Abstract
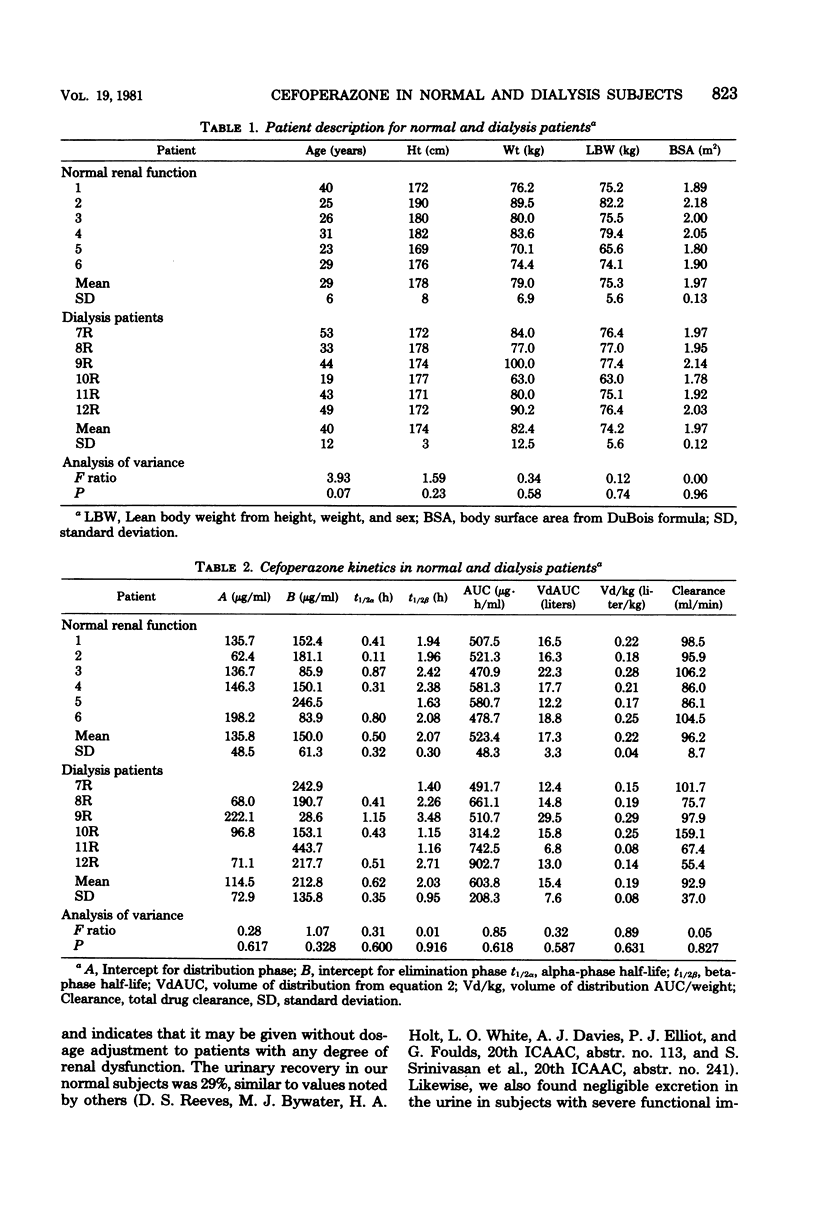
We examined the pharmacokinetics of the new beta-lactam agent, cefoperazone, in normal and functionally anephric dialysis subjects with normal liver function. All subjects received 3 g of cefoperazone intravenously, and serial serum and urine samples were taken thereafter for up to 36 h. The serum levels, volume of distribution (0.22 liter/kg, normal; 0.19 liter/kg; dialysis), beta half-life (2.07 h, normal; 2.03 h, dialysis), and total body clearance (96.2 ml/min, normal; 92.9 ml/ min, dialysis) were all not significantly different between the two groups. Cefoperazone may be administered without adjustment of dose for any degree of renal dysfunction to patients with normal hepatic function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borobio M. V., Aznar J., Jimenez R., Garcia F., Perea E. J. Comparative in vitro activity of 1-oxa-beta-lactam (LY127935) and cefoperazone with other beta-lactam antibiotics against anaerobic bacteria. Antimicrob Agents Chemother. 1980 Feb;17(2):129–131. doi: 10.1128/aac.17.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. In vitro activity of cefoperazone against nonfermenters and Aeromonas hydrophila. Antimicrob Agents Chemother. 1980 Sep;18(3):483–486. doi: 10.1128/aac.18.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. H., Opfer B. J., Gerding D. N. Comparative activities of the oxa-beta-lactam LY127935, cefotaxime, cefoperazone, cefamandole, and ticarcillin against multiply resistant gram-negative bacilli. Antimicrob Agents Chemother. 1980 Feb;17(2):273–279. doi: 10.1128/aac.17.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Barry A. L., Gavan T. L., Sommers H. M., Gerlach E. H. Cefoperazone (T-1551), a new semisynthetic cephalosporin: comparison with cephalothin and gentamicin. Antimicrob Agents Chemother. 1980 Apr;17(4):743–749. doi: 10.1128/aac.17.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Kobasa W., Kaye K. Susceptibilities of anaerobic bacteria to cefoperazone and other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):957–960. doi: 10.1128/aac.17.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz T. O., Winston D. J., Hindler J. A., Young L. S., Hewitt W. L., Martin W. J. Comparative in vitro activity of moxalactam, cefotaxime, cefoperazone, piperacillin, and aminoglycosides against gram-negative bacilli. Antimicrob Agents Chemother. 1980 Oct;18(4):645–648. doi: 10.1128/aac.18.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara N., Minami S., Matsuhashi M., Takaoka M., Mitsuhashi S. Affinity of cefoperazone for penicillin-binding proteins. Antimicrob Agents Chemother. 1980 Jul;18(1):195–199. doi: 10.1128/aac.18.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara N., Minami S., Muraoka T., Saikawa I., Mitsuhashi S. In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1979 Dec;16(6):731–735. doi: 10.1128/aac.16.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrogie J. J., Rogers J. D., Yeh K. C., Davies R. O., Holmes G. I., Skeggs H., Martin C. M. Pharmacokinetics and comparative pharmacology of cefoxitin and cephalosporins. Rev Infect Dis. 1979 Jan-Feb;1(1):90–98. doi: 10.1093/clinids/1.1.90. [DOI] [PubMed] [Google Scholar]
- Spyker D. A., Rugloski R. J., Vann R. L., O'Brien W. M. Pharmacokinetics of amoxicillin: dose dependence after intravenous, oral, and intramuscular administration. Antimicrob Agents Chemother. 1977 Jan;11(1):132–141. doi: 10.1128/aac.11.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyker D. A., Spyker J. M. Response model analysis for cross-fostering studies: prenatal versus postnatal effects on offspring exposed to methylmercury dicyandiamide. Toxicol Appl Pharmacol. 1977 Jun;40(3):511–527. doi: 10.1016/0041-008x(77)90077-1. [DOI] [PubMed] [Google Scholar]



