Abstract
Ever-increasing evidence in the literature suggests that the antiinflammatory and cytoprotective properties of activated protein C (APC) are mediated through its endothelial protein C receptor (EPCR)-dependent cleavage of protease-activated receptor 1 (PAR-1) on endothelial cells. However, recent results monitoring the cleavage rate of PAR-1 on human umbilical vein endothelial cells, transfected with an alkaline phosphatase–PAR-1 fusion reporter construct, have indicated that the catalytic activity of thrombin toward PAR-1 is several orders of magnitude higher than that of APC. Because thrombin is required for generation of APC, and because it also functions in the proinflammatory pathways through the activation of PAR-1, it has been difficult to understand how APC can elicit protective cellular responses through the activation of PAR-1 when thrombin is present. In this study we provide a plausible answer to this question by demonstrating that the critical receptors required for both protein C activation (thrombomodulin and EPCR) and APC cellular signaling (EPCR and PAR-1) pathways are colocalized in the membrane lipid rafts in endothelial cells. We further show that the APC cleavage of PAR-1 on cells transfected with a PAR-1 cleavage reporter construct is not sensitive to the cofactor function of EPCR. Thus, the colocalization of EPCR and PAR-1 in lipid rafts is a key requirement for the cellular signaling activity of APC. Thrombomodulin colocalization with these receptors on the same membrane microdomain can also recruit thrombin to activate the EPCR-bound protein C, thereby eliciting PAR-1 signaling events that are involved in the APC protective pathways.
Keywords: endothelial protein C receptor, protease-activated receptor 1, thrombin, thrombomodulin
Activated protein C (APC) is a multidomain plasma serine protease that down-regulates thrombin generation by inactivating procoagulant cofactors Va and VIIIa by limited proteolysis (1–3). The anticoagulant function of APC in degradation of both cofactors is stimulated by protein S (3, 4). The importance of APC in the regulation of the blood coagulation cascade can be illustrated by the observation that a heterozygous protein C deficiency is associated with a high risk of venous thrombosis, and its homozygous deficiency causes purpura fulminans, which is fatal unless treated by protein C replacement therapy (5). In addition to its essential role in the regulation of thrombin generation, recent results have indicated that APC also possesses antiinflammatory and cytoprotective properties (6–12), which have led to the approval of recombinant APC by the FDA as a therapeutic drug for treating severely septic patients (13). The protective activities of APC are thought to be mediated through its interaction with endothelial protein C receptor (EPCR) and the subsequent cleavage of protease-activated receptor 1 (PAR-1) on endothelial cells. Because the specificity of PAR-1 cleavage by thrombin is several orders of magnitude higher than that of APC and thrombin is required for generation of APC, it is not understood how APC can initiate protective signaling events in endothelial cells through the same receptor that can be cleaved with a much higher efficiency by thrombin (14). Moreover, because both protein C and APC can interact with EPCR with a similar affinity of ≈30 nM (15), it is not readily clear how physiological concentrations of APC (<10 nM) can compete with the zymogen protein C (≈80 nM) for binding to EPCR to exert its protective cellular effects. A partial answer to this question was recently provided by the observation that the cytoprotective activity of the endogenous APC generated by the thrombin–thrombomodulin (TM) complex is significantly higher than that of the exogenous APC, suggesting that endogenous protein C activation by thrombin is linked to efficient PAR-1-dependent protective signaling events in endothelial cells (16). One possible explanation of why the endogenous APC can exert a more efficient cytoprotective effect in endothelial cells might be that protein C zymogen activation by the thrombin–TM complex and the subsequent PAR-1 signaling events take place in the same microenvironment on the membrane surface. For example, a variety of G protein-coupled receptor signaling molecules and tyrosine kinases are known to be compartmentalized within the cholesterol/glycosphingolipid-rich cell membrane lipid rafts and caveolae (17, 18). In fact, there is some evidence for EPCR being targeted to such membrane microdomains as demonstrated by a higher rate of proteolytic shedding of soluble EPCR (sEPCR) in the caveolin-overexpressing cells (19). Whether EPCR, PAR-1, and TM are colocalized in the membrane microdomains of endothelial cells is not known.
To investigate this question, we isolated the lipid rafts of immortalized human umbilical vein endothelial cells (EA.hy926) and analyzed their protein contents by SDS/PAGE and Western blotting with antibodies specific to EPCR, PAR-1, and TM.
Results
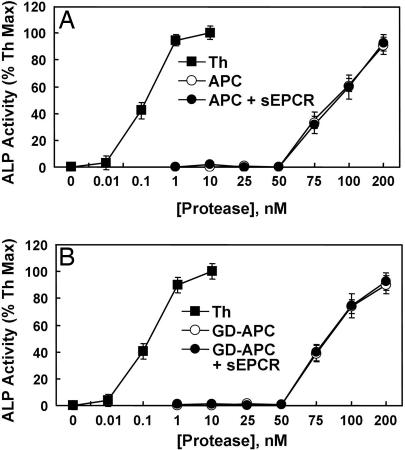
To investigate the mechanism by which EPCR enables APC to elicit cytoprotective signaling responses by the cleavage of PAR-1 in endothelial cells, we developed a PAR-1 cleavage reporter construct in which the NH2 terminus exodomain of PAR-1 has been fused to the COOH terminus of soluble alkaline phosphatase (ALP). This construct also contains the membrane-spanning domain of TF, fused to the COOH terminus of the PAR-1 exodomain, so that the ALP–PAR-1 fusion protein is anchored to the membrane surface upon its transfection to mammalian cells. A similar ALP–PAR-1 construct was recently used to demonstrate that thrombin cleaves its target scissile bond on the PAR-1 exodomain 3–4 orders of magnitude more efficiently than APC (14). Because thrombin is required for the generation of APC from protein C on endothelial cells, such a dramatic difference between the catalytic efficiencies of the two proteases toward PAR-1 has cast doubt on whether APC cleavage of PAR-1 in the presence of thrombin is relevant to eliciting protective signaling responses in endothelial cells under physiological conditions (14). To investigate this question and determine whether the PAR-1 cleavage by APC requires the cofactor effect of EPCR in this model system, we transfected either EA.hy926 or HEK-293 cells with ALP–PAR-1 and compared the cleavage rate of PAR-1 by either thrombin or APC in both the absence and presence of sEPCR. In agreement with previous results, thrombin exhibited ≈3 orders of magnitude higher efficiency toward PAR-1 in either cell line transfected with this construct (Fig. 1A, shown for EA.hy926 cells only). However, sEPCR did not influence the activity of APC toward PAR-1 in this model system.
Fig. 1.
Thrombin and APC concentration dependence of PAR-1 cleavage on endothelial cells transfected with the PAR-1 cleavage reporter construct. (A) PAR-1 cleavage by various concentrations of either thrombin (■) or APC in the absence (○) and presence (●) of a saturating concentration of sEPCR (500 nM) was monitored by an alkaline phosphatase assay as described in Materials and Methods. (B) The same as in A except that Gla-domainless APC was used in the cleavage reaction. The activities with APC are normalized to percentage maximal activity observed with 10 nM thrombin (Th).
The EPCR-dependent cellular activity of APC is mediated through the interaction of the Gla domain of the protease with the receptor on the endothelial cells (20, 21). To determine whether Gla-dependent EPCR interaction contributes to the efficiency of PAR-1 cleavage in this model system, we examined the activity of the Gla-domainless APC toward the cleavage of PAR-1 on cells transfected with the cleavage reporter construct. Interestingly, we found that the APC cleavage of PAR-1 in this system is EPCR-independent. The Gla-domainless APC exhibited an activity that was essentially identical to that observed with wild-type APC (Fig. 1B).
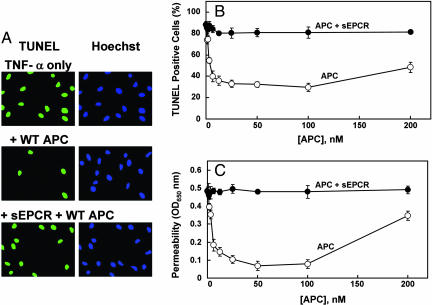
Next, we used two established cellular assays to compare the cytoprotective activities of the same APC derivatives in EA.hy926 cells not transfected with the reporter construct in the absence and presence of sEPCR. In the first assay we evaluated the antiapoptotic activities of the APC derivatives in the TNF-α-induced apoptosis assay. As shown in Fig. 2 A and B, APC inhibited the apoptotic cell death in a concentration-dependent manner. Neither the Gla-domainless APC nor the catalytically inactive S195A mutant of APC exhibited activity in this assay (data for mutants are not shown). However, unlike the results with ALP–PAR-1 cleavage reporter presented above, sEPCR effectively inhibited the cytoprotective function of APC. In agreement with Feistritzer and Riewald (22), the cytoprotective activity of APC, at concentrations much higher than physiological, was diminished.
Fig. 2.
Antiapoptotic and cytoprotective activities of APC in TNF-α-induced apoptosis and permeability assays in EA.hy926 cells. (A) Confluent monolayers of EA.hy926 cells were treated with APC (10 nM) in either the absence or presence of a saturating concentration sEPCR (500 nM) for 24 h followed by induction of apoptosis with TNF-α (10 ng/ml) for 4 h. The cells were fixed with paraformaldehyde and incubated with the TUNEL reaction mixture followed by Hoechst 33342 to stain the apoptotic cells (green) and the total number of nuclei (blue), respectively. (B) The same as above except the number of apoptotic cells is expressed as the percentage of TUNEL-positive cells of the total number of nuclei (P < 0.001) as a function of various concentrations of APC in the absence (○) and presence (●) of sEPCR. (C) The APC concentration dependence of inhibition of thrombin-induced permeability in the absence (○) and presence (●) of sEPCR was monitored from the flux of Evans blue-bound albumin across EA.hy926 cells as described in Materials and Methods.
Previous studies measuring the flux of albumin in a dual-chamber system have indicated that thrombin disrupts the permeability barrier of EA.hy926 cells and that APC has a potent protective effect (22). This assay was used to further assess the activity of APC derivatives in the absence and presence of sEPCR. In agreement with previous results, treatment of EA.hy926 cells with either TNF-α or thrombin resulted in an enhanced permeability that was effectively reversed by wild-type APC, which was not seen with either Gla-domainless APC or the S195A mutant mentioned above (Fig. 2C, shown only for wild-type APC in the presence of TNF-α). As with the antiapoptotic activity assay, a saturating concentration of sEPCR effectively inhibited the barrier protective effect of APC. Function-blocking antibodies to either EPCR or PAR-1 eliminated the cytoprotective effect of APC in both assays (data not shown). These results raised the possibility that, unlike the case with ALP–PAR-1-transfected cells (Fig. 1), the colocalization of PAR-1 and EPCR in the plasma membrane is required for APC to cleave PAR-1 and elicit cellular responses in endothelial cells.
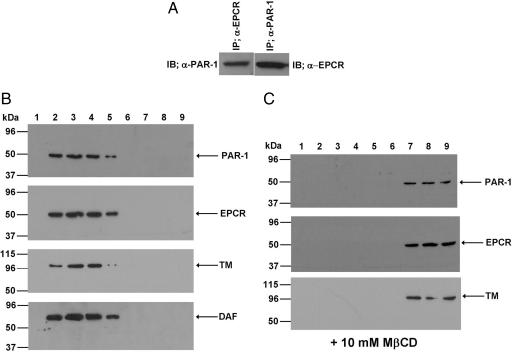
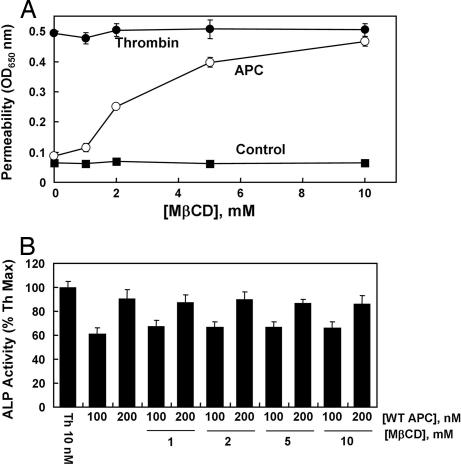
To explore this possibility, we immunoprecipitated the total protein extract of EA.hy926 cells with either anti-EPCR or anti-PAR-1 antibody and applied it on a 10% reducing SDS/PAGE as described in Materials and Methods. Western blot analyses of the immunoprecipitates indicated that the antibody specific for either receptor can coimmunoprecipitate both receptor proteins (Fig. 3A), suggesting that both EPCR and PAR-1 are either physically interacting or they are colocalized on the membrane surface of endothelial cells. To determine whether other components in protein C activation are also restricted to the same membrane microdomains, we isolated lipid rafts of EA.hy926 cells and analyzed the protein contents of different low- and high-buoyancy fractions by immunoblotting using antibodies to EPCR, PAR-1, and TM. Interestingly, we found that all three receptors of the protein C activation and PAR-1 signaling pathways are colocalized in the same low-density fractions representing the lipid rafts (Fig. 3B). As a positive control for the raft localization, the same membrane fractions were also immunoblotted with an antibody directed to the glycosylphosphatidylinositol-anchored decay-accelerating factor (Fig. 3B), a protein that is known to separate into the low-density lipid raft fractions (23). Consistent with the colocalization of all three receptors in lipid rafts, the treatment of endothelial cells with the cholesterol-depleting molecule methyl-β-cyclodextrin (MβCD) disrupted the colocalization of all three receptors in the lipid rafts as evidenced by their separation into the high-density sucrose gradient fractions (Fig. 3C). These results correlated well with the functional data. The treatment of endothelial cells with MβCD effectively eliminated the cytoprotective signaling activity of APC, without influencing the PAR-1-dependent permeability disruptive effect of thrombin (Fig. 4A). These results suggest that the colocalization of EPCR and PAR-1 in lipid rafts is required for APC to cleave PAR-1 and elicit protective cellular responses in endothelial cells. However, thrombin can cleave PAR-1 independent of its location in lipid rafts on the endothelial cell surface. In contrast to these results, MβCD did not have any effect on the APC cleavage of the PAR-1 on EA.hy926 or HEK-293 cells transfected with ALP–PAR-1, which is expressed on the cell surface but not in lipid rafts. These findings lead us to conclude that that receptor colocalization in lipid rafts is required for the cellular effect of APC, but that PAR-1 cleavage is independent of EPCR on endothelial cells transfected with the reporter construct (Fig. 4B).
Fig. 3.
Coimmunoprecipitation of EPCR and PAR-1 and immunoblotting of lipid rafts derived from EA.hy926 cells with anti-PAR-1, anti-EPCR, and anti-TM antibodies. (A) Immunoblots of total cellular proteins immunoprecipitated with either anti-EPCR or anti-PAR-1 antibody. (B) SDS/PAGE and immunoblotting of membrane fractions prepared by discontinuous sucrose gradient ultracentrifugation with antibodies directed to PAR-1, EPCR, TM, and decay-accelerating factor (DAF). (C) The same as in B except that the fractions were derived from EA.hy926 cells treated with 10 mM MβCD.
Fig. 4.
Effect of different concentrations of MβCD on the protective activity of APC in thrombin-induced permeability assay and on the PAR-1 cleavage rate in cells transfected with the PAR-1 cleavage reporter. (A) EA.hy926 cells were treated with the indicated concentrations of MβCD before evaluating the barrier protective effect of APC (○) in a thrombin-induced permeability assay. Controls are cells treated with thrombin only (●) and cells not treated with either thrombin or APC (■). (B) The rate of PAR-1 cleavage by APC (100 and 200 nM) monitored by ALP activity on EA.hy926 cells transiently transfected with the PAR-1 cleavage reporter at different concentrations of MβCD (1–10 mM) as described in the legend of Fig. 1.
Discussion
Recent results have indicated that the antiinflammatory effect of APC, at least partially, is mediated through the EPCR-dependent cleavage of PAR-1 in endothelial cells (9, 11, 12). PAR-1 has been identified as the primary target receptor for thrombin on platelets and endothelial cells (24). The cleavage of PAR-1 by thrombin elicits potent prothrombotic and proinflammatory responses (24, 25). Because thrombin is responsible for the activation of protein C and it also cleaves PAR-1 with a catalytic efficiency that is 3–4 orders of magnitude higher than that of APC, whether APC in the presence of thrombin produces physiologically significant protective signaling events by PAR-1 cleavage is controversial (14). Nevertheless, an interesting recent study demonstrated that the endogenous APC, generated by thrombin on the endothelial cell surface, exhibits much greater cellular effects than exogenous APC, suggesting that the activation of protein C on the cell surface may be mechanistically linked to its PAR-1-dependent protective signaling mechanism (16). Noting that APC and protein C both interact with EPCR with a similar affinity, Feistritzer et al. (16) hypothesized that thrombin can increase the local concentrations of EPCR-bound APC, thus channeling the protease directly into the signaling pathway. The findings of the present study, that all three receptors involved in both protein C activation and APC signaling are colocalized in the lipid rafts, explain how thrombin effectively channels endogenous APC to the protective signaling pathways.
A recent study, which monitored the activation of protein C and the cleavage rate of PAR-1 by thrombin on human umbilical vein cells transfected with an alkaline phosphatase–PAR-1 cleavage reporter, concluded that PAR-1 cleavage by APC may not play a role in the signaling activity of APC because no difference in the PAR-1 cleavage rate of the reporter on endothelial cells was observed in the presence of protein C incubated with different concentrations of thrombin (14). However, as demonstrated in this study with a similar reporter construct, the PAR-1 cleavage by thrombin and APC in this model system cannot mimic the real physiological conditions because the overexpressed PAR-1 fusion protein is not likely to be colocalized in the lipid rafts with TM and EPCR. This was confirmed by the EPCR-independent cleavage of PAR-1 by APC in endothelial cells transiently transfected with ALP–PAR-1. The results with the Gla-domainless APC provided further evidence for this proposal because the mutant cleaved PAR-1 from ALP–PAR-1 with an efficiency that was similar to that of wild-type APC, although the mutant did not confer any protective activity on nontransfected cells. The observation that MβCD eliminated the cytoprotective activity of exogenous APC without affecting its activity on cells transfected with ALP–PAR-1 supports the conclusion that the PAR-1 colocalization with EPCR in lipid rafts is required for the protective signaling effect of APC, but thrombin can cleave PAR-1 independent of its location in lipid rafts on endothelial cell membranes.
Thrombin utilizes the basic residues of exosite-1 to interact with the hirudin-like sequence on PAR-1 (24, 25) or the acidic residues of epidermal growth factor-like domains on TM (26, 27) to function in either the procoagulant/proinflammatory or the anticoagulant/antiinflammatory pathways, respectively (24, 28). Noting the high affinity of thrombin for TM (<1 nM) (29), physiological concentrations of thrombin may primarily interact with TM in lipid rafts, thereby blocking its interaction with PAR-1 and activating EPCR-bound protein C that is located next to PAR-1 in the same lipid raft and/or PAR-1 recruited from another nearby raft through a regulated mechanism. In support of a primarily antiinflammatory role for thrombin under normal physiological conditions, it is known that the infusion of a low concentration of thrombin to primates exhibits a protective effect by increasing the concentration of APC in circulation without increasing the markers of platelet activation (30). Similarly, a low concentration of thrombin has been demonstrated to block the lethal inflammatory effect of endotoxin (31). Noting that both fast and slow forms of thrombin can effectively activate protein C (32), but only the fast form of thrombin can optimally activate PAR-1 (32), it is possible that the colocalization of TM with EPCR and PAR-1 in the lipid rafts can tip the balance of thrombin in favor of interaction with TM, thereby mediating the PAR-1-dependent cellular signaling effects through the protective APC pathway under physiological conditions.
The observation that TM, EPCR, and PAR-1 are localized in the lipid rafts derived from the membrane fractions of unstimulated cells in the absence of an agonist suggests that all three receptors constitutively reside in the lipid rafts. However, whether all three receptors are colocalized in the same lipid raft or are segregated into different rafts is not known. This question becomes relevant because it is known that different classes of lipid rafts with different compositions can exist on cell membranes (17). Thus, the distribution of a receptor into separate lipid rafts can influence the specificity of signaling events by facilitating the association of that receptor with different downstream signaling molecules (17). Because the activation of protein C by thrombin requires the cofactor function of both TM and EPCR, it is anticipated that both of these receptors are colocalized in the same lipid rafts on endothelial cells. However, PAR-1 may or may not be colocalized with the other two receptors in the same lipid raft. A ligand-induced fusion of different lipid rafts can play a key role in cellular signal transduction mechanisms (17, 18). The localization of PAR-1 in different lipid rafts, enriched with different G proteins and related downstream signaling molecules, can endow specificity for PAR-1 signaling through either the thrombin or APC pathways. For instance, it is possible that thrombin interaction with TM, which is in the same lipid raft with EPCR, induces the fusion of a PAR-1-containing lipid raft that has a G protein(s) that is distinct from that of a lipid raft PAR-1 that is target for thrombin alone. Although highly hypothetical for thrombin and APC, there are precedents for this mechanism of lipid raft-associated G protein-coupled receptor signaling in the literature (17). This mechanism, or other similar subtle signaling mechanisms facilitated by lipid rafts (17, 18), may explain how thrombin and APC can function in opposite disruptive and protective pathways through the activation of the same receptor in endothelial cells. Thus, our findings that the receptors of both protein C activation and protective APC signaling pathways are colocalized in the membrane lipid rafts of endothelial cells pave the way for designing new approaches to understanding the details of this important question.
Materials and Methods
Antibodies blocking the activation of PAR-1 (H-111) or nonblocking antibody (S-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The function-blocking anti-EPCR antibody (clone RCR-252) was purchased from Cell Sciences (Canton, MA). All antibodies were used at 25 μg/ml. TNF-α was purchased from R & D Systems (Minneapolis, MN). The chromogenic substrate Spectrozyme PCa was purchased from American Diagnostica (Greenwich, CT).
Recombinant Proteins.
Expression and purification of wild type, a Gla-domainless mutant (GD-PC), and a Ser-195 to Ala (S195A) substitution mutant of protein C in HEK-293 have been described previously (33). Both wild-type and mutant zymogens were purified to homogeneity by a combination of immunoaffinity and ion-exchange chromatography using the Ca2+-dependent monoclonal antibody HPC4 coupled to Affi-Gel-10 (Bio-Rad, Hercules, CA) and FPLC Mono Q column (GE Healthcare, Little Chalfont, Buckinghamshire, UK) as described (34). Recombinant thrombin (29) and protein C inhibitor (35) were expressed and purified as described. Recombinant sEPCR and the monoclonal antibody to human TM (RSV TM GT 261) were generous gifts from C. T. Esmon (Oklahoma Medical Research Foundation, Oklahoma City, OK). All recombinant proteins were tested for homogeneity by SDS/PAGE.
Alkaline Phosphatase–PAR-1-Tissue Factor (ALP–PAR-1) Fusion Plasmid and PAR-1 Cleavage Assay.
The cDNA encoding secreted human tissue nonspecific alkaline phosphatase (36) (a generous gift from William Sly, Saint Louis University School of Medicine), lacking the last 19 COOH-terminal residues, was fused to a synthetic DNA fragment encoding the exodomain of PAR-1 (Thr-37 to Ser-99) and the membrane-spanning domain of tissue factor (Arg-218 to Lys-244). The ALP–PAR-1 cDNA was inserted into the HindIII/XbaI cloning sites of the mammalian expression vector pRc/RSV (Invitrogen, San Diego, CA). EA.hy926 or HEK-293 cells at 90% confluence in 24-well plates were transiently transfected with pRc/RSV containing ALP–PAR-1 cDNA in antibiotic-free Opti-MEM medium using Lipofectamine (Invitrogen, Carlsbad, CA). On the following day, cells were washed and incubated in serum-free medium for 5 h. Cells were then incubated for an additional 1 h with various concentrations of thrombin or APC plus hirudin (4 units/ml). Conditioned medium was collected and centrifuged to remove cell debris. Supernatant was collected, and alkaline phosphatase activity was measured by using the EnzoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA) according to the manufacturer's instructions.
Zymogen Activation.
Two milligrams of protein C derivatives was incubated with thrombin (50 μg) in 0.1 M NaCl, 0.02 M Tris·HCl (pH 7.4) (TBS buffer) containing 5 mM EDTA for 2 h at 37°C. The APC derivatives were separated from thrombin by an FPLC Mono Q column developed with a 40-ml linear gradient from 0.1 to 1.0 M NaCl/0.02 M Tris·HCl (pH 7.4) as described (34). The concentrations of proteases were determined from the absorbance at 280 nm and extinction coefficient (E1%1cm) of 14.5 (assuming a molecular mass of 56 kDa for APC and 50 kDa for Gla-domainless APC), by an amidolytic activity assay, and by stoichiometric titration of enzymes with known concentrations of protein C inhibitor as described (35).
Cell Culture.
EA.hy926 cells (kindly provided by C. Edgell, University of North Carolina, Chapel Hill, NC) were cultured to confluence in a humidified atmosphere at 37°C in DMEM supplemented with 10% FBS (HyClone, Logan, UT) and antibiotics (penicillin G and streptomycin).
Apoptosis Assay.
EA.hy926 cells (0.5 × 106) were seeded onto coverslips coated with gelatin as described (9). After 24 h at 37°C, the medium was replaced and cells were incubated with APC (10 nM) for another 24 h. Then, the cells were incubated with 10 ng/ml TNF-α (or 5 μM staurosporine) for 4 h. Cells were fixed with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100/0.1% sodium citrate, and incubated for 1 h in the dark with a TUNEL reaction mixture (Roche, Mannheim, Germany) for in situ detection of cell death. After washing with PBS, cells were incubated with Hoechst 33342 (Sigma–Aldrich, St. Louis, MO) for 15 min. The number of apoptotic cells was expressed as the percentage of TUNEL-positive cells of the total number of nuclei determined by Hoechst staining. The number of TUNEL-positive cells in the absence of staurosporine or TNF-α was 10–15%. Both TNF-α and staurosporine yielded similar results in the apoptosis assays.
Permeability Assay.
Permeability was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional EA.hy926 cell monolayers using a modified two-compartment chamber model as previously described (22). Briefly, EA.hy926 cells were plated (5 × 104 per well) in transwells of 3-μm pore size and 12-mm diameter for 4–6 days. The confluent monolayers were incubated with APC (10 nM) for 3 h followed by activation by either 5 nM thrombin for 10 min or 10 ng/ml TNF-α for 18 h as described (22). Inserts were washed with PBS (pH 7.4) before adding 0.5 ml of Evans blue (0.67 mg/ml) (Sigma) diluted in growth medium containing 0.4% BSA. Fresh growth medium was added to the lower chamber, and the medium in the upper chamber was replaced with Evans blue/BSA. After 10 min, the optical density at 650 nm was measured in the lower chamber. For the function-blocking antibody treatments of the monolayers, medium was removed and antibodies were added for 30 min in serum-free medium followed by analysis of the permeability. Experiments were performed in triplicate and repeated multiple times.
Immunoprecipitation, SDS/PAGE, and Western blotting.
Total cellular proteins were extracted with PBS containing 25 μM of proteasome inhibitor MG132 (Sigma–Aldrich) and complete protease inhibitor mixture (Roche Molecular Systems, Summerville, NJ) and were sonicated (set at 10% of maximum speed) for 10 sec followed by 1-min incubation on ice. This process was repeated five times. Lysates were combined with 3 μg of specific antibody and incubated for 2 h at 4°C. Immunoprecipitates were collected with Protein A/G Agarose (Santa Cruz Biotechnology) and washed with PBS containing 25 μM MG132 and protease inhibitor mixture. Immunoprecipitates were fractionated by SDS/10% PAGE, transferred to membranes, and subjected to Western blotting with appropriate primary and secondary antibodies. The immunoreactive protein bands were visualized by SuperSignal West Pico (Pierce, Rockford, IL).
Isolation of Lipid Rafts.
Lipid rafts were isolated by slight modification of a detergent-free procedure as described previously (37). Briefly, cells were grown to near confluence in 150-mm Petri dishes. After washing with ice-cold PBS, cells were scraped into Tris lysis buffer (25 mM Tris, 250 mM sucrose, and 2 mM EDTA). Cell pellets were then homogenized with a tight-fitting Dounce homogenizer followed by three 20-sec bursts of ultrasonic sonicator on ice. The lysate was adjusted to 45% sucrose by the addition of an equal volume of 90% sucrose in Mes-buffered saline [25 mM Mes and 0.15 M NaCl (pH 6.5)] and placed at the bottom of an ultracentrifuge tube. Two solutions (1.7 ml each) of 35% and 5% sucrose were laid sequentially on the top of the 45% sucrose solution. After ultracentrifugation at 35,000 rpm with a Beckman SW Ti55 rotor for 16–20 h, 10 0.5-ml fractions were collected from the top of tubes, and a portion of each fraction was analyzed by SDS/PAGE, transferred to nitrocellulose membranes, and subjected to Western blotting with appropriate primary and secondary antibodies. The immunoreactive protein bands were visualized as described above. The same methods were used to isolate the membrane fractions from endothelial cells treated for 1.5 h with the cholesterol-depleting molecule MβCD (Sigma–Aldrich).
Statistical Analysis.
Results are expressed as mean ± SEM, and t tests (paired or independent) were used to assess data. Differences were considered statistically significant at P values of <0.05. Statistics were performed by using the software package SPSS, version 14.0 (SPSS, Chicago, IL).
Acknowledgments
We thank Audrey Rezaie for proofreading of the manuscript and Tracey Baird for technical assistance in preparing the manuscript for publication. The research discussed herein was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health Grants HL 62565 and HL 68571 (to A.R.R.).
Abbreviations
- APC
activated protein C
- EPCR
endothelial protein C receptor
- sEPCR
soluble EPCR
- PAR-1
protease-activated receptor 1
- TM
thrombomodulin
- ALP
soluble alkaline phosphatase
- MβCD
methyl-β-cyclodextrin.
Footnotes
The authors declare no conflict of interest.
References
- 1.Esmon CT. Thromb Haemostasis. 1993;70:29–35. [PubMed] [Google Scholar]
- 2.Stenflo J. Semin Thromb Hemostasis. 1984;10:109–121. doi: 10.1055/s-2007-1004413. [DOI] [PubMed] [Google Scholar]
- 3.Walker FJ, Fay PJ. FASEB J. 1992;6:2561–2567. doi: 10.1096/fasebj.6.8.1317308. [DOI] [PubMed] [Google Scholar]
- 4.Dahlback B. Thromb Haemostasis. 1991;66:49–61. [PubMed] [Google Scholar]
- 5.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. J Clin Invest. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor FB, Jr, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 7.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 8.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 9.Mosnier LO, Griffin JH. Biochem J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 13.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 14.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 15.Regan LM, Stearns-Kurosawa DJ, Kurosawa S, Mollica J, Fukudome K, Esmon CT. J Biol Chem. 1996;271:17499–17503. doi: 10.1074/jbc.271.29.17499. [DOI] [PubMed] [Google Scholar]
- 16.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, Riewald M. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 17.Pike LJ. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Zajchowski LD, Robbins SM. Eur J Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Qu D, Esmon NL, Esmon CT. J Biol Chem. 2000;275:6038–6044. doi: 10.1074/jbc.275.8.6038. [DOI] [PubMed] [Google Scholar]
- 20.Oganesyan V, Oganesyan N, Terzyan S, Qu D, Dauter Z, Esmon NL, Esmon CT. J Biol Chem. 2002;277:24851–24854. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 21.Preston RJ, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlback B, Lane DA. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 22.Feistritzer C, Riewald M. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 23.Dietzen DJ, Page KL, Tetzloff TA. Blood. 2004;103:3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin SR. J Thromb Haemostasis. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 25.Ayala YM, Cantwell AM, Rose T, Bush LA, Arosio D, Di Cera E. Proteins. 2001;45:107–116. doi: 10.1002/prot.1130. [DOI] [PubMed] [Google Scholar]
- 26.Fuentes-Prior P, Iwanaga Y, Huber R, Pagila R, Rumennik G, Seto M, Morser J, Light DR, Bode W. Nature. 2000;404:518–525. doi: 10.1038/35006683. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Rezaie AR. J Biol Chem. 2003;278:10484–10490. doi: 10.1074/jbc.M211797200. [DOI] [PubMed] [Google Scholar]
- 28.Ruf W, Dorfleutner A, Riewald M. J Thromb Haemostasis. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 29.Ye J, Rezaie AR, Esmon CT. J Biol Chem. 1994;269:17965–17970. [PubMed] [Google Scholar]
- 30.Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A. J Clin Invest. 1993;92:2003–2012. doi: 10.1172/JCI116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor FB, Jr, Chang A, Hinshaw LB, Esmon CT, Archer LT, Beller BK. Thromb Res. 1984;36:177–185. doi: 10.1016/0049-3848(84)90339-6. [DOI] [PubMed] [Google Scholar]
- 32.Page MJ, Macgillivray RT, Di Cera E. J Thromb Haemostasis. 2005;3:2401–2408. doi: 10.1111/j.1538-7836.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 33.Rezaie AR, Esmon CT. J Biol Chem. 1992;267:26104–26109. [PubMed] [Google Scholar]
- 34.Chen L, Manithody C, Yang L, Rezaie AR. Protein Sci. 2004;13:431–442. doi: 10.1110/ps.03406904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Manithody C, Rezaie AR. Biochemistry. 2002;41:6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 36.Weiss MJ, Henthorn PS, Lafferty MA, Slaughter C, Raducha M, Harris H. Proc Natl Acad Sci USA. 1986;83:7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Casey L, Pike LJ. Biochem Biophys Res Commun. 1998;245:684–690. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]