Abstract
CD4+CD25+Foxp3+ regulatory T cells (T regs) are important for preventing autoimmune diabetes and are either thymic-derived (natural) or differentiated in the periphery outside the thymus (induced). Here we show that β-cell peptide-pulsed dendritic cells (DCs) from nonobese diabetic (NOD) mice can effectively induce CD4+CD25+Foxp3+ T cells from naïve islet-specific CD4+CD25− T cells in the presence of TGF-β1. These induced, antigen-specific T regs maintain high levels of clonotype-specific T cell receptor expression and exert islet-specific suppression in vitro. When cotransferred with diabetogenic cells into NOD scid recipients, T regs induced with DCs and TGF-β1 prevent the development of diabetes. Furthermore, in overtly NOD mice, these cells are able to significantly protect syngeneic islet grafts from established destructive autoimmunity. These results indicate a role for DCs in the induction of antigen-specific CD4+CD25+Foxp3+ T cells that can inhibit fully developed autoimmunity in a nonlymphopoenic host, providing an important potential strategy for immunotherapy in patients with autoimmune diabetes.
Keywords: antigen-presenting cells, autoimmunity, type 1 diabetes, nonobese diabetic (NOD) mice
The nonobese diabetic (NOD) mouse models the pathogenesis of human type 1 diabetes and allows the study of potential therapeutics (1). In NOD mice, the thymic-derived CD4+CD25+ regulatory T cells (T regs) expressing the transcription factor Foxp3 suppress autoimmunity and delay the development of diabetes (2). Considerable effort has focused on expanding the small numbers of these so-called “natural” CD4+CD25+ T regs in the NOD and other models (3–6). Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that can effectively expand and sustain antigen-specific CD4+CD25+ T regs (3, 4). However, in humans, only the infrequent cells with high CD25 expression have regulatory function (7). Alternatively, the more abundant CD4+CD25− T cells can be differentiated into CD4+CD25+ T regs that express Foxp3 by stimulation with mitogenic antibodies in the presence of TGF-β1, although it is not known whether these induced cells are functionally identical to T regs that develop in the thymus (8). Such “induced T regs” with islet specificity can prevent diabetes in lymphopoenic models (9), but their ability to induce tolerance at late pathogenic stages of autoimmunity, such as in already-diabetic NOD mice, has not been fully addressed.
A separate issue that remains to be addressed is the requirement for APCs in the induction of T regs with TGF-β1. The use of DCs instead of mitogenic stimuli to differentiate T regs de novo from CD4+CD25− T cells has numerous potential advantages, including selection and maintenance of antigen specificity (10, 11); provision of paracrine TGF-β1 (12–14), which is known to play an important role in T reg homeostasis by its ability to induce Foxp3 (8, 15); and/or provision of costimulatory signals such as CD80/86 for CTLA-4 ligation, which is necessary for TGF-β1-dependent induction of Foxp3 (16, 17).
In this study, we examined the ability of DCs from NOD mice to induce islet antigen-specific CD4+CD25+Foxp3+ T cells from naive CD4+CD25−Foxp3− T cells. By using T cells from BDC2.5 mice, a well described diabetogenic CD4+ TCR transgenic system (18), we show that DCs, together with specific peptide and TGF-β1, induce CD4+CD25+Foxp3+ T cells that maintain islet-antigen specificity. The stimulation with DCs and TGF-β1 results in T cells that have high levels of Foxp3 as well as specific TCR expression, and these T cells are able to suppress proliferation and cytokine responses in vitro. Importantly, the DC+TGF-β1-induced CD4+CD25+Foxp3+ T cells also are potent suppressors of ongoing autoimmune diabetes in vivo and provide significant protection for syngeneic islet grafts in diabetic mice from established autoimmune destruction.
Results
Splenic CD11c+ DCs Induce Differentiation of CD4+CD25+Foxp3+ T Regs in the Presence of TGF-β1.
To test the role of DCs in the de novo differentiation of CD4+CD25+Foxp3+ T regs, we used CD4+CD25− T cells from the BDC2.5 TCR transgenic NOD mice. These T cells respond to both an unidentified autoantigen expressed in the secretory granules of islet β cells and a mimetope peptide (BDC) (18, 19). We isolated CD11c+ DCs from splenocytes of NOD mice and characterized them for expression of CD40, CD86, and MHCII (Fig. 1A). Compared with LPS-matured DCs, these freshly isolated DCs express weaker CD40 and MHC II and significantly less CD86 (data not shown), suggesting a relatively immature phenotype. We also characterized a starting population of CD4+CD25−CD62L+ BDC2.5 T cells, after FACS sorting to a purity of >95%, and found the expected small fraction of Foxp3+ cells (Fig. 1B). We then added the DCs together with the BDC peptide to the CD4+CD25− BDC2.5 T cells in the presence or absence of TGF-β1. After 6 days of culture, we saw good induction of Foxp3 mRNA (Fig. 1D) and protein (Fig. 1B), but only when TGF-β1 was included in the cultures. In the presence of TGF-β1, the frequency of Foxp3+ cells increased to 88.3 ± 7% (n = 3; Fig. 1B), and their numbers increased ≈50- to 100-fold (Fig. 1C). The newly formed Foxp3+ cells appeared by 2 days of culture, and the total number of Foxp3+ cells peaked at 4 days and decreased after 6 days. Because of the rapid appearance of the Foxp3+ cells, the increase observed is unlikely to be caused by the expansion of the few Foxp3+ cells in the starting population. In the absence of TGF-β1, the percentage of Foxp3+ cells remained close to baseline, although CD25 expression was still induced (Fig. 1B). Quantification of Foxp3 mRNA expression by real-time RT-PCR demonstrated that the level induced by the DCs and TGF-β1 was equivalent to that found in naturally occurring T regs (Fig. 1D). The induction of Foxp3+ cells by TGF-β1 was dose-dependent, being readily evident at 0.01 ng/ml and peaking at 1 ng/ml (Fig. 1E). Therefore, DCs can serve as effective APCs for differentiating Foxp3+ T cells from CD4+CD25−Foxp3− precursors in the presence of low-dose TGF-β1.
Fig. 1.
Splenic DCs efficiently induce Foxp3 expression from naïve CD4+CD25− T cells. (A) Cell surface expression of CD40, CD86, and MHC class II (I-Ag7) of freshly isolated NOD splenic CD11c+ DCs. (B) Foxp3 expression by precultured sorted CD4+CD25−CD62L+ BDC2.5 T cells and induction in T cells after culture with or without 2 ng/ml TGF-β1 on day 6 of culture. Expression of CD62L by precultured CD4+CD25− BDC2.5 T cells is also shown. (C) Time-course of induction of CD4+CD25+Foxp3+ BDC2.5 T cells from naïve CD4+CD25−Foxp3− BDC2.5 T cells in the presence of 2 ng/ml TGF-β1. (Upper) Total number of Foxp3+ T cells per well. (Lower) Percentages of Foxp3+ T cells determined by intracellular staining on days 2, 3, 4, 5, 6, and 10 of DC-T cultures. The isotype control for day 3 is shown. (D) Quantification of Foxp3 mRNA by real-time RT-PCR. Samples were prepared from enriched CD25+ fractions of the resulting T cells from cocultures with or without 2 ng/ml TGF-β1 or freshly isolated CD4+CD25+ and CD4+CD25− BDC2.5 T cells. Values were standardized by 18s RNA and expressed as fold of increase compared with precultured freshly isolated CD4+CD25− cells. (E) Dose-response of TGF-β1 determined on day 6 of DC-T cocultures at indicated concentrations of TGF-β1. The isotype control for the 0.01 ng/ml dose is shown. All results are representative of two to four separate experiments.
Characterization of CD4+CD25+Foxp3+ T Regs Induced by DCs and TGF-β1.
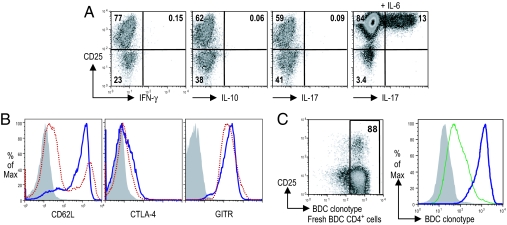
We next determined cytokine expression by T cells cultured with splenic DCs and TGF-β1. No IFN-γ or IL-10 was observed (Fig. 2A). Because TGF-β1 is able to induce IL-17-secreting cells or Foxp3+ cells depending on the presence or absence of IL-6 (20), we also tested IL-17 production. T cells cultured with splenic DCs and TGF-β1 were negative for IL-17 expression, whereas as a positive control, T cells cultured with IL-6 and TGF-β1 did express IL-17 (Fig. 2A).
Fig. 2.
Characterization of CD4+CD25+Foxp3+ BDC T cells induced by DCs and TGF-β1. (A) Intracellular expressions of IFN-γ, IL-10, and IL-17 on day 6 of DC-T coculture. The addition of 20 ng/ml IL-6 induced IL-17 expression. (B) Expression of CD62L, CTLA-4, and GITR on day 6 of DC-T coculture. Cells cultured in the absence of TGF-β1 were also shown for comparison. Shaded histogram, isotype control; blue line, +TGF-β1; red dotted line, −TGF-β1. (C) BDC clonotype expression. (Left) Freshly isolated BDC2.5 CD4+ T cells. (Right) T cells on day 6 after culture. Shaded histogram, isotype control; blue line, T cells cultured with DCs and TGF-β1; green line, T cells cultured with anti-CD3, anti-CD28, and TGF-β1. All results are representative of two to four separate experiments.
Next, we assessed the expression of proteins associated with T reg function in the CD4+CD25+Foxp3+ T cells differentiated by splenic DCs and TGF-β1. As shown in Fig. 2B, a greater percentage of T cells cultured in the presence of TGF-β1 retained CD62L expression compared with those cultured in the absence of TGF-β1. T cells cultured either with or without TGF-β1 up-regulated both CTLA-4 and glucocorticoid-induced TNF receptor (GITR; Fig. 2B), whereas freshly purified T cells were negative for both (data not shown).
Because the level of TCR expression contributes to the avidity of T cell responses, we measured BDC clonotype expression before and after stimulation. Approximately 90% of freshly isolated CD4+ BDC2.5 T cells were clonotype+ (Fig. 2C). Stimulation with splenic DCs pulsed with the BDC peptide maintained high levels of clonotype expression. In contrast, nonspecific stimulation with anti-CD3/CD28 resulted in much lower clonotype expression (Fig. 2C). These observations indicate that the DC and TGF-β1-induced T regs share many features with natural T regs.
In Vitro Suppression of CD4+CD25+Foxp3+ T Regs Induced by DCs and TGF-β1.
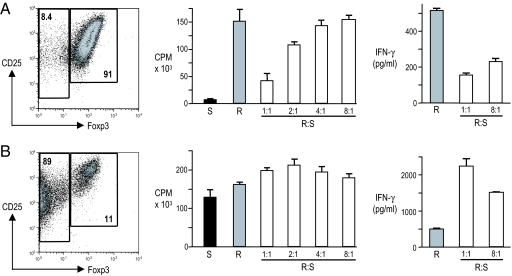
To test for the suppressive function of the CD4+CD25+Foxp3+ T cells induced with splenic DCs and TGF-β1, we first used an in vitro assay. We depleted CD11c+ cells and enriched the CD25+ T cell fraction from 6-day cultures with DCs and added the resulting CD4+CD25+ T cells in graded doses to responder CD4+CD25− BDC2.5 T cells. We then measured the inhibition of proliferation to BDC peptide presented by whole NOD splenic APCs. As shown in Fig. 3A, in the presence of TGF-β1, the DC-induced CD4+CD25+ T cells were 91% Foxp3+ after enrichment. They were anergic in response to BDC peptide and were able to block the proliferation of naïve BDC2.5 T cells to BDC peptide (70% suppression at a 1:1 ratio). This ratio of T regs needed for suppression was greater than required for DC-expanded natural T regs, which gave similar suppression of proliferation with 4-fold fewer T regs (4). Suppression of IFN-γ secretion was also observed and at a responder/suppressor ratio as low as 8:1 (Fig. 3A). In contrast, in the absence of TGF-β1, the resulting CD4+CD25+ T cells were only 11% Foxp3+ after enrichment. They showed vigorous proliferation to BDC peptide themselves, were unable to suppress proliferation of naïve BDC2.5 T cells, and led to augmented IFN-γ secretion (Fig. 3B). Therefore, the induced Foxp3+ T regs have some suppressive function in vitro, although they are less potent than natural T regs after expansion with DCs (4).
Fig. 3.
CD4+CD25+Foxp3+ BDC T cells induced by DCs and TGF-β1 suppress BDC-specific T cell proliferation and cytokine production. After 6 days of culture with (A) or without (B) TGF-β1, CD11c+ cells were depleted and CD25+ cells were enriched. FACS plots constructed after the enrichment are shown (Left). Proliferation assays were set up with CD4+CD25− cells from BDC2.5 mice [responders (R)], APCs from NOD mice, and BDC peptide (100 ng/ml). Increasing numbers of the induced CD4+CD25+ cells [suppressors (S)] were added at ratios indicated. Proliferation was assessed by [3H]thymidine uptake (Center), and IFN-γ was measured from culture supernatants (Right) as described in Materials and Methods.
CD4+CD25+Foxp3+ T Regs Induced by DCs and TGF-β1 Suppress Autoimmune Diabetes in Vivo.
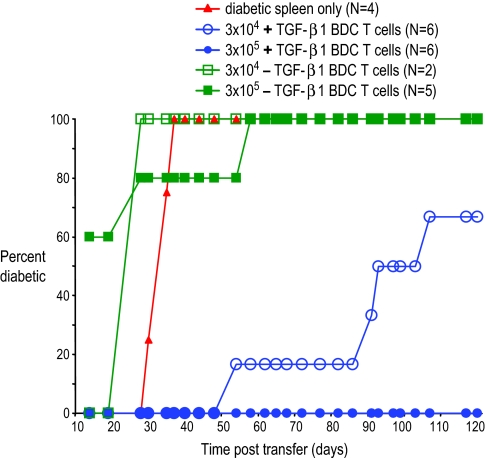
A more critical function for naturally occurring CD4+CD25+ T regs is the suppression of autoimmunity in vivo. To test whether the T regs induced by splenic DCs and TGF-β1 could inhibit autoimmune diabetes, we first used adoptive transfer in NOD.scid recipients. In this model, injection of diabetogenic splenocytes from acutely diabetic NOD mice into NOD.scid recipients results in rapid autoimmune destruction of the native pancreas and consequent development of hyperglycemia (21). Because a known quantity of diabetogenic splenocytes is injected into the NOD.scid recipients, this model allows for a measurement of the potency of the DC-induced T regs according to the kinetics of diabetes development after injection. As shown in Fig. 4, i.v. coinjection of 3 × 105 of the DC+TGF-β1-induced CD4+CD25+ T cells along with 107 diabetogenic splenocytes resulted in complete protection from the development of diabetes (0/6 recipients developed diabetes at 120 days after injection). In addition, 10-fold fewer (3 × 104) of the same T cells coinjected with 107 diabetogenic splenocytes still resulted in a significant delay of onset and a lower incidence of diabetes. In contrast, injection of either dose of the CD4+CD25+ T cells from cultures without TGF-β1 along with 107 diabetogenic splenocytes resulted in accelerated onset of diabetes in NOD.scid recipients.
Fig. 4.
CD4+CD25+Foxp3+ BDC T cells induced by DCs and TGF-β1 block the development of diabetes in NOD.scid recipients. NOD.scid mice were injected i.v. with 107 spleen cells from diabetic NOD females with either nothing (red triangles) or the indicated numbers of CD4+CD25+ BDC T cells from cultures with splenic DCs in the presence (+TGF-β1 BDC T, blue circles) or absence (−TGF-β1 BDC T, green squares) of TGF-β1. P < 0.0001, control vs. 3 × 105 +TGF-β1 BDC T cells; P < 0.0001, control vs. 3 × 104 +TGF-β1 BDC T cells.
Next, we tested the efficacy of these T regs in blocking autoimmune destruction of a syngeneic islet graft in spontaneously diabetic NOD recipients. This model represents a clinically relevant scenario of diabetes pathogenesis in which islet-specific effector cells are already present in a nonlymphopoenic host. Without intervention, ongoing autoimmunity directed toward the transplanted islet β cells results in graft destruction within 5–17 days after transplantation (22). To avoid the confounding factor of T reg cell trafficking, we chose to directly deposit the T regs at the site of the islet graft, i.e., the kidney subcapsular space. When we cotransplanted 3 × 105 DC+TGF-β1-induced CD4+CD25+ T cells along with the syngeneic islets into the kidney subcapsular space of diabetic NOD recipients, there was a significant prolongation of graft survival (Fig. 5). In contrast, CD4+CD25+ T cells induced in the absence of TGF-β1-accelerated isograft destruction.
Fig. 5.
CD4+CD25+Foxp3+ BDC T cells induced by DCs and TGF-β1 protect syngeneic islet grafts from ongoing autoimmune destruction in spontaneously diabetic NOD recipients. A total of 500 NOD islets were transplanted into kidney subcapsular space either alone (red) or with 3 × 105 +TGF-β1 BDC T cells (blue) or −TGF-β1 BDC T cells (green). Day 0 indicates the day of islet transplantation. (A) Blood glucose levels post transplantation. Each line represents one mouse. (B) Summary of graft survival after transplantation for individual mice is shown in (A). P < 0.0001 for comparison of graft survival among all three groups; P = 0.0069, −TGF-β1 BDC T vs. islets only; P = 0.0001, +TGF-β1 BDC T vs. islets only. Data represent the combination of three separate transplant experiments with cells from three separate cultures.
In both the adoptive transfer NOD.scid model and the spontaneous diabetes NOD model, the CD4+CD25+Foxp3+ T regs induced by DCs and TGF-β1 were therefore able to effectively block autoimmunity mediated by a diverse repertoire of autoreactive TCR specificities, analogous to the previously reported properties of “natural” T regs.
Discussion
In this study, we have shown two unique findings: (i) DCs can differentiate Foxp3− cells en masse to Foxp3+ cells ex vivo in the presence of TGF-β1 and (ii) in vivo, islet-specific T regs differentiated with DCs and TGF-β1 can protect islet grafts from established destructive autoimmunity in diabetic NOD mice.
Demonstration of the in vivo potency of the DC+TGF-β1-induced T regs in the NOD model is important because although these ex vivo-induced T regs express similar levels of Foxp3 as the thymic-derived T regs, they may not be functionally identical a priori. In fact, the in vitro suppression of T regs induced with DCs and TGF-β1 is not as potent as previously observed with natural T regs (Fig. 3 and ref. 4). This finding suggests that factors other than Foxp3 expression levels may be important for T reg function. Here we show that despite this decreased in vitro potency, DC+TGF-β1-induced T regs with islet specificity can suppress pathogenic T cells of other specificities and inhibit diabetes development, as had been shown for naturally occurring T regs (4, 23).
Identifying DCs as an APC population for differentiation of regulatory cells has important implications both for understanding of the basic biology of how T regs may be induced in vivo and for the ability to translate this system for clinical applications. A recent study reports that the targeted delivery of antigen to DCs in vivo induces de novo differentiation of CD4+CD25+Foxp3+ cells from naïve CD4+CD25−Foxp3− T cells (24). Although this approach provides a limited yield of T regs and has not yet been used to suppress autoimmune disease, it suggests that the process we are describing here with DCs and TGF-β1 in vitro also occurs in vivo. DCs presenting antigen induce effector cells, but here we show that the addition of one cytokine, TGF-β1, shifts the differentiation to suppressor cells. With the system described here, we can now define molecular signals that DCs may provide to ensure efficient TGF-β1-mediated induction of Foxp3.
Antigen-specific T regs are more potent suppressors of autoimmunity than polyclonal T regs; therefore, methods for the expansion of islet-specific T regs will likely contribute to human therapy of type 1 diabetes. Direct injection of either DCs from pancreatic draining lymph nodes or β cell antigen-pulsed immature DCs has been shown to protect prediabetic NOD recipients from developing diabetes, possibly through the in vivo induction of regulatory T cells (25, 26). In vitro, beads coated with complexes of islet peptide-MHC class II molecules can expand islet-specific CD4+CD25+ T regs from a polyclonal NOD T reg population (6). However, the lack of defined peptides in the human disease may limit this approach. Conditions that would allow islet-loaded DCs to selectively enrich islet-specific T regs from autoimmune individuals should be investigated.
In allogeneic transplantation models, donor-specific T regs also have been shown to mediate transplant tolerance and to prevent graft rejection (27). Studies with therapeutic manipulations of transplant recipients have shown in vivo generation and/or expansion of alloantigen-specific T regs as a potential mechanism for transplant tolerance induction (28). An important difference between alloimmune and autoimmune tolerance induction is that alloreactive T cells are found at greater initial frequencies than autoreactive T cells, presumably because of cross-reactivities between numerous allo-MHC peptide complexes and self-MHC molecules. Consequently, T reg therapies in transplant models, such as skin allograft and graft-versus-host disease models, typically required large numbers (106 to 107) of T regs to achieve alloantigen-specific suppression in vivo (29–31). Therefore, strategies to expand T regs may also be important for the treatment of transplant rejection. The role of DCs in the ex vivo expansion of donor-specific T regs only recently has begun to be elucidated (11). Early studies have reported that the injection of donor MHC class I allopeptide-pulsed host DCs prolong the survival of rat islet allografts (32) and have implicated CD4+CD25+ T regs in the salutary effect on graft survival (33). The system we describe here, in which we use DCs and TGF-β1 to differentiate large numbers of antigen-specific CD4+CD25+Foxp3+ T regs, also may provide a clinically applicable tool for cell therapy in transplant tolerance induction.
In summary, our findings show that DCs can be harnessed for efficient ex vivo differentiation of islet-specific CD4+CD25+ Foxp3+ T regs from naïve CD4+CD25− T cells. Therapy with such ex vivo-generated T regs may provide additional treatments for autoimmune diseases and for transplantation rejection (34).
Materials and Methods
Mice.
NOD, NOD.scid, and BDC2.5 TCR transgenic mice were purchased from The Jackson Laboratory (West Grove, PA). Mice were used according to institutional guidelines and protocols approved by the institutional Animal Care and Use Committees at Northwestern University, Cornell University, and The Rockefeller University.
Antibodies.
Biotinylated mAbs for CD8 (53–6.7), CD25 (7D4), Ly-76 (Ter-119), Gr1 (RB6–8C5), CD49b/Pan-NK (DX5), B220 (RA3–682), and CD11b (M1/70); FITC-conjugated anti-CD4 (GK1.5), CD11c (HL3), I-Ag7 (OX-6); PE-conjugated anti-IL-17 (TC11–18H10), IL-10 (JES5–16E3), CD25 (PC61), CD86 (GL1), GITR (DTA-1); and APC-conjugated anti-CD62L (MEL-14) were from BD Biosciences (Franklin Lakes, NJ). PE-conjugated anti-CD152 (UC10–4B9) and Foxp3 (FJK-16s) in addition to APC-conjugated anti-CD25 (PC61) were from eBioscience (San Diego, CA). A hybridoma expressing the anti-clonotype antibody that is specific for BDC2.5 TCR (BDC) was provided by O. Kanagawa (Washington University, St. Louis, MO), and the antibody was purified and biotinylated (35). Recombinant human TGF-β1 was obtained from R&D Systems, (Minneapolis, MN).
Cell Purifications.
Splenic DCs were isolated from NOD males as described in ref. 36. In brief, spleens from normoglycemic NOD males between the ages of 4 and 10 weeks were first digested with collagenase D (Sigma–Aldrich, St. Louis, MO) followed by the enrichment of the DC fraction with 30% BSA density gradient. The CD11c fraction was then purified with magnetic microbeads (Miltenyi Biotec, Auburn, CA). The purity of DCs was routinely >90%. DCs were irradiated with 15 Gy before their use as APCs. Naïve CD4+CD25− cells were purified from BDC2.5 spleen and lymph nodes. Cells were first enriched by depletion (anti-CD8, CD25, Ter119, Gr1, DX5, B220, CD11b) followed by FACS sorting for the CD4+CD25−CD62L+ population to >95% purity.
Cell Culture, Proliferation Assays, and Cytokine Detection.
The sequence of the mimetope peptide used (BDC) was RVRPLWVRME (18). A total of 2 × 104 per well of CD4+CD25−BDC2.5 T cells were cultured for 6–10 days with splenic DCs at a 3:1 T/DC cell ratio in 96-well plates with 100 ng/ml BDC peptide, either with or without TGF-β1 at indicated concentrations. Alternatively, T cells were cultured with 10 μg/ml anti-CD3 (a gift from Terrence Barrett, San Jose, CA) and 2 μg/ml anti-CD28 (BD Biosciences, San Jose, CA) for 6 days. For functional tests, DCs were removed by CD11c antibody-conjugated magnetic microbeads, and CD25+ cells were further enriched by labeling them with PE-conjugated anti-CD25 (PC61) and anti-PE microbeads. For proliferation assays, freshly isolated CD4+CD25− BDC2.5 T cells were cultured with NOD whole spleen cells at a T/APC cell ratio of 1:5 and 100 ng/ml BDC peptide. [3H]thymidine (1 μCi per well; PerkinElmer, San Jose, CA) was added for the last 18 h of a 72-h assay. For suppression assays, the cultured CD25+ T cells were added to proliferation assays at the indicated ratios and [3H]thymidine uptake was measured. For cytokine assays, culture supernatants were tested with the LiquiChip Mouse 10-cytokine assay kit (Qiagen, Valencia, CA). For intracellular cytokine detection, cells were first stimulated with leukocyte activation mixture (BD Biosciences) for 4 h before being stained in accordance with the manufacturer's protocol.
Real-Time PCR.
Total RNA was extracted with the RNeasy mini kit (Qiagen). The sequences of primers and probe for mouse Foxp3 were as follows: sense 5′-AGGAGAAGCTGGGAGCTATGC- 3′; anti-sense 5′-TGGCTACGATGCAGCAA GAG-3′; probe 5′-FAM AGCGCCATCTTCCCAGCCAGG TAMRA-3′. The final quantity of mRNA was calculated as copies per microgram of RNA and standardized according to 18s RNA.
Diabetes Experiments.
For NOD.scid experiment, diabetes was induced in 5- to 9-week-old NOD.scid mice with an i.v. injection of 107 spleen cells from female NOD mice with diabetes. Indicated numbers of the induced CD4+CD25+ T cells were coinjected where indicated. Urine glucose was checked two to three times per week, and the development of diabetes in NOD.scid mice was defined as three consecutive positive urine glucose readings.
The spontaneous development of diabetes in female NOD mice was defined as two consecutive blood glucose levels of >250 mg/dl. Islet isolation and transplantation were performed as described in ref. 22. In brief, islets were handpicked to a purity of >80% for transplant, and 500–600 islets were transplanted into the kidney subcapsular space of newly diabetic NOD female mice. For cell and islet cotransplants, the indicated numbers of T cells were mixed with islets immediately before transplantation. Graft destruction was diagnosed by the recurrence of hyperglycemia (blood glucose level >250 mg/dl on two consecutive readings).
Statistical Analysis.
For analysis of real-time PCR data, we used the Student's t test. To analyze the incidence of diabetes in NOD.scid and syngeneic islet graft survival in NOD recipients, we used analysis of variance. We considered P < 0.05 to be significant.
Acknowledgments
We thank Dr. Stephen D. Miller for helpful discussions and critical review of the manuscript. This work was supported in part by a fellowship award from the American Society of Transplantation and by National Institutes of Health (NIH) Career Awards 1K08DK070029-01 (to X.L.) and 5K01DK071586-02 (to K.V.T.), NIH Grants R21 DK60186 (to M.S.) and AI 51573 (to R.M.S.), and a program project grant from the Juvenile Diabetes Research Foundation (to R.M.S.).
Abbreviations
- NOD
nonobese diabetic
- T reg
regulatory T cell
- DC
dendritic cell
- APC
antigen-presenting cell
- BDC peptide
mimetope peptide for BDC2.5 TCR transgenic T cells
- GITR
glucocorticoid-induced TNF receptor.
Footnotes
The authors declare no conflict of interest.
References
- 1.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, et al. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. J Immunol. 2005;175:3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, et al. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Hawiger D, Nussenzweig MC. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki S, Patel M, Harper A, Bonito A, Fukuyama H, Pack M, Tarbell KV, Talmor M, Ravetch JV, Inaba K, et al. Proc Natl Acad Sci USA. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W. Front Biosci. 2006;11:1360–1370. doi: 10.2741/1889. [DOI] [PubMed] [Google Scholar]
- 13.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobl H, Knapp W. Microbes Infect. 1999;1:1283–1290. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 15.Marie JC, Letterio JJ, Gavin M, Rudensky AY. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 17.Chatenoud L, Primo J, Bach JF. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 18.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 19.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Christianson SW, Shultz LD, Leiter EH. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, Yang H, Kim IS, Saint-Hilaire F, Thomas DA, De BP, Ozkaynak E, Muthukumar T, Hancock WW, Crystal RG, et al. Transplantation. 2005;79:1091–1096. doi: 10.1097/01.tp.0000161223.54452.a2. [DOI] [PubMed] [Google Scholar]
- 23.Thornton AM, Shevach EM. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 24.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 25.Lo J, Peng RH, Barker T, Xia CQ, Clare-Salzler MJ. Ann NY Acad Sci. 2006;1079:153–156. doi: 10.1196/annals.1375.023. [DOI] [PubMed] [Google Scholar]
- 26.Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. J Clin Invest. 1992;90:741–748. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldmann H, Chen TC, Graca L, Adams E, Daley S, Cobbold S, Fairchild PJ. Semin Immunol. 2006;18:111–119. doi: 10.1016/j.smim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Wood KJ, Sakaguchi S. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Int Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- 32.Ali A, Garrovillo M, Jin MX, Hardy MA, Oluwole SF. Transplantation. 2000;69:221–226. doi: 10.1097/00007890-200001270-00005. [DOI] [PubMed] [Google Scholar]
- 33.Oluwole OO, DePaz HA, Adeyeri A, Jin MX, Hardy MA, Oluwole SF. Transplantation. 2003;75:1136–1142. doi: 10.1097/01.TP.0000062842.47597.13. [DOI] [PubMed] [Google Scholar]
- 34.Akl A, Luo S, Wood KJ. Transpl Immunol. 2005;14:225–230. doi: 10.1016/j.trim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Kanagawa O, Militech A, Vaupel BA. J Immunol. 2002;168:6159–6164. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 36.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]