Abstract
Pancreatic β cell mass and function increase in conditions of enhanced insulin demand such as obesity. Failure to adapt leads to diabetes. The molecular mechanisms controlling this adaptive process are unclear. Fas is a death receptor involved in β cell apoptosis or proliferation, depending on the activity of the caspase-8 inhibitor FLIP. Here we show that the Fas pathway also regulates β cell secretory function. We observed impaired glucose tolerance in Fas-deficient mice due to a delayed and decreased insulin secretory pattern. Expression of PDX-1, a β cell-specific transcription factor regulating insulin gene expression and mitochondrial metabolism, was decreased in Fas-deficient β cells. As a consequence, insulin and ATP production were severely reduced and only partly compensated for by increased β cell mass. Up-regulation of FLIP enhanced NF-κB activity via NF-κB-inducing kinase and RelB. This led to increased PDX-1 and insulin production independent of changes in cell turnover. The results support a previously undescribed role for the Fas pathway in regulating insulin production and release.
Keywords: diabetes, IL-1, insulin
A decrease in functional insulin-producing pancreatic β cells contributes to the establishment of not only type 1 but also type 2 diabetes (1, 2). Fas (CD95) is a cell surface receptor that plays a central role in the regulation of death in many cell types, including β cells, and may be implicated in the development of type 1 and type 2 diabetes (3–5). Glucose-induced β cell apoptosis in human islets involves IL-1β-mediated up-regulation of Fas and subsequent interaction with the constitutively expressed Fas ligand (FasL) on neighboring β cells (3, 6–8). Fas/FasL interaction leads to cleavage of procaspase-8, the most upstream caspase in the Fas apoptotic pathway. However, elevated glucose concentrations have a dual effect on β cell turnover, inducing proliferation in the short-term and apoptosis in the long-term (3, 9, 10). This dual effect of glucose may be explained by the pivotal role of FLICE-inhibitory protein (FLIP) on Fas signaling. FLIP structurally resembles caspase-8 but lacks its proteolytic activity (11). FLIP is expressed in human pancreatic β cells and is decreased in the pancreatic tissue of type 2 diabetic patients as well as in cultured islets after prolonged exposure to elevated glucose concentrations. Up-regulation of FLIP switches Fas-mediated glucose signaling in β cells from being apoptotic to favoring cell replication (12).
The transcription factor NF-κB is an essential component of cytokine signaling. Interestingly, in β cells NF-κB activation can also be triggered by glucose (6, 13). Increasing evidence points to noninflammatory effects of NF-κB, including regulation of insulin secretion (14, 15). NF-κB is activated by signaling through many receptors, which can be grouped into the classical and nonclassical pathways of NF-κB activation (16). In the classical pathway, upstream signals induce phosphorylation of IκBα bound to cytosolic NF-κB. Phosphorylation is carried out by the IκB kinase (IKK) complex, which is composed of IKK-γ and two catalytic subunits, IKK-α and IKK-β. Phosphorylation tags IκBα for ubiquitination and, ultimately, for proteasomal degradation, liberating NF-κB (p50 and RelA or p65) for translocation to the nucleus and subsequent activation of target genes. The nonclassical pathway is controlled through NF-κB-inducing kinase (NIK), which phosphorylates IKK-α; this regulation occurs independent of the classical IKK complex and leads to processing of p100, generating p52–RelB heterodimers, which translocate to the nucleus.
The β cell-specific transcription factor PDX-1 predominantly regulates β cell differentiation and secretory function. In particular, PDX-1 controls several events in glucose stimulated insulin secretion including mitochondrial metabolism (17–20). Several observations point to a role for the Fas pathway in the regulation of cell cycle-independent events including T cell activation (21), renal tubular epithelial integrin function (22), and protection against neurodegeneration (23). However, a direct interaction between the Fas pathway and PDX-1 has not been investigated.
In addition to its effect on β cell turnover, chronic hyperglycemia impairs β cell secretory function (2, 24–26). This glucotoxic effect is evident before apoptosis leads to a significant decrease in β cell mass. This is most striking in vitro, where a 4-day exposure of human islets to elevated glucose concentrations leads to almost complete ablation of β cell secretory function, although <1% of β cells are apoptotic (6). Because hyperglycemia regulates Fas expression (3, 7), we hypothesize that the Fas pathway may not solely mediate glucose-induced changes in cell turnover, but also changes in β cell secretory function.
Results
Fas Regulates β Cell Function.
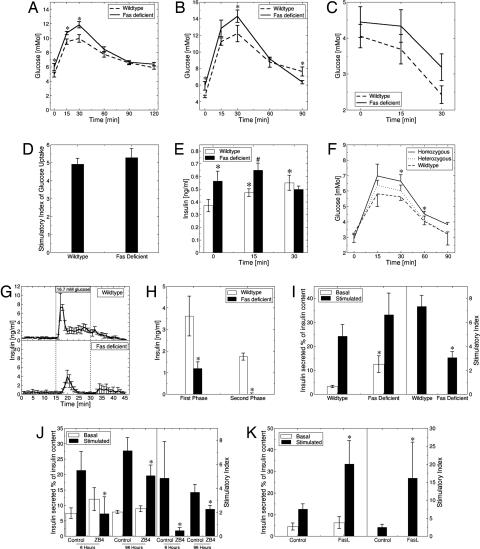
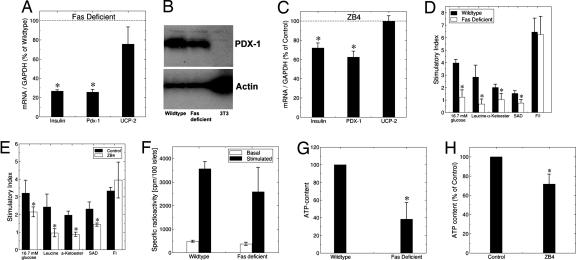
To assess the role of Fas on β cell secretory function, we first used mice with a natural Fas mutation [lpr (27)] transferred onto a C57BL/6j inbred strain background. In contrast to the original MRL/MpJlpr mice, the B6.MRLlpr mice did not develop any detectable sign of autoimmunity even at 13 weeks of age, as described previously (28). This was confirmed by the lack of detectable morphological and histological changes in the spleen, liver, kidney, lung, and lymph nodes, as well as by the failure to detect circulating anti-nuclear antibodies or increased circulating cytokine (IL-1β, IFNγ, IL-6, and TNFα) levels in Fas-deficient mice between 7 and 13 weeks of age (data not shown). At 8 weeks of age, Fas-deficient B6.MRLlpr mice displayed impaired glucose tolerance after an i.p. glucose load (Fig. 1 A and B). Assessment of insulin sensitivity of the Fas-deficient mice by insulin tolerance test (Fig. 1C) and insulin-stimulated glucose uptake into isolated adipocytes (Fig. 1D) failed to detect major changes in insulin resistance under these conditions. Baseline circulating insulin was higher in Fas-deficient mice but did not increase in response to the glucose load, whereas in wild-type mice there was a significant increase (Fig. 1E). To exclude a nonspecific effect related to the genetic background or an indirect effect due to Fas-deficiency in non-β cells, a second set of glucose tolerance tests was performed in mice with a β cell-specific knockout of Fas. Because these mice are on the nonobese diabetic (NOD) genetic background, care was taken to analyze the animals at 6 weeks of age, before the onset of insulitis (data not shown). Compared with their wild-type NODβFas+/+ littermates, heterozygous NODβFas+/− mice showed a slight, albeit nonsignificant impairment of glucose tolerance, whereas the homozygous NODβFas−/− mice displayed a significantly impaired glucose tolerance, thus confirming the selective effect of Fas deficiency (Fig. 1F). Of note, the NODβFas+/+ mice were RipCre-positive to ensure an appropriate control. Finally, the consequence of Fas deficiency was confirmed by a third animal model, i.e., in mice with a β cell-specific knockout of Fas on a C57BL/6j genetic background (data not shown). To characterize the defect in insulin secretion, we performed in situ pancreas perfusion experiments. In Fas-deficient mice, the first phase insulin secretory response to a 15-min perfusion of 16.7 mM glucose was delayed and blunted, followed by an ablation of second phase insulin release (Fig. 1 G and H). The secretory defect apparent in the Fas-deficient mouse pancreas was also present in isolated islets. Indeed, the ratio of high to low glucose-stimulated insulin release was 45% less than in wild-type islets (Fig. 1I). The defect was also apparent in islets after 24 h of culture (2-fold less glucose stimulated insulin secretion in the Fas-deficient islets). The basal secretion was elevated in Fas-deficient islets, which is in agreement with the in vivo levels of insulin secretion and may reflect the compensatory increase in islet β cell mass due to defective secretion. The role of Fas in glucose-stimulated insulin secretion was also verified in human islets by means of the antagonistic anti-Fas antibody ZB4. In cultured human islets isolated from pancreata of organ donors, ZB4 inhibited glucose-stimulated insulin release already after 6 h (Fig. 1J), whereas it was enhanced by FasL after 48 h (Fig. 1K), supporting the notion that Fas activation is necessary for normal β cell function. Pancreata from 8-week-old Fas-deficient mice revealed a normal islet structure and ratio of β to α cells. However, islet β cell mass was increased as compared with wild-type mice (2.10 ± 0.48 mg in Fas-deficient vs. 0.59 ± 0.15 mg in wild-type mice; P < 0.01), with no change in pancreas weight (0.27 ± 0.02 g in Fas-deficient vs. 0.22 ± 0.01 g in wild-type mice). Despite the ≈3-fold increase in islet β cell mass of Fas-deficient mice, insulin content per pancreatic wet weight and per isolated islet remained unchanged (data not shown) probably because of a severe decrease in insulin mRNA per β cell (Fig. 2A). In parallel, expression of PDX-1 was decreased at mRNA and protein levels (Fig. 2 A and B), whereas mRNA levels of uncoupling protein 2 (used as a control) were not significantly changed (Fig. 2A). Similarly, in cultured human islets, the antagonistic anti-Fas antibody ZB4 inhibited insulin and PDX-1 mRNA expression, demonstrating a direct regulation of these genes by Fas (Fig. 2C). Furthermore, expression levels of FLIP mRNA were similar in Fas-deficient and wild-type mice (data not shown). To characterize the secretory pathway regulated by Fas signaling, insulin release was stimulated with metabolic secretagogues, generating mitochondrial coupling factors such as ATP. The energy substrate leucine and the cell-permeable dimethyl esters of α-ketoglutarate and succinic acid were less effective in stimulating insulin release from Fas-deficient mice and ZB4-treated human islets (Fig. 2 D and E). In contrast, the secretory responses in Fas-deficient or ZB4-treated islets were restored in the presence of the phosphodiesterase inhibitors 3-isobutyl-1-methylxanthine and the activator of adenylyl cyclase forskolin that increase cAMP levels (Fig. 2 D and E). Furthermore, acute glucose-stimulated insulin synthesis (which reflects translation) was normal (Fig. 2F). This suggests that the exocytotic machinery and insulin translation are fully functional in the absence of Fas signaling and indicates a defect in the stimulus–secretion coupling in addition to persistently decreased insulin mRNA expression. Consistent with this hypothesis, ATP concentration was decreased in Fas-deficient and ZB4-treated islets (Fig. 2 G and H).
Fig. 1.
Fas regulates β cell secretory function. Blood glucose levels after i.p. injection of 2 mg of glucose (A) and 1 mg of glucose (B) per gram of body weight in male Fas-deficient B6.MRLlpr and wild-type C57BL/6j mice aged 7–8 weeks. ∗, P < 0.05, Fas-deficient versus wild type (n = 15 and 10 for each group in A and B, respectively). (C) Blood glucose levels after i.p. injection of 0.5 units of insulin per kilogram of body weight in male Fas-deficient and wild-type mice. ∗, P < 0.05, Fas-deficient versus wild type (n = 15 for each group). (D) Insulin-stimulated 2-deoxyglucose uptake in adipocytes isolated from Fas-deficient and wild-type mice (n = 2, each in hexaplicate). (E) Insulin levels after i.p. injection of 2 mg of glucose per gram of body weight in male Fas-deficient and wild-type mice (n = 15 for each group). ∗, P < 0.05 relative to wild type at time point 0 min; #, P < 0.05 relative to wild type at time point 15 min. (F) Blood glucose levels after i.p. injection of glucose in male prediabetic NODβFas−/− (homozygous), NODβFas+/− (heterozygous), and NODβFas+/+ (wild-type) littermate mice aged 5–6 weeks. ∗, P < 0.05 (n = 5–10 for each group). (G and H) Glucose-induced insulin secretion in perfused pancreata from Fas-deficient and wild-type mice. Pancreata were perfused with basal solution (2.8 mM glucose) for 30 min, and then glucose was increased to 16.7 mM for the indicated period (n = 3). (I) Percentage of islet insulin content released during a 1-h incubation at 3.3 mM (basal) and 16.7 mM (stimulated) glucose after an 8-day culture period of islets isolated from Fas-deficient and wild-type mice (Left) and the corresponding stimulatory index of insulin secretion (Right) (n = 4, each in hexaplicate). Insulin content was 0.12 ± 0.02 ng/ml for wild-type mice and 0.14 ± 0.03 ng/ml for Fas-deficient mice. ∗, P < 0.001. (J) Percentage of islet insulin content released during successive 1-h incubations at 3.3 mM (basal) and 16.7 mM (stimulated) glucose after 6-h and 4-day incubation periods of islets isolated from human pancreata in the presence of 500 ng/ml isotype IgG (control) or 500 ng/ml antagonistic anti-Fas antibody (ZB4) (Left) and the corresponding stimulatory index (Right) (n = 3, each in triplicate). Insulin content for control islets was 0.04 ± 0.01 and 0.03 ± 0.002 ng/ml, and insulin content for ZB4-treated islets was 0.05 ± 0.01 and 0.03 ± 0.01 ng/ml after 6 h and 4 days, respectively. ∗, P < 0.001. (K) Percentage of islet insulin content released during successive 1-h incubations at basal and stimulated glucose after incubation of human islets in the presence of FasL for 48 h. Insulin content for control was 0.04 ± 0.01 ng/ml, and insulin content for FasL-treated islets was 0.06 ± 0.01 ng/ml (n = 2, each done in quintuplicate).
Fig. 2.
Fas-deficient islets have decreased insulin and PDX1 expression and mitochondrial metabolism. (A) RT-PCR detection of insulin, PDX-1, and uncoupling protein 2 (UCP-2) mRNA expression in Fas-deficient and wild-type islets (n = 5 for each group). ∗, P < 0.05. (B) Immunoblotting of PDX-1 and actin of Fas-deficient and wild-type islets and from the 3T3 cell line (negative control). One of two experiments is shown. (C) RT-PCR detection of insulin, PDX-1, and uncoupling protein 2 (UCP-2) in human islets cultured for 4 days with 500 ng/ml isotype IgG (control) or 500 ng/ml antagonistic anti-Fas antibody (ZB4) (n = 3, each in duplicate). (D and E) Stimulatory index of insulin secretion during successive 1-h incubation at 3.3 mM (basal) and 16.7 mM (stimulated) glucose, 20 mM leucine, 10 mM α-ketoglutarate dimethyl ester (α-Ketoester), 10 mM succinic acid dimethyl ester (SAD), or 10 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (F/I), after a 4-day culture period of islets isolated from Fas-deficient and wild-type mice (D) and after a 4-day culture period of human islets in the presence of 500 ng/ml isotype IgG (control) or 500 ng/ml antagonistic anti-Fas antibody (ZB4) (E) (n = 3 from three different isolations/donors, each in triplicate). ∗, P < 0.05 vs. wild type or controls. (F) Islets isolated from wild-type and Fas-deficient mice were radiolabeled (25 min) with [3H]leucine in 2.8 mM (basal) and 16.7 mM (stimulated) glucose. Radiolabeled proinsulin plus insulin is presented as specifically immunoprecipitable radioactivity as a function of islet number (cpm per 100 islets) (n = 3). (G) After 1 day in culture, islets isolated from Fas-deficient and wild-type mice were incubated successively for 30 min in 2.8 mM glucose followed by an additional 10 min in 16.7 mM glucose and analyzed for stimulated ATP content per islet. ∗, P < 0.001 (n = 3, each in triplicate). (H) After a 4-day culture period, human islets cultured in the presence of 500 ng/ml isotype IgG (control) or 500 ng/ml antagonistic anti-Fas antibody (ZB4) were incubated successively for 30 min in 2.8 mM glucose followed by an additional 10 min in 16.7 mM glucose and analyzed for stimulated ATP content per islet. ∗, P < 0.05. Data are means of percentage relative to control for islets from three different donors, each plated in triplicate.
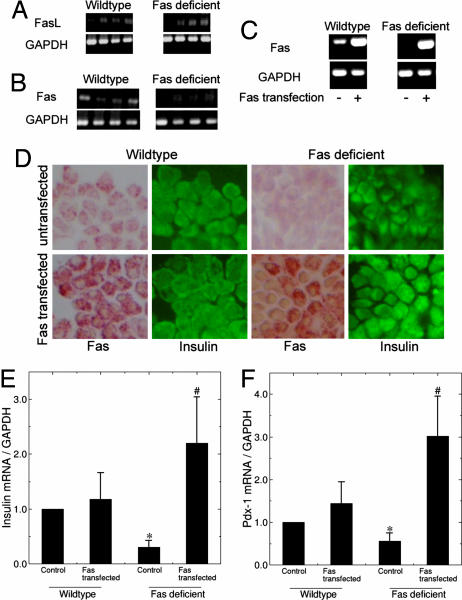
Fas and FasL Are Expressed in Islets and Regulate Insulin and PDX-1 mRNA Expression.
FasL and, at low levels, the Fas receptor are normally expressed in β cells, as previously shown in human islets and in the present study in mouse islets (3, 8) (Fig. 3 A–D). To examine whether the effects of the Fas pathway indeed influence the phenotype of the β cell, wild-type and Fas-deficient mouse islets were transfected with a vector encoding Fas (Fig. 3 C and D). Transfection efficiency in β cell monolayers was ≈30–50% (Fig. 3D). Restoration of Fas in Fas-deficient islets significantly stimulated insulin and PDX-1 mRNA expression, whereas overexpression of Fas at similar levels in wild-type mouse islets did not affect these genes possibly because endogenous Fas was sufficient to maximally stimulate steady-state PDX-1 levels (Fig. 3 E and F).
Fig. 3.
Fas and FasL are expressed in islets and regulate insulin and PDX-1 mRNA expression. (A and B) Shown is RT-PCR analysis of FasL (A) and Fas (B) expression in wild-type and Fas-deficient islets. Each lane represents an individual animal. GAPDH was used as control. (C–F) Fas-deficient and wild-type islets were transfected with a vector encoding for Fas and incubated for 8 days. (C) RT-PCR of Fas and GAPDH (control) expression in Fas-deficient and wild-type islets. (D) Immunostaining for Fas and insulin. (E and F) Quantitative RT-PCR detection of insulin (E) and PDX-1 (F) mRNA expression normalized to GAPDH (n = 3 for each group). ∗, P < 0.05 relative to wild type; #, P < 0.05 relative to Fas-deficient control.
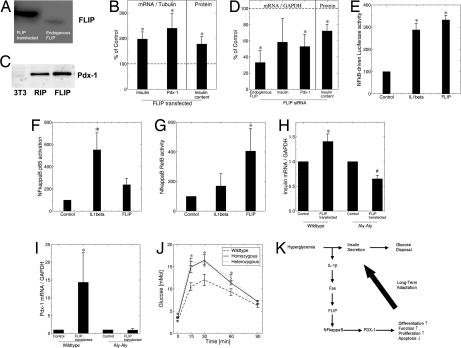
FLIP Regulates Insulin and PDX-1 mRNA Expression via the Alternative Pathway of NF-κB Activation.
Next we investigated the mediators of Fas-regulated insulin production. Transfection efficiency of FLIP in β cell monolayers was ≈30–50%, as previously shown (12). Overexpression of FLIP significantly increased islet insulin and PDX-1 mRNA and protein expression (Fig. 4 A–C). Next we tested whether FLIP can also regulate insulin production in Fas-deficient mouse islets. As expected, transfection of FLIP in Fas-deficient islets enhanced islet insulin content by 63 ± 24% (n = 3; P = 0.05). The functional role of FLIP in insulin production was then investigated by RNAi. siRNA to FLIP suppressed endogenous FLIP expression by 70%, leading to a 50% decrease in insulin and PDX-1 expression with a concomitant decrease in insulin content (Fig. 4D), whereas scrambled siRNA had no effect on these genes (data not shown). β cell proliferation and apoptosis remained unaffected by transfection with FLIP at baseline 11.1 mM glucose (data not shown), in support of the concept that Fas-FLIP can affect β cell function independent of changes in cell turnover. To test whether FLIP acts via NF-κB, INS-1E cells were transfected with an NF-κB-driven luciferase construct. Cotransfection with FLIP strongly induced NF-κB activity, reaching similar levels to IL-1β (Fig. 4E). Nevertheless, detection of p50-RelA (p65) binding to an NF-κB consensus site revealed no significant change after transfection with FLIP (Fig. 4F). However, using an anti-RelB antibody, which detects binding to an NF-κB consensus site, revealed a robust induction of RelB by FLIP, indicating NF-κB activation via the alternative pathway (Fig. 4G). To support these results, we used islets isolated from alymphoplastic aly/aly mice, which are defective in activation of the alternative NF-κB pathway because of a point mutation in NF-κB-inducing kinase (29). In contrast to wild-type islets, in aly/aly mice, FLIP failed to induce insulin and PDX-1 mRNA expression (Fig. 4 H and I). Finally, aly/aly mice displayed impaired glucose tolerance (Fig. 4J), supporting the concept that NF-κB activity is required for β cell secretory function (14, 15).
Fig. 4.
FLIP regulates insulin and PDX-1 mRNA expression via the alternative pathway of NF-κB activation. INS-1E (A and E–G), wild-type, and aly/aly (B–D, H, and I) mouse islet cells were transfected with a mock vector or a vector encoding for FLIP (FLIP transfected) or exposed to siRNA to FLIP. (A) Immunoblotting of FLIP. (B and D) RT-PCR detection of insulin, PDX-1, and FLIP mRNA expression and islet insulin content. The level of mRNA expression was normalized to tubulin or GAPDH, and the results are expressed as mRNA levels relative to controls. (C) Immunoblotting of PDX-1 in islets and in 3T3 cells (negative control) transfected with the RIP vector alone and with RIP-FLIP. One of two experiments is shown. (E–G) Analysis of NF-κB activity by detection of luciferase activity after transfection of an NF-κB-driven firefly luciferase construct normalized to a cotransfected constitutive Renilla luciferase construct (E) and by detection of p50–p65 (F) and RelB (G) binding to an NF-κB consensus site. The effect of FLIP was compared with 150 pg/ml IL-1β (n = 4, each in duplicate). (H and I) RT-PCR of insulin (H) and PDX-1 (I) mRNA normalized to GAPDH (n = 3–4). ∗, P < 0.05 relative to wild-type control; #, P < 0.05 relative to aly/aly control. (J) Blood glucose levels after i.p. injection of glucose (2 mg/g of body weight) in male homozygous and heterozygous aly/aly mice and C57BL/6 (wild-type) mice. ∗, P < 0.05 homozygous, heterozygous vs. wild type (n = 6, 5, and 5, respectively, for each group). (K) Hypothetical model illustrating the consequence of limited hyperglycemia on β cell production of IL-1β in parallel with insulin secretion. The paracrine effect of IL-1β induces Fas engagement, which, in the presence of FLIP, leads to β cell proliferation, differentiation, and increased function.
Discussion
Hitherto, activation of the Fas death receptor was thought to mainly activate the apoptotic cascade. Recently we have shown that in human β cells FLIP may switch Fas signaling toward proliferation (12). Here we demonstrate that Fas is also a regulator of β cell function. This is supported by impaired glucose tolerance in Fas-deficient animals and by an abnormal response to glucose in perfused pancreata and isolated islets. In particular, impaired β cell function in the perfused pancreas was characterized by a delayed and reduced first and second phase of insulin secretion. This defect is partly explained by decreased ATP synthesis and suggests a mitochondrial defect. Indeed, decreased total cellular ATP mainly reflects overall mitochondrial dysfunction (30). This notion is supported by the failure of energy substrates generating mitochondrial coupling factors to produce a normal insulin release. In contrast, nonfuel stimuli evoked normal insulin secretion, suggesting that metabolism–secretion coupling is defective in the absence of Fas signaling while the exocytotic machinery is fully functional. However, in addition to the regulation of ATP production, Fas signaling has certainly further important effects on β cells. Indeed, the dramatic increase in β cell mass and in basal insulin secretion observed in the absence of Fas signaling cannot be solely attributed to ATP deficiency.
The defective metabolism–secretion coupling may be a consequence of decreased PDX-1 expression. PDX-1 has recently been shown to be an essential regulator of several events in insulin secretion, including mitochondrial metabolism and in particular ATP production (18, 20). In line with these findings, we observed a decrease in PDX-1 expression in the islets of Fas-deficient animals. Moreover, Fas and FLIP up-regulation increased insulin and PDX-1 expression. Interestingly, mutations in PDX-1 have been associated with type 2 diabetes (31, 32), and high glucose dramatically lowers DNA-binding activity of PDX-1 (33).
The endocrine pancreas has a remarkable capacity to adapt to conditions of increased insulin demand, as encountered in obesity and pregnancy, by increasing its functional β cell mass (34). Taken together with previous findings, we propose a hypothesis for long-term β cell plasticity, attributing a central role to the Fas pathway (Fig. 4K). According to this hypothesis, long-term adaptation of the β cells to conditions of increased demand may be triggered by limited hyperglycemic excursions (35). These excursions elicit β cell production of IL-1β (6) followed by Fas up-regulation (3, 7). At low concentrations of IL-1β and in the presence of FLIP, Fas engagement leads to β cell proliferation (36) and enhanced function via NF-κB and PDX-1, as shown in the present study.
The contribution of insufficient insulin secretion to the development of type 2 diabetes is now widely accepted (1, 2). However, opinions diverge regarding the relative contribution of a decrease in β cell mass versus an intrinsic defect in the secretory machinery of the β cell. Here we demonstrate that the Fas pathway regulates β cell secretory function in addition to its known role in β cell turnover. It follows that the adaptive mechanisms of function and mass share common regulatory pathways and will therefore act in concert. However, β cell adaptation is not solely dependent on the Fas pathway because Fas-deficient animals exhibited a compensatory increase in β cell mass. Nevertheless, the results support a novel role for the Fas pathway in regulating β cell secretory function.
Materials and Methods
Animals.
Ethical approval was granted by the Zurich Cantonal Animal Experimentation Committee. C57BL/6j wild-type mice and mice with a natural Fas mutation backcrossed for >10 generations onto this same C57BL/6j inbred strain background (B6.MRLlpr) were obtained from The Jackson Laboratory. NODβFas−/− and BL6βFas−/− mice were produced by introduction of LoxP sites flanking exon IX of Fas and breeding to NOD and C57BL/6j rat insulin promoter (RIP)-Cre mice, respectively. The aly/aly (alymphoplasia or Map3k14aly) mice were bred in-house (37).
i.p. Glucose and Insulin Tolerance Tests.
Mice were injected i.p. with 1 or 2 mg of glucose per gram of body weight or with 0.5 milliunits/g recombinant human insulin (Novo Nordisk, Bagsværd, Denmark) for the glucose or insulin tolerance test, respectively. Blood samples were obtained from tail-tip bleedings, and blood glucose concentration was measured with a Glucometer (Disetronic Medical Systems, Burgdorf, Switzerland).
In Situ Pancreas Perfusion.
Mice were anesthetized and prepared as previously described (38). The pancreas was perfused at 37°C with 1.5 ml/min Krebs–Ringer Hepes buffer supplemented with 2.8 mM glucose and with 16.7 mM glucose for stimulation. Effluent of the first 30 min was not collected, and then effluent was collected in 1-min fractions.
Islet Cell Culture.
Islets were cultured on extracellular matrix-coated plates (Novamed, Jerusalem, Israel). Mouse islets were cultured in RPMI medium 1640 containing 11.1 mM glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamycin, and 10% FCS (Invitrogen, Carlsbad, CA), and human islets were cultured in CMRL 1066 medium containing 5.5 mM glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. In some experiments the medium was supplemented with 500 ng/ml mouse antagonistic anti-Fas antibody (ZB4; MBL, Nogoya, Japan) or 500 ng/ml isotype control.
Liposome-Mediated Transfection.
Lipofectamine 2000 (Invitrogen)–DNA complexes were prepared by using a RIP-FLAG-tagged-FLIP-long-DNA (provided by J. Tschopp, University of Lausanne, Lausanne, Switzerland), RIP-plasmid-DNA (control), or a CMV-Fas-plasmid-DNA (provided by S. Nagata, Osaka University Medical School, Osaka, Japan). The solution was added at a final concentration of 3 μg of DNA per milliliter. The islets were assayed 8 days after transfection.
Immunostaining and Histochemical Analysis.
Tissue sections were incubated with guinea pig anti-insulin, fluorescein-conjugated rabbit anti-guinea pig and mouse anti-glucagon antibodies (Dako, Carpinteria, CA), and donkey anti-mouse Cy3-conjugated antibody (Jackson ImmunoResearch, West Grove, PA). β cell mass was assessed by the fraction of the cross-sectional area of pancreatic tissue positive for insulin staining and multiplying this by the pancreatic weight.
Anti-Nuclear Antibodies.
Anti-nuclear antibodies were detected by incubation of a mouse liver tissue section with test serum followed by detection with anti-mouse Cy3-conjugated antibody (Jackson ImmunoResearch). Serum of wild-type mice and of systemic lupus erythematosus-diseased mice was used as negative and positive controls, respectively.
Cytokine Assay.
Sera IL-1β, IFNγ, IL-6, and TNFα were measured by using Luminex technology (Labodia, Préverenges, Switzerland).
Cell Replication and Apoptosis.
Islets were double-stained with antibodies against mouse Ki-67 antigen (Zymed, San Francisco, CA) or by TUNEL (Roche Molecular Biochemicals, Germany) and for insulin as described (6).
Insulin Release and Insulin and ATP Contents.
Acute insulin release in response to glucose was tested as described (36). For ATP content, islets were preincubated for 30 min in KRB followed by a 10-min stimulation with 16.7 mM glucose KRB. ATP was quantified with a bioluminescence-based kit (Roche).
RNA Extraction and Quantitative RT-PCR.
We extracted total RNA with Rneasy mini kit (QIAGEN, Basel, Switzerland) and performed RT-PCR with SuperScript Double-Stranded cDNA synthesis kit (Life Technologies/Gibco, Gaithersburg, MD). For quantitative analysis, we used the Light Cycler quantitative PCR system (Roche, Basel, Switzerland) with a commercial kit (Light Cycler-DNA Master SYBR Green I; Roche). Mouse primers used were as follows: 5′-TACGGGGTTTGTGAAAGGAG-3′ and 5′-CACATCATTCCCCAGGAAAC-3′ (insulin); 5′-GAGGACCCGTACAGCCTACA-3′ and 5′-CGTTGTCCCGCTACTACGTT-3′ (PDX-1); 5′-CTAAATTTGGTTGCCCCAGA-3′ and 5′-CTCCCATTATGGAGCCTGAA-3′ (FLIP long); 5′-CAGCCAGCGCCCAGTACC-3′ and 5′-CAATGCGGACGGAGGCAAAGC-3′ (uncoupling protein 2); 5′-GTGGCAGTGA-TGGCATGGAC-3′ and 5′-CAGCACCAGTGGATGCAGGG-3′ (GAPDH); and 5′-AGAGTCGCGCTGGTAAGAAGC-3′ and 5′-CCCCAATGGTCTTGTCACTT-3′ (tubulin). Human primers used were as follows: 5′-CTCTACAATGGGCTGGTTGC-3′ and 5′-TTGGTATCTCCGACCACCTC-3′ (uncoupling protein 2); 5′-CCACCTTGGGACCTGTTTAG-3′ and 5′-TGATGCCAGAGGAAGAGGAG-3′ (PDX-1); 5′-GCTGGTAGAGGGAGCAGATG-3′ and 5′-CTCACACCTGGTGGAAGCTC-3′ (insulin); 5′-AGAGTCGCGCTGTAAGAAGC-3′ and 5′-TGGTCTTGTCACTTGGCATC-3′ (α-tubulin); and 5′-AACAGCGACACCCACTCCTC-3′ and 5′-GGAGGGGAGATTCAGTGTGGT-3′ (GAPDH).
Western Blot.
Membranes were exposed to a rat monoclonal antibody to FLIP (Dave-2; Alexis Biochemicals), a rabbit anti-PDX-1 antibody (kindly donated by H. Edlund, University of Umea, Sweden), and a mouse anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
RNAi.
RNAs to FLIPlong (Silencer Predesigned siRNA) and scrambled siRNA were synthesized by Ambion (Austin, TX). siRNA was transfected by using SiPortAmine, and transfection efficiency was estimated with cy3-labeled siRNA by using the Silence siRNA Labeling Kit (Ambion), as described (39).
NF-κB Activation.
INS-1E cells were transfected with 1 μg of plasmid DNA. NF-κB-dependent gene transcription was analyzed with 0.4 μg of the PathDetect NFκB cis-Reporting System (Stratagene, San Diego, CA). The luciferase reporter gene was selectively regulated by 5× synthetic NF-κB promoter enhancer elements. Cells were also transfected with an empty vector (pcDNA3; Invitrogen) as control. The promoter activity was analyzed by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Detection of specific activation of NF-κB was performed by using attached oligonucleotides binding to a NF-κB consensus site and detected by an anti-p65 or anti-p50 subunit antibody and by an anti-Relb subunit antibody (TransAM NFκB and TransAM NFκB family; Active Motif, Carlsbad, CA).
Determination of Glucose Uptake.
Glucose uptake measurements into isolated white adipocytes were carried out as described (40).
Acute Glucose-Stimulated Insulin Synthesis.
Islets were preincubated for 15 min at 37°C and resuspended in KRB-Hepes-BSA with either 2.8 or 16.7 mM glucose and containing 590 μCi/ml [3H]leucine (American Radiolabeled Chemicals, St. Louis, MO). Islets were radiolabeled for 25 min at 37°C, and labeling was stopped with 0.9 ml of ice-cold KRB-Hepes-BSA, 2.8 mM glucose, and 1 mM leucine. The islet pellet was extracted by freeze–thawing and sonication on ice in 200 μl of 0.2 M glycine/0.25% BSA (pH 8.8). Supernatants were analyzed for proinsulin and insulin as described (41).
Statistical Analysis.
Samples were evaluated in a randomized manner blinded to the treatment conditions. Data are means ± SE and were analyzed by Student's t test or by ANOVA with a Bonferroni correction for multiple-group comparisons.
Acknowledgments
This work was supported by the Swiss National Science Foundation, a European Foundation for the Study of Diabetes/MSD Research Award, the Juvenile Diabetes Research Foundation, the American Diabetes Association, the University of Zurich Research Priority Program “Integrative Human Physiology,” the Larry Hillblom Research Foundation, and a European Network Grant (GrowBeta) through the Swiss Office for Education and Science. D.M.S. is the recipient of a South African National Research Foundation Scholarship.
Abbreviations
- FasL
Fas ligand
- IKK
IκB kinase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rhodes CJ. Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 2.Donath MY, Halban PA. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 3.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 4.Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. J Mol Med. 2003;81:455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- 5.Nolsoe RL, Hamid YH, Pociot F, Paulsen S, Andersen KM, Borch-Johnsen K, Drivsholm T, Hansen T, Pedersen O, Mandrup-Poulsen T. Genes Immun. 2006;7:316–321. doi: 10.1038/sj.gene.6364300. [DOI] [PubMed] [Google Scholar]
- 6.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. J Biol Chem. 2003;278:2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 8.Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG. Diabetes. 1998;47:727–732. doi: 10.2337/diabetes.47.5.727. [DOI] [PubMed] [Google Scholar]
- 9.Donath MY, Gross DJ, Cerasi E, Kaiser N. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 10.Hoorens A, Van de Casteele M, Kloppel G, Pipeleers D. J Clin Invest. 1996;98:1568–1574. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Nature. 1997;10:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 12.Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, et al. Proc Natl Acad Sci USA. 2002;99:8236–8241. doi: 10.1073/pnas.122686299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal-Mizrachi E, Wen W, Shornick M, Permutt MA. Diabetes. 2002;51(Suppl 3):S484–S488. doi: 10.2337/diabetes.51.2007.s484. [DOI] [PubMed] [Google Scholar]
- 14.Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller DG, Halban PA. J Biol Chem. 2005;280:30630–30637. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- 15.Norlin S, Ahlgren U, Edlund H. Diabetes. 2005;54:125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 16.Siebenlist U, Brown K, Claudio E. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson J, Carlsson L, Edlund T, Edlund H. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 18.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS. J Clin Invest. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier BR, Brun T, Sarret EJ, Ishihara H, Schaad O, Descombes P, Wollheim CB. J Biol Chem. 2004;279:31121–31130. doi: 10.1074/jbc.M405030200. [DOI] [PubMed] [Google Scholar]
- 21.Tai TS, Fang LW, Lai MZ. Cell Death Differ. 2004;11:69–79. doi: 10.1038/sj.cdd.4401316. [DOI] [PubMed] [Google Scholar]
- 22.Jarad G, Wang B, Khan S, DeVore J, Miao H, Wu K, Nishimura SL, Wible BA, Konieczkowski M, Sedor JR, Schelling JR. J Biol Chem. 2002;277:47826–47833. doi: 10.1074/jbc.M204901200. [DOI] [PubMed] [Google Scholar]
- 23.Landau AM, Luk KC, Jones ML, Siegrist-Johnstone R, Young YK, Kouassi E, Rymar VV, Dagher A, Sadikot AF, Desbarats J. J Exp Med. 2005;202:575–581. doi: 10.1084/jem.20050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weir GC, Clore ET, Zmachinski CJ, Bonner-Weir S. Diabetes. 1981;30:590–595. doi: 10.2337/diab.30.7.590. [DOI] [PubMed] [Google Scholar]
- 25.Unger RH, Grundy S. Diabetologia. 1985;28:119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP. Diabetes. 1989;38:1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 28.Pisetsky DS, Caster SA, Roths JB, Murphy ED. J Immunol. 1982;128:2322–2325. [PubMed] [Google Scholar]
- 29.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 30.Maechler P, Wollheim CB. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane WM, Frayling TM, Ellard S, Evans JC, Allen LI, Bulman MP, Ayres S, Shepherd M, Clark P, Millward A, et al. J Clin Invest. 1999;104:R33–R39. doi: 10.1172/JCI7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hani EH, Stoffers DA, Chevre JC, Durand E, Stanojevic V, Dina C, Habener JF, Froguel P. J Clin Invest. 1999;104:R41–R48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, Cerasi E, Melloul D. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 34.Bonner-Weir S. J Mol Endocrinol. 2000;24:297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- 35.Chick WL, Like AA. Diabetologia. 1970;6:243–251. doi: 10.1007/BF01212233. [DOI] [PubMed] [Google Scholar]
- 36.Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY. Diabetes. 2006;55:2713–2722. doi: 10.2337/db05-1430. [DOI] [PubMed] [Google Scholar]
- 37.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 38.Maechler P, Gjinovci A, Wollheim CB. Diabetes. 2002;51(Suppl 1):S99–S102. doi: 10.2337/diabetes.51.2007.s99. [DOI] [PubMed] [Google Scholar]
- 39.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Proc Natl Acad Sci USA. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudich A, Konrad D, Torok D, Ben-Romano R, Huang C, Niu W, Garg RR, Wijesekara N, Germinario RJ, Bilan PJ, Klip A. Diabetologia. 2003;46:649–658. doi: 10.1007/s00125-003-1080-1. [DOI] [PubMed] [Google Scholar]
- 41.Halban PA, Wollheim CB, Blondel B, Renold AE. Biochem Pharmacol. 1980;29:2625–2633. doi: 10.1016/0006-2952(80)90077-5. [DOI] [PubMed] [Google Scholar]