant Disease. 1982;66:142–144. [Google Scholar]
- 26.Zhao G, Ping M, Chu P. J Agric Biotech. 2005;13:230–234. [Google Scholar]
- 27.Godfree RC, Chu PWG, Woods MJ. Aust J Bot. 2004;51:1–11. [Google Scholar]
- 28.Godfree RC, Vivian LM, Lepschi BJ. Biol Invasions. 2006;8:1159–1178. [Google Scholar]
- 29.Norton MR, Johnstone GR. Aust J Agric Res. 1998;49:723–728. [Google Scholar]
- 30.Lane LA, Ayres JF, Lovett JV. Aust J Exp Agric. 1997;37:831–839. [Google Scholar]
- Caswell H. Matrix Population Models: Construction, Analysis, and Interpretation. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 32.Turkington R, Cahn MA, Vardy A, Harper JL. J Ecol. 1979;67:231–243. [Google Scholar]
- 33.Bouton JH, Woodfield DR, Hoveland CS, McCann MA, Caradus JR. Crop Sci. 2005;45:1596–1602. [Google Scholar]
- 34.Ellstrand NC, Prentice HC, Hancock JF. Annu Rev Ecol Syst. 1999;30:539–563. [Google Scholar]
- 35.Thrall PH, Burdon JJ. Plant Pathol. 2000;49:767–773. [Google Scholar]
- 36.Bariana HS, Shannon AL, Chu PWG, Waterhouse PM. Phytopathology. 1994;84:1201–1205. [Google Scholar]
- 37.Sasaya T, Shimizu T, Nozu Y, Nishigchi M, Inouye N, Koganezawa H. Phytopathology. 1997;87:1014–1019. doi: 10.1094/PHYTO.1997.87.10.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.