Abstract
In ecdysozoan protostomes, including arthropods and nematodes, transcription factors of the GATA family specify the endoderm: Drosophila dGATAb (ABF/Serpent) and Caenorhabditis elegans END-1 play important roles in generating this primary germ layer. end-1 is the earliest expressed endoderm-specific gene known in C. elegans and appears to initiate the program of gene expression required for endoderm differentiation, including a cascade of GATA factors required for development and maintenance of the intestine. Among vertebrate GATA proteins, the GATA-4/5/6 subfamily regulates aspects of late endoderm development, but a role for GATA factors in establishing the endoderm is unknown. We show here that END-1 binds to the canonical target DNA sequence WGATAR with specificity similar to that of vertebrate GATA-1 and GATA-4, and that it functions as a transcriptional activator. We exploited this activity of END-1 to demonstrate that establishment of the vertebrate endoderm, like that of invertebrate species, also appears to involve GATA transcriptional activity. Like the known vertebrate endoderm regulators Mixer and Sox17, END-1 is a potent activator of endoderm differentiation in isolated Xenopus ectoderm. Moreover, a dominant inhibitory GATA-binding fusion protein abrogates endoderm differentiation in intact embryos. By examining these effects in conjunction with those of Mixer- and Sox17β-activating and dominant inhibitory constructs, we further establish the likely relationships between GATA activity and these regulators in early development of the vertebrate endoderm. These results suggest that GATA factors may function sequentially to regulate endoderm differentiation in both protostomes and deuterostomes.
In all triploblastic metazoans, an inner embryonic germ layer, the endoderm, is generated during gastrulation. Evidence from the ecdysozoan protostomes (1) Drosophila and Caenorhabditis elegans indicates that activation of endoderm development is regulated by transcription factors of the GATA family in these species. GATA proteins are key regulators of gene expression and cell differentiation in a wide range of species. They carry the defining zinc-finger motif CXNCX17CNXC, which confers binding specificity for the nucleotide sequence WGATAR (2); sequence similarities outside this domain are often limited.
The C. elegans end-1 gene is expressed exclusively and early in the endoderm lineage (3), is able to program nonendodermal embryonic blastomeres to endodermal fate, and is the first zygotically transcribed gene known to specify endoderm in worms (4). END-1 carries a single zinc finger that is similar to the DNA-binding domain of all GATA proteins (3). ELT-2, a second gut-specific transcription factor expressed slightly later in the C. elegans endoderm, has two presumptive zinc fingers, one of which is similar to the GATA motif (5), and is required for maintenance of intestinal cells (6). Similarly, mutations that eliminate the Drosophila GATA factor ABF/Serpent (dGATAb) result in the complete absence of the midgut, the only endoderm derivative in flies (7, 8). dGATAc, which contains two GATA-type zinc fingers, is expressed later in the developing Drosophila midgut (9), among other tissues. These observations have led investigators to speculate that specification and subsequent differentiation of the invertebrate endoderm is regulated sequentially by subsets of GATA factors (8). Members of the vertebrate GATA-4/5/6 subfamily are expressed in the developing and adult gut (10, 11), as well as in the heart and other sites. They regulate transcription of markers of the mature gut epithelium (12, 13), and GATA proteins likely contribute toward specification of hepatocyte identity (14). However, their role in specifying the early endoderm is unknown. Absence of either GATA-4 or GATA-6 in mice (15–19) or of GATA-5 in zebrafish (20) results in embryonic death, with abnormal development of endoderm derivatives, but specification of this primary germ layer is preserved in all three cases.
Distinct families of transcription factors are known to regulate endoderm differentiation in Xenopus laevis. The T-box gene VegT/Brat/Xombi/Antipodean is transcribed maternally, expressed in mesendodermal precursor cells, and required for endoderm differentiation (21–25). Effectors of VegT function may include members of the Bix family of paired-type homeodomain proteins (26). The zygotic factors Sox17α and β and Mixer/Mix.3 localize to the presumptive endoderm and induce ectopic endoderm in animal cap explants (27–29). Mixer expression is restricted to gastrula stages and up-regulates expression of Sox17 genes; a dominant inhibitory Sox17β protein blocks Mixer-induced endoderm differentiation (28). Mixer thus appears to function upstream of Sox17 in one transcriptional pathway of endoderm specification. The Mixer-related genes Mix.1 and Milk are also expressed in vegetal blastomeres in Xenopus embryos, show weak induction of endoderm in animal cap explants, and suppress endogenous differentiation of mesoderm (30, 31). The functions of Mixer and Sox17 were recently confirmed in zebrafish (32), but a role for GATA proteins in vertebrate early endoderm development has not been described.
We are investigating the hypothesis that molecular mechanisms of early endoderm development may be conserved in all triploblastic metazoans. Here we show that END-1 is a potent sequence-specific transcriptional activator, with DNA-binding preference akin to that of GATA-1 and -4, and hence is a useful molecular probe for GATA-factor activity. END-1 activates ectopic endoderm differentiation in Xenopus embryos, comparable to the effects of Xenopus Mixer and Sox17, and a dominant inhibitory End-1 construct disrupts endogenous endoderm development. Our studies further suggest a role for GATA activity early in development of the vertebrate endoderm, and within the framework of the Mixer/Sox17 transcriptional pathway. These results suggest that establishment of the vertebrate endoderm may require early activity of GATA proteins.
Materials and Methods
Plasmid Constructs.
end-1 cDNA (3) was amplified by PCR and subcloned in pCS2, a plasmid derivative of pcDNA3 (33); the truncation mutant End-1Δ3′ contains nucleotides 1–504 of the end-1 cDNA. Mixer/Mix.3 (28, 29) and Xenopus GATA-4, -5, and -6 cDNAs were kindly provided as constructs in pcDNA3 by Paul Mead (Children's Hospital, Boston, MA) and Todd Evans (Albert Einstein College of Medicine, New York), respectively. For fusion constructs with the Engrailed repressor (EnR) domain, bases encoding aa 2–296 of Drosophila Engrailed (34) were amplified by PCR and subcloned in pCS2; nucleotides encoding aa 1–168 of Mixer (28) or aa 106–221 of END-1 (3) with an in-frame ATG codon were inserted upstream of this fragment. End-1∷EnRMut was generated by PCR with the alteration of two Cys residues to yield the sequence GAIEGNGASLY instead of GAIECNGCSLY. Sox17β and Sox17β∷EnR constructs (27) were kindly provided by Clare Hudson and Hugh Woodland (University of Warwick, Coventry, U.K.).
Electrophoretic Mobility Shift Assay.
Proteins were overexpressed in COS cells by transfection of 5–7 × 106 cells with 20 μg of plasmid DNA, by using 2.5 μl of FuGENE6 (Boehringer Mannheim) per μg of DNA. Nuclear extracts were prepared as described (35) and protein expression was confirmed by immunoblot with rabbit antiserum (1:500 dilution) against the C-terminal END-1 peptide SPTPEDSKLCHNTTPLQNIPSQHFS. Electrophoretic mobility shift assays were performed at 4°C by using 10 μg of nuclear extract and [γ-32P]ATP end-labeled double-stranded oligonucleotide with the sequence 5′-CTGGGGACAGATAAGCTACAGC-3′. A 100-fold excess of competitor oligonucleotides (GATC, 5′-CTGGGGACAGATCAGCTACAGC-3′; CTTA, 5′-CTGGGGACACTTAAGCTACAGC-3′) or 1 μl of anti-END-1 or preimmune serum were incubated with the extracts for 20 min before addition of the probe. Reaction products were resolved on 5% PAGE at 200V for 2 h at 4°C.
Transactivation Assays.
Transient transfection assays were performed in COS cells. The αD3 and αD4 promoter constructs (36), provided by Todd Evans (Albert Einstein College of Medicine, New York), contain the sequence AGATAA or ACTGAA, respectively, upstream of firefly luciferase cDNA. Transfections were done in triplicate by using FuGENE6 (Boehringer Mannheim), 0.025 μg of β-galactosidase plasmid, 0.05 μg of reporter plasmid, and 0.1–0.3 μg of GATA expression plasmid or empty vector. After 48 h, cells were lysed at 4°C in 25 mM Tris (pH 7.8)/2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid/10% glycerol/1% Triton X-100/2 mM DTT/0.3 mM PMSF/2 μg/ml aprotinin, and assayed at a 1:10 dilution for β-galactosidase activity in 40 mM NaH2PO4/60 mM Na2HPO4/10 mM KCl/1 mM MgSO4/4 mM β-mercaptoethanol/8 mM o-nitrophenyl β-d-galactoside by measurement of A415. Corresponding luciferase activity was measured by luminometry after diluting the lysate 1:10 in 25 mM glycyl glycine/15 mM MgSO4/15 mM K2HPO4/4 mM EGTA/40 μM ATP/40 μM DTT/0.3 μg/ml of luciferin. Relative luciferase activity was determined by comparing averaged luciferase:β-galactosidase ratios, and expressed relative to results from control transfections with the empty vector.
Microinjection and Embryo Manipulations.
Plasmids were linearized and capped mRNA was transcribed in vitro by using the mMESSAGE Machine kit (Ambion, Austin, TX). Xenopus oocytes were fertilized in vitro, dejellied in 3% Cys, rinsed in 0.1× Modified Marc's Ringer's (MMR) solution (37), and transferred to 3% Ficoll in 0.5× MMR. RNA (4.6 nanoliter; ≤700 pg) was injected into the animal pole at the one-cell stage. Animal caps were dissected between Nieuwkoop-Faber stages 8.5 and 9.5 (38), cultured overnight in 0.5× MMR supplemented with 50 units/ml penicillin and 50 μg/ml streptomycin, transferred to 0.1× MMR, and cultured at 21°C until untreated tadpoles reached the desired stage. Alternatively, embryos were injected at two points in the vegetal hemisphere with 4.6 nl RNA (≤1.2 ng). After 8–10 h in 3% Ficoll/0.5× MMR, embryos were either cultured whole in 0.1× MMR or vegetal hemispheres were explanted and cultured in 0.7× MMR until the equivalent of stage 35.
Reverse Transcription–PCR (RT-PCR).
Total RNA was extracted from animal or vegetal explants or whole embryos by using RNAzolB (Tel-Test, Friendswood, TX), reverse transcribed with oligo(dT) primers, and used for PCR (annealing temperature 61°C) with 0.1 μCi [α-32P]dCTP radiotracer. The number of PCR cycles was varied to ensure that amplification was always in the linear range, and PCR products were resolved on 4% PAGE. Primers and sizes of the amplified products: EF-1α (221 bp) 5′-CCTGAATCACCCAGGCCAGATTAA-3′ and 5′-GAGGGTAGTCTGAGAAGCTCTCCACG-3′; Endodermin (340 bp) 5′-ATAACGTTCCCCACCCCAAAGA-3′ and 5′-TTGGGTTGCTGATGGGAATGT-3′; IFABP (299 bp) 5′-CAAGT-TTACCCTTGCACAACCC-3′ and 5′-CAACTTCATCCC-AGCCCAATCA-3′; LFABP (145 bp) 5′-ACCGAGATTGA-ACAGAATGG-3′ and 5′-CCTCCATGTTTACCACGGAC-3′; Mixer (373 bp) 5′-GGAGGCACCCAGGAGAAAGT-3′ and 5′-TAGCGTGAGGTTTAGAGATG-3′; ornithine decarboxylase (ODC) (234 bp) 5′-AATGGATTTCAGAGACCA-3′ and 5′-CCAAGGCTAAAGTTGCAG-3′; XSox17α (176 bp) 5′-GGACGAGTGCCAGATGATG-3′ and 5′-CTGGCAAGTACATCTGTCC-3′; XlHbox8 (432 bp) 5′-CAACTTCATCCCAGCCCAATCA-3′ and 5′-TTTCCCTTCCCCTAATAACCCG-3′; Xbra (188 bp) 5′-GGATCGTTATCACCTCTG-3′ and 5′-GTGTAGTCTGTAGCAGCA-3′; Xtwist (302 bp) 5′-AGAAACTGGAGCTGGATC-3′ and 5′-GGCTTCAAAGGCACGACT-3′.
In Situ Hybridization.
Whole-mount in situ hybridization was performed in the InsituPro (Abimed Analysentechnik, Langenfeld, Germany). Animal caps were fixed for 1.5 h in MEMFA [100 mM Mops (pH 7.4)/2 mM EGTA/1 mM MgSO4/3.7% formaldehyde], stored overnight at 4°C in ethanol, rehydrated, bleached for 1 h in 10% H2O2, rinsed in PTween (PBS and 0.1% Tween-20), treated for 2 min with 10 μg/ml proteinase K, and refixed for 20 min in 4% paraformaldehyde in PTween. After prehybridization for 4 h, samples were probed overnight at 65°C with digoxigenin-labeled endodermin antisense RNA, prepared by using the DIG Labeling Kit (Boehringer Mannheim); endodermin cDNA (39) was a gift from Eddy De Robertis (University of California at Los Angeles, CA). Caps were washed at a final stringency of 0.1× SSC at 65°C, preincubated for 1.5 h in blocking solution (Boehringer Mannheim), and incubated for 6 h with alkaline phosphatase-conjugated anti-digoxigenin antibody (Boehringer Mannheim) at room temperature. After 3 h of serial washing in 100 mM maleic acid, 150 mM NaCl (pH 7.6), caps were stained with nitroblue tetrazolium/ 5-bromo-4-chloro-3-indolyl-phosphate chromogenic substrates (Boehringer Mannheim) for 3 h.
Results
END-1 Is a Potent Sequence-Specific Activator of Transcription.
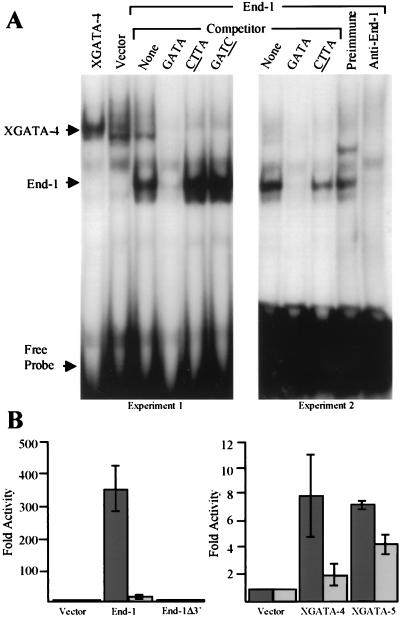
In C. elegans embryos homozygous for partial deletions of chromosome V, the cell lineage of the E blastomere, the sole endoderm precursor, is completely transformed to that of the C blastomere, which produces ectoderm and muscle. The putative GATA factor End-1 was identified based on its ability to restore endoderm differentiation in these mutants (3). Whereas the canonical GATA zinc-finger motif is defined by the amino acid sequence CXNCX17CNXC, the corresponding region of END-1 contains an atypical CX4C sequence in place of CX2C at the NH2 terminus (3), possibly suggesting divergent functions. We therefore tested END-1 activity in electrophoretic mobility shift and transcriptional activation assays. END-1 shows binding specificity for the consensus GATA sequence, as judged by competition from GATA- but not GATC- or CTTA-containing oligonucleotides (Fig. 1A). Specific abrogation of binding by END-1 antiserum confirms that the observed activity represents END-1. A luciferase reporter gene under the control of a promoter containing the GATA sequence is strongly activated by END-1, compared with consistent but lower levels of activation by Xenopus GATA-4 and GATA-5 in transfected COS cells (Fig. 1B). Transactivation is significantly reduced if the reporter construct contains the mutant site ACTGAA instead of AGATAA. A truncation mutant, END-1Δ3′, which lacks the C-terminal 18 aa and likely disrupts the structure of the zinc finger, does not bind DNA (data not shown), as predicted from structural studies of GATA proteins (40), and exhibits no transactivation (Fig. 1B). These properties of END-1, resembling vertebrate GATA factors, highlight its utility as a probe for conserved functions of GATA proteins.
Figure 1.
END-1 is a sequence-specific DNA-binding protein and transcriptional activator. (A) Electrophoretic mobility shift assay with a radiolabeled GATA probe and nuclear extracts from COS cells transfected with XGATA-4 or END-1 cDNA. Cold competitor oligonucleotides (100× excess) either had the same sequence as the probe (GATA) or replacement of this sequence by GATC or CTTA. In the last two lanes, reactions included preimmune or End-1-specific rabbit antiserum. (B) Fold activation of the GATA-driven luciferase reporter construct αD3 (black bars) or the mutant reporter construct αD4 (in which the GATA site in the promoter is replaced by CTGA; gray bars) after transfection of plasmids encoding END-1, END-1Δ3′, XGATA-4, or XGATA-5.
end-1 Activates Endoderm Differentiation in Vertebrate Embryos and May Mimic a Similar Endogenous Activity.
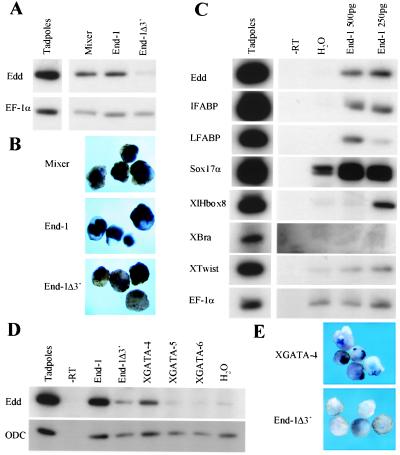
To test whether GATA factors might specify endoderm in vertebrates, we expressed end-1 mRNA in Xenopus embryos and assayed for ectopic endoderm differentiation in animal cap explants. Both RT-PCR and in situ hybridization analysis (Fig. 2 A and B) indicate that END-1 induces expression of endodermin mRNA, a specific marker of endoderm (39). This activity is comparable to that of Xenopus Mixer/Mix.3 (28) and is not seen with the inert truncation mutant END-1Δ3′. All endoderm-specific markers tested, including the intestine- and liver-specific fatty acid binding proteins IFABP (41) and LFABP, Sox17α, and transcription factor XlHbox8 (42), are similarly activated in animal cap explants expressing END-1 (Fig. 2C). These effects are consistently seen with mRNA injections over 200 pg; however, dose dependence of induced expression of XlHbox8 in ectodermal explants is aberrant, as was noted with Mixer (28). Thus, END-1 is a potent activator of vertebrate endoderm differentiation and may mimic a similar endogenous activity. In contrast, there is no induction of brachyury and only weak induction of Xtwist, markers of the mesoderm; we attribute this to effects that may mimic, for example, XGATA-6, a known regulator of cardiac muscle differentiation (43).
Figure 2.
Induction of endoderm in Xenopus ectodermal explants by GATA proteins. Embryos were injected with 250–500 pg of mRNA into the animal pole, and animal cap explants were cultured until sister tadpoles reached Stage 40. RNA was analyzed by RT-PCR (A, C, and D) or whole-mount in situ hybridization (B and E) for endodermin (Edd), IFABP, LFABP, Sox17α, XlHbox8, brachyury (Xbra), and Xtwist. Equal input of RNA samples was established by RT-PCR for elongation factor (EF)-1α or ODC. A low background of endodermin and Xsox17α is occasionally observed in controls.
The vertebrate GATA-4/5/6 proteins are expressed in the developing and adult gut and have been implicated principally in late endoderm development (13, 16, 17, 19). Although a low level of GATA-5 mRNA is expressed maternally, this subfamily is largely expressed later in Xenopus development than might be expected for proteins that specify endoderm (44). Nevertheless, it includes potential candidates for the endogenous activity mimicked by END-1. We observe that GATA-4, in particular, does induce endodermin expression in ectodermal explants (Fig. 2 D and E), albeit more weakly than END-1. END-1 may thus mimic the function either of some combination of the known vertebrate GATA factors or of a novel family member.
A Dominant Inhibitory END-1 Fusion Protein Represses Differentiation of the Endogenous Endoderm in Xenopus Embryos.
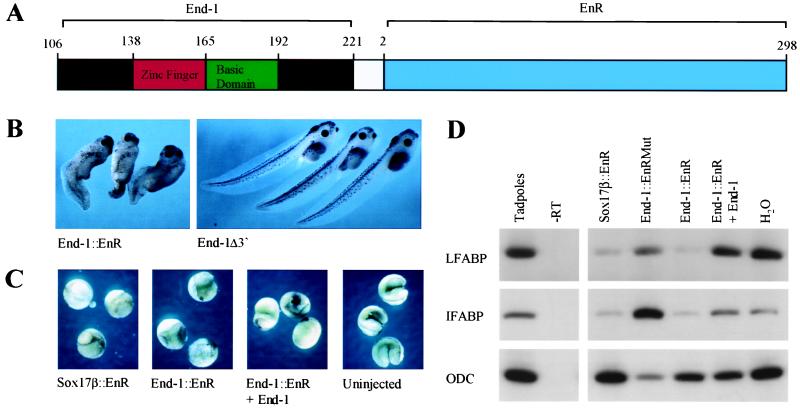
Loss-of-function studies provide a powerful, independent means to test the role of endogenous GATA activity in endoderm development. We therefore constructed a dominant inhibitory GATA-binding protein End-1∷EnR (Fig. 3A) by fusing the C-terminal 116 aa of END-1 to the NH2-terminal repressor domain of the Drosophila Engrailed protein (34). This general strategy has yielded useful insights into requirements for sequence-specific transcriptional regulators in Xenopus development (24, 27, 28). Expression of End-1∷EnR in the vegetal pole of early Xenopus embryos results in dose-dependent effects on endoderm development that are very similar to those seen on expression of dominant inhibitory Mixer and Sox17 fusion constructs (27, 28). At mRNA doses exceeding 200 pg, embryos fail to gastrulate beyond formation of the blastopore lip (Fig. 3C), whereas a dose of 100 pg results in reproducible phenotypes that reflect defective endoderm differentiation. These defects include relative expansion of the posterior region, reduced endoderm mass, replacement of portions of the endoderm by cysts, and absence of gut coiling in advanced embryos (Fig. 3B). Although the dorsal axis is formed normally, the antero-posterior axis is considerably shortened. Normal development is completely rescued, even in embryos receiving high doses of End-1∷EnR, by coinjection of end-1, but not end-1Δ3′ mRNA (Fig. 3C); this confirms the specificity of the repressive effects of End-1∷EnR.
Figure 3.
A dominant inhibitory GATA-binding protein represses endoderm development in Xenopus embryos. (A) Design of the dominant-inhibitory construct End-1∷EnR fusing the putative DNA-binding domain of END-1 (aa 106–221) to the transcriptional repression domain (aa 2–298) of Drosophila Engrailed. (B) Phenotype of Xenopus embryos at stage 42/43 after microinjection of 100 pg of End-1∷EnR or control (End-1Δ3′) mRNA into the vegetal hemisphere at the one-cell stage. (C) Rescue of lethal developmental effects of vegetal expression of End-1∷EnR by coinjection of wild-type End-1. Two-cell embryos were injected in the vegetal hemisphere of each blastomere with 400 pg of Sox17β∷EnR or End-1∷EnR mRNA, and 400 pg of either End-1 (panel 3) or End-1Δ3′ (panels 1 and 2) mRNA. (D) Down-regulation of mature markers of Xenopus endoderm in vegetal explants treated with End-1∷EnR. Two-cell embryos were injected as above with 250 pg of Sox17β∷EnR or End-1∷EnR mRNA and 250 pg of End-1 (lane 6) or End-1Δ3′ (lanes 3–5) mRNA. Vegetal explants were cultured until the equivalent of Stage 35. RT-PCR for ODC serves as a control for equal RNA input.
Dominant inhibitory forms of Mixer and Sox17 significantly repress endogenous expression of selected endoderm markers in isolated vegetal explants (27, 28). Similarly, End-1∷EnR results in reduced levels of mRNAs encoding the late endodermal markers LFABP and IFABP (Fig. 3D). A control fusion, End-1∷EnRMut, in which two Cys residues in the zinc-finger motif are replaced, fails to produce this effect, and endogenous levels of both markers are restored by coinjection of end-1 mRNA, again confirming specificity of the repression. We conclude that an endogenous GATA-type activity that is repressed specifically by End-1∷EnR is essential for proper endoderm development in Xenopus embryos.
GATA Activity Functions Early in the Pathway of Endoderm Differentiation.
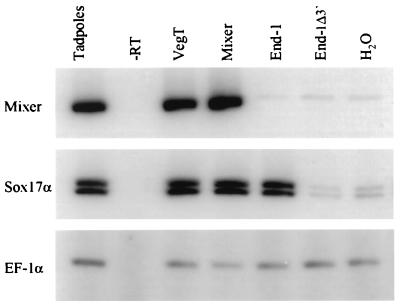
VegT is the best characterized maternally expressed transcription factor known to regulate Xenopus endoderm development (25), whereas Mixer and Sox17α and β are the earliest known zygotic factors (27, 28). Accordingly, ectopic expression of VegT in Xenopus ectodermal explants induces expression of both Mixer and Sox17α mRNAs (Fig. 4). In contrast, END-1 induces rapid (Nieuwkoop stage 11.5) expression of Sox17α but not of Mixer. Thus, both VegT and END-1 activate early expression of Sox17α, implicating GATA function early in endoderm development, and likely upstream of the Sox17 genes. The results further suggest that induction of Sox17α by END-1 is either independent of Mixer, or that the proposed linear pathway from Mixer to Sox17 (28) in part involves a GATA-factor intermediary.
Figure 4.
GATA activity functions early in vertebrate endoderm development. One-cell Xenopus embryos were injected with 250 pg of mRNA, animal cap explants were cultured until sister tadpoles reached Stage 11.5, and RNA was analyzed by RT-PCR for the early endoderm markers Mixer and XSox17α and for EF-1α.
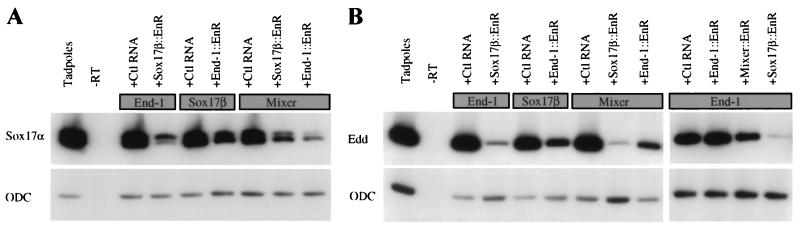
To analyze these relationships further, we compared the effects of various dominant inhibitory fusion proteins on early (Stage 15) and late (Stage 40) endoderm differentiation in Xenopus animal caps. Because these experiments involve estimation of mRNA expression by RT-PCR, they are not strictly quantitative, and there are degrees of repression by different constructs; we therefore adopt the most conservative interpretation of the following representative results. Like Sox17β∷EnR (27), End-1∷EnR significantly represses endoderm induction by Mixer, whereas Mixer∷EnR reduces slightly but does not block induction by END-1 (Fig. 5 A and B), just as is seen when Sox17β is the inducer (28). Together with the failure of END-1 to activate Mixer expression (Fig. 4), these results place the putative GATA activity in endoderm differentiation either downstream or in parallel to Mixer, rather than upstream of it. The EnR domain itself does not appear to impart nonspecific repressive toxicity because coinjection of END-1 and End-1∷EnR, for example, does not reduce endodermin (Fig. 5B) and other (data not shown) mRNAs. Moreover, like Mixer∷EnR (28), End-1∷EnR causes consistently lower inhibition of endoderm induction by Sox17β than Sox17β∷EnR causes repression of the inductive activity of either END-1 (Fig. 5 A and B) or Mixer (28) (and data not shown). This observation also suggests that GATA activity functions upstream of the Sox17 genes, and that the proposed transcriptional pathways in vertebrate early endoderm development might converge on the Sox17 genes.
Figure 5.
Placing GATA activity within the context of Mixer/Mix.3 and Sox17 functions in Xenopus endoderm development. One-cell embryos were injected with 200 pg of endoderm-inducing mRNAs, and 400 pg of either mRNA-encoding dominant-inhibitory EnR fusion proteins or EF-1α “filler” mRNA. Animal cap explants were cultured and RNA analyzed by RT-PCR for Sox17α when sister tadpoles reached Nieuwkoop-Faber Stage 15 (A), for endodermin (Edd) at the equivalent of Stage 40 (B), and for ODC as a loading control.
Discussion
Genetic studies in C. elegans establish END-1 as a pivotal regulator of endoderm specification (3, 4). END-1 shares sequence homology with the GATA family of proteins, many of which are established lineage-restricted transcriptional regulators of cell differentiation (6–9, 16–19, 45–48). These features of END-1 prompted us to explore the possible importance of a similar activity in vertebrate endoderm development. Despite the atypical nature of its solitary GATA-type zinc finger, END-1 is a transcriptional activator with the predicted specificity in DNA binding. END-1 exhibits a striking capacity to promote endoderm differentiation in vertebrate embryos, comparable to that of the known endodermal regulators Mixer/Mix.3 and Sox17α and β. Moreover, a dominant inhibitory End-1∷EnR construct perturbs endoderm development in a fashion similar to dominant inhibitory Mixer and Sox17 constructs (27, 28). These results establish the relevance of GATA factor-mediated endoderm differentiation in vertebrate embryos.
The Xenopus maternal T-box protein VegT induces ectopic endoderm differentiation in animal cap explants (24), and depletion of VegT mRNA virtually eliminates endoderm development (25). Consistent with predictions from these studies, we show that VegT induces expression of both Mixer and Sox17α (Fig. 4). Also, ectopic expression of Mixer increases Sox17 mRNA levels, whereas Sox17 genes do not induce Mixer expression (28). Considered together, these data support an apparently linear model wherein VegT induces expression of Mixer, which in turn activates Sox17 genes. Our studies point to a role for GATA transcriptional activity within the framework of this pathway. END-1 does not activate Mixer expression, whereas its ability to induce Sox17α is comparable to that of both VegT and Mixer (Figs. 4 and 5A). Mixer-induced endoderm differentiation is blocked completely by Sox17β∷EnR, whereas repression of Sox17- (28) or END-1- (Fig. 5) induced endoderm differentiation by Mixer∷EnR is much weaker. Furthermore, Sox17β∷EnR blocks induction by END-1 or Mixer equally effectively (Fig. 5). This correlates with the consistent induction of Sox17α by END-1 (Figs. 2C, 4, and 5A), and confirms that GATA activity functions upstream of Sox17.
These results are best interpreted in the light of two possibilities. Either Mixer is the predominant early zygotic regulator of vertebrate endoderm specification and functions upstream of both GATA and Sox17, or Mixer and some GATA factor(s) function in parallel pathways that converge on Sox17α/β. Alternatively, vertebrate endoderm development may be regulated through complex networks rather than linear cascades of transcription factors. Our findings are perhaps most consistent with the possibility of concurrent pathways, one of which might include VegT, Mixer, and Sox17 genes, whereas a second, possibly parallel, pathway involves the Sox17 genes and a GATA factor. Such a regulatory network is reminiscent of the redundant transcriptional pathway used to specify the endoderm in C. elegans (3).
Transcription factors of the hepatocyte nuclear factor (HNF)- 3/forkhead family are important regulators of foregut differentiation in mice, flies, and worms (49–51). The vertebrate GATA-4/5/6 subfamily is implicated in aspects of fetal gut development (11, 16–19, 44), although germ-line absence of either GATA-4 or GATA-6 does not result in failure of endoderm specification in mice. This implies that the known GATA factors are either not required for this function or are functionally redundant, and raises the question of which GATA proteins fulfill this role in vivo. Interestingly, whereas all known vertebrate GATA factors contain two canonical zinc fingers, only two of the three known Drosophila factors, dGATAa/Pannier and dGATAc, are similar in this regard; the third, ABF/Serpent (dGATAb), carries a single GATA-type zinc-finger motif (8, 9, 52, 53). Likewise, 10 of the 11 C. elegans GATA proteins have a single GATA-type zinc finger; only ELT-1 resembles the structure of vertebrate GATA factors (54). Whereas the sequence of END-1 does not particularly resemble that of ABF/Serpent, we are intrigued by the functional homology between these GATA proteins, which have a single zinc finger and are required for endoderm specification. Indeed, the potent induction of vertebrate endoderm differentiation by END-1 correlates well with the established requirement for these GATA proteins in the development of flies and worms. Our observations further suggest that molecular mechanisms underlying early endoderm development are conserved across the wide phylogenetic gulf of chordates and ecdysozoan protostomes, spanning nearly the entire radiation of triploblastic animals. Some of our present efforts are directed toward isolating members of a potential class of vertebrate GATA proteins with a single zinc finger and with functions in early development.
Acknowledgments
We thank Jeremy Green and Isabel Dominguez for helpful discussions, and Todd Evans, Paul Mead, Len Zon, Clare Hudson, Hugh Woodland, and Eddy DeRobertis for cDNA constructs. This work was supported in part by the Dana–Farber Cancer Institute-Novartis Program in Drug Discovery and by Fellowships (R.A.S.) from the Dolphin Trust, Harcourt General Charitable Foundation, and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation.
Abbreviations
- MMR
Modified Marc's Ringer's solution
- RT-PCR
reverse transcription–PCR
- ODC
ornithine decarboxylase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Aguinaldo A M, Turbeville J M, Linford L S, Rivera M C, Garey J R, Raff R A, Lake J A. Nature (London) 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- 2.Orkin S H. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 3.Zhu J, Hill R J, Heid P J, Fukuyama M, Sugimoto A, Priess J R, Rothman J H. Genes Dev. 1997;11:2883–2896. doi: 10.1101/gad.11.21.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Fukushige T, McGhee J D, Rothman J H. Genes Dev. 1998;12:3809–3814. doi: 10.1101/gad.12.24.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins M G, McGhee J D. J Biol Chem. 1995;270:14666–14671. doi: 10.1074/jbc.270.24.14666. [DOI] [PubMed] [Google Scholar]
- 6.Fukushige T, Hawkins M G, McGhee J D. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- 7.Reuter R. Development (Cambridge, UK) 1994;120:1123–1135. doi: 10.1242/dev.120.5.1123. [DOI] [PubMed] [Google Scholar]
- 8.Rehorn K P, Thelen H, Michelson A M, Reuter R. Development (Cambridge, UK) 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- 9.Lin W H, Huang L H, Yeh J Y, Hoheisel J, Lehrach H, Sun Y H, Tsai S F. J Biol Chem. 1995;270:25150–25158. doi: 10.1074/jbc.270.42.25150. [DOI] [PubMed] [Google Scholar]
- 10.Arceci R J, King A A, Simon M C, Orkin S H, Wilson D B. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laverriere A C, MacNeill C, Mueller C, Poelmann R E, Burch J B, Evans T. J Biol Chem. 1994;269:23177–23184. [PubMed] [Google Scholar]
- 12.Tamura S, Wang X H, Maeda M, Futai M. Proc Natl Acad Sci USA. 1993;90:10876–10880. doi: 10.1073/pnas.90.22.10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Sedgwick T, Shi Y B, Evans T. Mol Cell Biol. 1998;18:2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossard P, Zaret K S. Development (Cambridge, UK) 1998;125:4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- 15.Soudais C, Bielinska M, Heikinheimo M, MacArthur C A, Narita N, Saffitz J E, Simon M C, Leiden J M, Wilson D B. Development (Cambridge, UK) 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- 16.Kuo C T, Morrisey E E, Anandappa R, Sigrist K, Lu M M, Parmacek M S, Soudais C, Leiden J M. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 17.Molkentin J D, Lin Q, Duncan S A, Olson E N. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 18.Morrisey E E, Tang Z, Sigrist K, Lu M M, Jiang F, Ip H S, Parmacek M S. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. Development (Cambridge, UK) 1999;126:723–732. [PubMed] [Google Scholar]
- 20.Reiter J F, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier D Y. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, King M L. Development (Cambridge, UK) 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
- 22.Lustig K D, Kroll K L, Sun E E, Kirschner M W. Development (Cambridge, UK) 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- 23.Stennard F, Carnac G, Gurdon J B. Development (Cambridge, UK) 1996;122:4179–4188. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- 24.Horb M E, Thomsen G H. Development (Cambridge, UK) 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Houston D W, King M L, Payne C, Wylie C, Heasman J. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- 26.Casey E S, Tada M, Fairclough L, Wylie C C, Heasman J, Smith J C. Development (Cambridge, UK) 1999;126:4193–4200. doi: 10.1242/dev.126.19.4193. [DOI] [PubMed] [Google Scholar]
- 27.Hudson C, Clements D, Friday R V, Stott D, Woodland H R. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 28.Henry G L, Melton D A. Science. 1998;281:91–96. doi: 10.1126/science.281.5373.91. [DOI] [PubMed] [Google Scholar]
- 29.Mead P E, Zhou Y, Lustig K D, Huber T L, Kirschner M W, Zon L I. Proc Natl Acad Sci USA. 1998;95:11251–11256. doi: 10.1073/pnas.95.19.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemaire P, Darras S, Caillol D, Kodjabachian L. Development (Cambridge, UK) 1998;125:2371–2380. doi: 10.1242/dev.125.13.2371. [DOI] [PubMed] [Google Scholar]
- 31.Ecochard V, Cayrol C, Rey S, Foulquier F, Caillol D, Lemaire P, Duprat A M. Development (Cambridge, UK) 1998;125:2577–2585. doi: 10.1242/dev.125.14.2577. [DOI] [PubMed] [Google Scholar]
- 32.Alexander J, Stainier D Y. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- 33.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 34.Han K Y, Manley J L. Genes Dev. 1993;7:491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- 35.Andrews N C, Faller D V. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans T, Felsenfeld G. Mol Cell Biol. 1991;11:843–853. doi: 10.1128/mcb.11.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay B K, Peng H B. Xenopus laevis: Practical Uses in Cell and Molecular Biology. San Diego: Academic; 1991. [PubMed] [Google Scholar]
- 38.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis. Amsterdam: North–Holland; 1967. [Google Scholar]
- 39.Sasai Y, Lu B, Piccolo S, De Robertis E M. EMBO J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- 40.Omichinski J G, Clore G M, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl S J, Gronenborn A M. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y B, Hayes W P. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- 42.Gamer L W, Wright C V E. Dev Biol. 1995;171:240–251. doi: 10.1006/dbio.1995.1275. [DOI] [PubMed] [Google Scholar]
- 43.Gove C, Walmsley M, Nijjar S, Bertwistle D, Guille M, Partington G, Bomford A, Patient R. EMBO J. 1997;16:355–368. doi: 10.1093/emboj/16.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Evans T. Dev Biol. 1996;174:258–270. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- 45.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D'Agati V, Orkin S H, Costantini F. Nature (London) 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 46.Tsai F Y, Keller G, Kuo F C, Weiss M, Chen J, Rosenblatt M, Alt F W, Orkin S H. Nature (London) 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 47.Ting C N, Olson M C, Barton K P, Leiden J M. Nature (London) 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 48.Page B D, Zhang W, Steward K, Blumenthal T, Priess J R. Genes Dev. 1997;11:1651–1661. doi: 10.1101/gad.11.13.1651. [DOI] [PubMed] [Google Scholar]
- 49.Ang S L, Wierda A, Wong D, Stevens K A, Cascio S, Rossant J, Zaret K S. Development (Cambridge, UK) 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 50.Horner M A, Quintin S, Domeier M E, Kimble J, Labouesse M, Mango S E. Genes Dev. 1998;12:1947–1952. doi: 10.1101/gad.12.13.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalb J M, Lau K K, Goszczynski B, Fukushige T, Moons D, Okkema P G, McGhee J D. Development (Cambridge, UK) 1998;125:2171–2180. doi: 10.1242/dev.125.12.2171. [DOI] [PubMed] [Google Scholar]
- 52.Ramain P, Heitzler P, Haenlin M, Simpson P. Development (Cambridge, UK) 1993;119:1277–1291. doi: 10.1242/dev.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 53.Winick J, Abel T, Leonard M W, Michelson A M, Chardon-Loriaux I, Holmgren R A, Maniatis T, Engel J D. Development (Cambridge, UK) 1993;119:1055–1065. doi: 10.1242/dev.119.4.1055. [DOI] [PubMed] [Google Scholar]
- 54.Spieth J, Shim Y H, Lea K, Conrad R, Blumenthal T. Mol Cell Biol. 1991;11:4651–4659. doi: 10.1128/mcb.11.9.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]