Abstract
Studies in rodents suggest an important role for the D3 dopamine receptor in regulating locomotor responses to spatial novelty and psychostimulants. The D3 receptor alternatively spliced variant D3nf produces a non-dopamine binding protein that may alter D3 receptor localization by dimerizing with the full-length receptor. In the high responder/low responder (HR/LR) model, the locomotor response to an inescapable, novel spatial environment predicts individual differences in the locomotor and rewarding effects of psychostimulants. We hypothesized that individual differences in D3 receptor expression could contribute to individual differences in the locomotor response to novelty in the HR/LR model. To test this hypothesis, we screened rats for response to a novel spatial environment and analyzed brain tissue for mRNA levels of the D3 receptor and D3nf by real-time RT-PCR. The ratios of D3/D3nf mRNA in prefrontal cortex and substantia nigra/ventral tegmentum were significantly lower in HRs than in LRs. There were no differences in relative expression of D3/D3nf between HRs and LRs in nucleus accumbens. These data further support a role for the D3 dopamine receptor in behavioral responses to novelty and, given the established relationship between novelty and psychostimulant responses, suggest that the D3 receptor may be an important target for assessment of drug abuse vulnerability. Additionally, these findings are consistent with the hypothesis that alternative splicing may contribute to regulation of D3 dopamine receptor function.
Keywords: Dopamine receptor, Drug dependence, RT-PCR, mRNA, Locomotor activity, Alternative splicing
1. Introduction
In the high responder/low responder (HR/LR) model, originally described by Piazza et al., individual differences in the locomotor response to an inescapable, novel spatial environment predict individual differences in the locomotor and rewarding effects of psychostimulants [27]. Thus, rats that exhibit relatively high locomotor activity in a novel environment also exhibit higher locomotor activity in response to amphetamine injection and acquire amphetamine self-administration more readily as compared to those with low locomotor responses to the novel environment. This finding has been advanced as a potential model of traits that may predispose certain individuals to drug taking or drug dependence. Support for this relationship is reported in human studies describing correlations between sensitivity to the physiological and subjective effects of amphetamine and novelty-seeking personality traits [14,35]. The HR/LR model thus provides a useful tool for studying potential neural substrates underlying individual differences in vulnerability to substance abuse.
The locomotor response to environmental stimuli is regulated in part by the mesolimbic dopamine system, which is activated by appetitive stimuli, stress, spatial novelty, and drugs of abuse. Behavioral studies suggest an important role for the D3 dopamine receptor in regulating locomotor responses to spatial novelty and psychostimulants [1,29,40]. Mice lacking the D3 dopamine receptor are transiently hyperactive in a novel spatial environment and are more sensitive to the locomotor effects of low dose cocaine and to the rewarding effects of amphetamine [1,40], though conflicting data on the behavioral phenotype of D3 knockout mice have also been reported [3]. Low doses of selective D3 receptor agonists inhibit novelty-stimulated locomotion in wild-type, but not D3 receptor mutant mice [29]. This effect is specific to novelty-stimulated locomotion, as no inhibition occurs in animals acclimated to the test chamber prior to injection. Additionally, there is a direct inverse correlation between individual differences in locomotor activation by amphetamine and locomotor inhibition by D3 receptor agonists [33]. In combination, these findings suggest that individual differences in D3 receptor inhibitory influence could significantly contribute to individual differences in the locomotor response to both novelty and psychostimulants.
The gene coding for the D3 receptor is organized to allow for the production of different transcripts via alternative splicing, and at least seven distinct alternative splicing variants of the D3 receptor have been identified [9,10,21,25]. The D3nf isoform, identified by Schmauss and colleagues [21,36], is among the best characterized of the D3 receptor splice variants. D3nf mRNA, first identified in cortical tissue from schizophrenia patients, is created by splicing at a non-canonical 3′-acceptor site. D3nf protein does not exhibit high-affinity dopamine binding, but alters D3 receptor localization by dimerizing with the full-length receptor [7,19,26]. Dimerization leads to intracellular sequestration and decreased dopamine binding to the full-length D3 receptor in vitro [7,19]. D3 and D3nf dimers and tetramers occur in the rat and primate brains in vivo [26]. Therefore, changes in the relative abundance of D3nf could modulate D3 receptor signaling in vivo [32].
To test the hypothesis that individual differences in D3 receptor alternative splicing and expression contribute to individual differences in the locomotor response to novelty, we examined mRNA levels for D3 and D3nf in the brains of HR and LR rats by real-time RT-PCR. We predicted that reduced D3 receptor-mediated inhibitory influence on locomotion, consistent with the HR phenotype, would be associated with decreased levels of D3 receptor mRNA and/or increased levels of D3nf mRNA in key regions of the mesolimbic dopamine system (prefrontal cortex, nucleus accumbens and substantia nigra/ventral tegmentum).
2. Methods
2.1. Subjects
Thirty-two male, Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 300–350 g at the time of the experiment, were housed three per polypropylene box, in a temperature and humidity controlled room on a 12 h light–dark cycle (on 06:00, off 18:00). Standard lab chow and water were available ad libitum. Animals remained undisturbed in the housing facility, except for cage changes, for 2 weeks prior to the start of the experiment. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Behavioral testing
Behavioral testing was performed in 30 custom-designed residential activity chambers (RACs), as previously described [31]. Each chamber consisted of a lighted, ventilated, sound-attenuated cabinet (Cline Builders, Covington, KY) housing a 40 cm × 40 cm × 38 cm Plexiglas enclosure. Lights inside the chambers were coordinated with the vivarium light cycle (on 06:00–18:00). Locomotion was monitored with a 16 × 16 photo beam array (San Diego Instruments, San Diego, CA) located 2.5 cm above the floor of the enclosure. Locomotion was expressed as crossovers, defined as entry into any of the active zones of the chamber, as previously described [29].
On day 1 of the experiment (08:00), all rats were transferred, in home cages, from the housing room to the testing room, where they were left undisturbed for 30 min prior to behavioral testing. This length of recovery time was chosen based on previous experiments, in which rats were resting quietly in the home cage within 30 min of transfer (unpublished observation). Each rat was then removed from the home cage and placed in a RAC, and locomotor behavior was recorded for 1 h to determine locomotor response to a novel spatial environment. Based on total crossovers during this hour, animals were separated into low (lowest 25%; LR), median (25% surrounding the median score; MR), or high (highest 25%; HR) responders. Animals whose locomotor activity scores fell outside these designations were excluded from further statistical analysis. Thus, a total of 24 animals (n = 8/group) were used for statistical analyses. After the spatial novelty test, rats were placed back in their home cages in the testing room.
One hour before the onset of dark phase on day 1, those animals designated as LR, MR or HR were placed back in the RACs, and their locomotor behavior was recorded for the duration of dark time (until 06:00 the following morning). The locomotor data from the first hour of this session were used as a measure of spontaneous, non-novelty-induced locomotor activity because subjects had previously been exposed to the RACs during the novelty test. All rats had free access to lab chow and water overnight and were left undisturbed in the RACs until the time of sacrifice on day 2 (see below).
Beginning at 08:00 on day 2, rats were removed from the RACs and sacrificed by rapid decapitation. Brains were removed, placed in ice-cold 0.9% saline for 5 min, then dissected using the rodent coronal brain matrix, as previously described [11]. The brain matrix was used to produce coronal sections, followed by freehand dissection of olfactory bulbs, prefrontal cortex (PFC), nucleus accumbens (N.Acc.), caudate/putamen, hippocampus, substantia nigra/ventral tegmentum (SN/VTA) and cerebellum. Dissected tissue pieces were snap frozen in liquid nitrogen and stored at −80 °C.
2.3. Tissue preparation and RT-PCR
Frozen tissue from individual animals was homogenized (Caframo Model RZR1 homogenizer) in 1.0 ml of Tri Reagent (Molecular Research Center, Cincinnati, OH) per 50–100 mg of tissue. Total RNA was then isolated and precipitated according to the manufacturer's instructions. Pellets were resuspended in nuclease-free water to give a concentration of approximately 1.0 μg/μl and stored at −20 °C.
D3 and D3nf mRNA levels were determined for each tissue sample using the Taq Man® One-Step RT-PCR method, which utilizes release of a fluorescent reporter from a fluorogenic probe as described by others [24]. Our PCR design follows the overall strategy developed by Schmauss et al. for selective amplification of D3 and D3nf by PCR [36]. We employed different sets of primers, however, based on results of preliminary studies. Primers and fluorogenic probes were designed using Primer Express v.2.0 software (Applied Biosystems, Foster City, CA) based on the rat mRNA sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GenBank accession number AF106860), D3 dopamine receptor (accession number NM 017140), and D3nf, which was determined from the full-length rat D3 receptor sequence using sequence homology to the splice junction previously described for human D3nf [36]. The reverse primer for D3 was located within the 98 nucleotide sequence deleted in D3nf, such that only the full-length D3 cDNA was amplified by the D3 primers. The forward primer for D3nf spanned the putative splice junction and therefore amplified only the D3nf cDNA. Sequences for D3 and D3nf primers and probes and their positions relative to the splice site are shown in Fig. 1. Forward and reverse primer and probe sequences are listed in Table 1. Each probe was conjugated to a TET reporter at the 5′-end and a TAMRA quencher at the 3′-end.
Fig. 1.

Partial mRNA sequence for the rat D3 DA receptor (GenBank accession number NM 017140). The splice junction for production of D3nf from rat D3 receptor mRNA is indicated by arrowheads. Primers for the full-length D3 receptor are shown in uppercase italics and probe for the full-length D3 receptor is shown in lower case italics. Primers for D3nf are indicated by underlined upper case, and probe for D3nf is indicated by underlined lower case.
Table 1.
Primer and probe sequences for quantitative RT-PCR
| Target | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Rat GAPDH | 5′-CTCAACTACATGGTCTACATGTTCCA-3′ | 5′-CTTCCCATTCTCAGCCTTGACT-3′ | 5′-CCCACGGCAAGTTCAACGGCA-3′ |
| Rat D3 | 5′-CGTGGAAAGGACTCGGAACTC-3′ | 5′-GTGGATAACCTGCCGTTGCT-3′ | 5′-CCCAAGCTCAGCTTAGAGGTTCGAA-3′ |
| Rat D3nf | 5′-GGAACTCCTTGAGTACCACTTCGA-3′ | 5′-CAATGAAGGCTCCAAGCACAA-3′ | 5′-AGAAGAAGGCCACCCAGATGGTGGTC-3′ |
Total RNA concentration from each sample was determined by A260 measurement. Samples were diluted in nuclease-free water to a final concentration of 200 ng/μl. A second dilution of 20 ng/μl was prepared for quantitation of the internal standard, GAPDH. Relative quantities of D3 and D3nf mRNA were calculated using the standard curve interpolation method described by Medhurst et al. [24], normalizing expression of the target mRNA of interest to levels of the housekeeping gene GAPDH. This method corrects for inter-sample differences in input RNA amount, as well as variability in cDNA quantity and quality. A seven point standard curve for each brain region was constructed using twofold serial dilutions of known concentrations of total RNA. Standard curves were prepared at 10-fold lower concentration from the same RNA samples for GAPDH. All samples were assayed in duplicate for each primer set. Animals for whom calculated mean mRNA levels were not within the limits of the standard curve were re-assayed, in duplicate, for D3, D3nf and GAPDH. In some cases, the amount of RNA available was insufficient for re-assay. In such cases, the animals were excluded from analysis. Numbers of animals included in analyses are noted in the figure legends.
Reactions were carried out in optical 96-well plates in a Perkin-Elmer GeneAmp 5700 Sequence Detection System (Applied Biosystems). Each reaction consisted of the following: 5 μl total RNA, 900 nM each primer, 250 mM probe, 1× Taq Man® One-Step RT-PCR Master Mix, 1× Multiscribe™ and RNAse inhibitor mix (Applied Biosystems) and nuclease-free water to a final volume of 50 μl. For reverse transcription, samples were heated to 48 °C for 30 min. For PCR, cycling parameters were as follows: 95 °C for 10 min (hot start), 95 °C for 30 s (melting), 60 °C for 1 min (annealing and extension). The last two steps were repeated for 45 cycles.
Because previous studies have suggested that human D3nf may regulate the function of the full-length D3 receptor protein in a dominant-negative fashion, we compared the relative proportions of the two mRNAs in HR, MR and LR groups. Thus, data are expressed as a ratio: relative quantity of D3 mRNA/relative quantity of D3nf mRNA. Behavioral and RT-PCR data were analyzed by single-factor ANOVA, followed by post hoc group comparisons using Fisher's LSD tests. The relationship between novelty-stimulated locomotor activity and D3/D3nf mRNA levels in individual animals was examined using linear regression analysis. All statistical analyses were performed using SPSS 13.0 software.
3. Results
3.1. Novelty-induced locomotor activity
Locomotor response to a novel spatial environment is shown in Fig. 2a. The 25% designation produced a significant main effect of group (F2,23 = 34.147, p < 0.001) and a significant difference in total crossovers between HRs and LRs (p < 0.001) and between MRs and LRs (p < 0.05). Locomotor activity was also analyzed during the first hour of the rats' second RAC exposure. As shown in Fig. 2b, no group differences were observed during the second exposure (F2,23 = 0.1747, p = 0.8409).
Fig. 2.
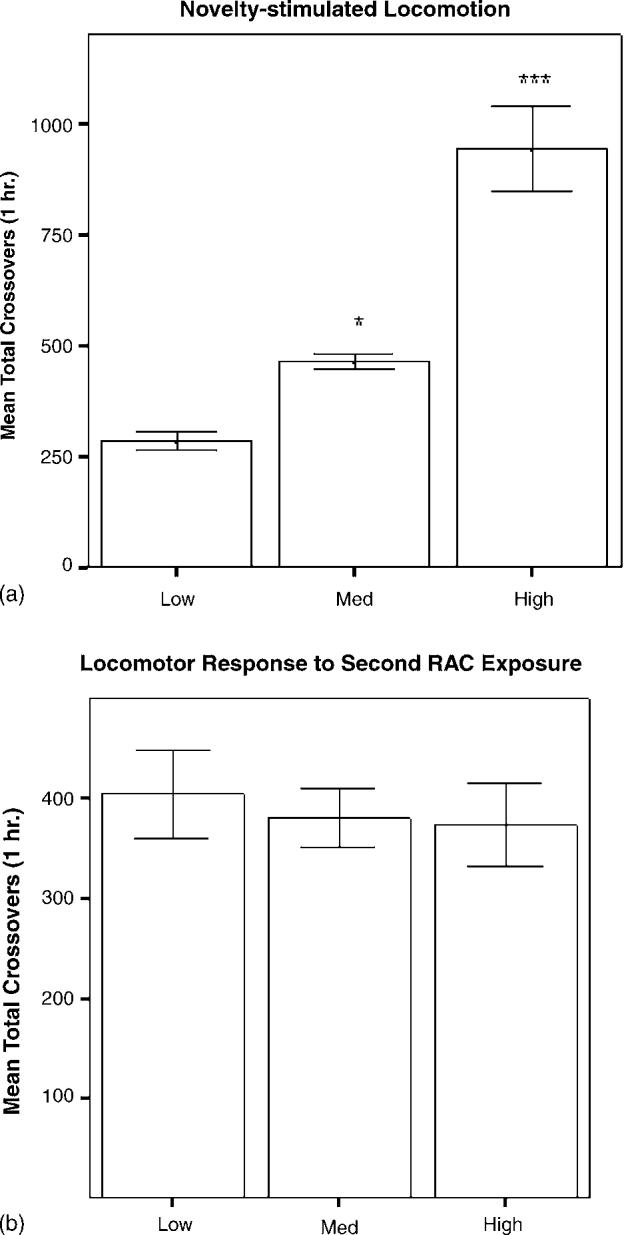
(a) Locomotor activity during the first hour of exposure to the RACs is expressed as mean ± S.E.M. of total crossovers for each group (n = 8/group): low (lowest 25%), median (25% surrounding the median crossover score), and high (highest 25%). LRs exhibited significantly less locomotor activity during the first hour in a novel environment than did MRs or HRs (*p < 0.05, ***p < 0.001, respectively). (b) Locomotor activity during the first hour of the second exposure to the RACs is expressed as mean ± S.E.M. of total crossovers for each group (n = 8/group). There were no differences in locomotor activity between groups (p = 0.8409).
3.2. D3 and D3nf mRNA levels
Expression levels for individual transcripts in each brain region examined are shown in Table 2. In PFC, there was a main effect of group for D3 mRNA expression (F2,19 = 4.445, p = 0.028). Post hoc analyses revealed that levels of D3 mRNA in PFC were significantly lower in HR rats than in LR rats (p < 0.05). There was no main effect of group for D3nf mRNA expression in PFC (F2,19 = 2.125, p = 0.144), nor for D3 (F2,23 = 0.1376, p = 0.8722) or D3nf (F2,23 = 1.512, p = 0.2434) mRNA levels in N.Acc. Similarly, there were no main effects of group for D3 (F2,22 = 0.9178, p = 0.4352) or D3nf (F2,18 = 2.293, p = 0.1331) mRNA levels in SN/VTA.
Table 2.
D3 and D3nf mRNA expression normalized to GAPDH
| PFC |
N.Acc. |
SN/VTA |
||||
|---|---|---|---|---|---|---|
| D3/GAPDH | D3nf/GAPDH | D3/GAPDH | D3nf/GAPDH | D3/GAPDH | D3nf/GAPDH | |
| LR | 8.33 (0.67) | 11.33 (1.31) | 6.81 (0.50) | 10.67 (1.61) | 17.06 (2.21) | 23.01 (2.42) |
| MR | 9.25 (1.51) | 16.67 (3.07) | 6.44 (0.68) | 11.91 (1.29) | 15.76 (1.40) | 71.34 (39.67) |
| HR | 6.01 (0.62)* | 12.60 (1.17) | 6.66 (0.20) | 8.96 (0.33) | 19.50 (2.08) | 156.08 (77.66) |
Data are expressed as mean (S.E.M.) of D3 or D3nf mRNA quantity/GAPDH quantity.
p < 0.05 vs. LR for same transcript in same region.
mRNA levels in HR and LR brain tissue, expressed as D3 normalized to GAPDH/D3nf normalized to GAPDH, are shown in Figs. 3-5. In PFC, the main effect of group was significant (F2,20 = 5.726, p = 0.012), and the D3/D3nf ratio was significantly greater in LRs than in HRs (p < 0.01) or MRs (p < 0.05; Fig. 3a). The main effect of group was also significant for SN/VTA (F2,18 = 5.966, p = 0.012), and the D3/D3nf ratio of LRs was increased relative to both MRs (p < 0.05) and HRs (p < 0.01; Fig. 4a). D3/D3nf ratios did not differ between groups in N.Acc. (F2,23 = 2.174, p = 0.139; Fig. 5a). Both D3 and D3nf mRNA were most abundant in the N.Acc., less abundant in SN/VTA and least abundant in PFC (data not shown).
Fig. 3.
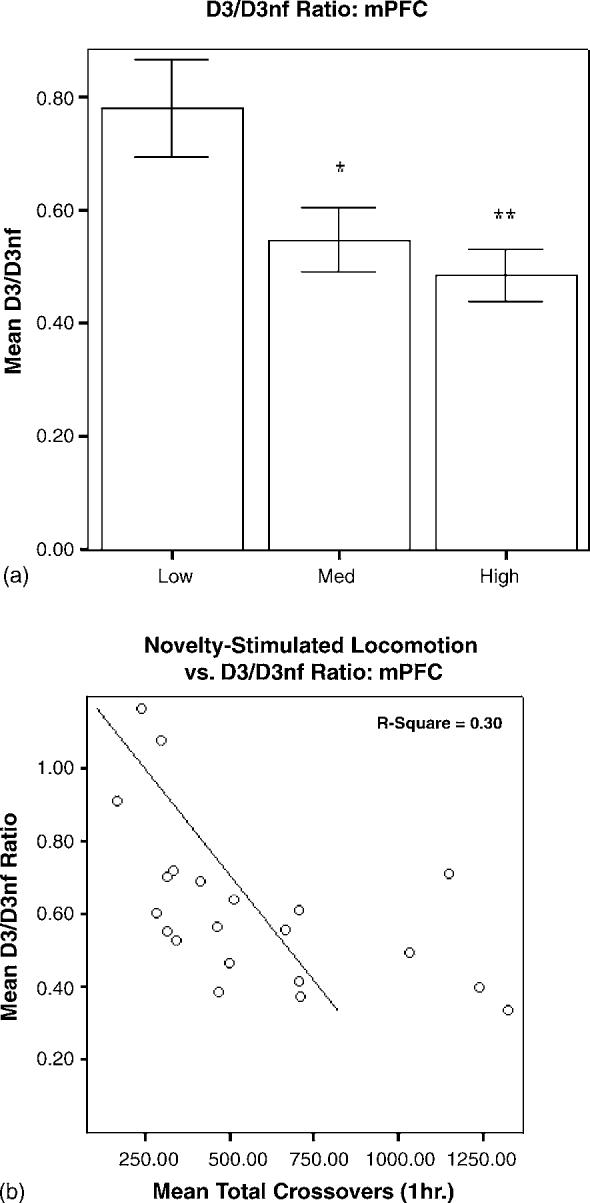
(a) Relative quantities of D3/D3nf mRNA in PFC are expressed as mean ± S.E.M. of the ratio of D3 (normalized to GAPDH)/D3nf (normalized to GAPDH). The D3/D3nf ratio for LRs (n = 8) was significantly higher than that for HRs (n =8, **p < 0.01) and MRs (n =5, *p < 0.05). (b) Novelty-stimulated locomotor activity and D3/D3nf mRNA ratios for individual animals were negatively correlated (R 2 = 0.30, p = 0.01).
Fig. 5.
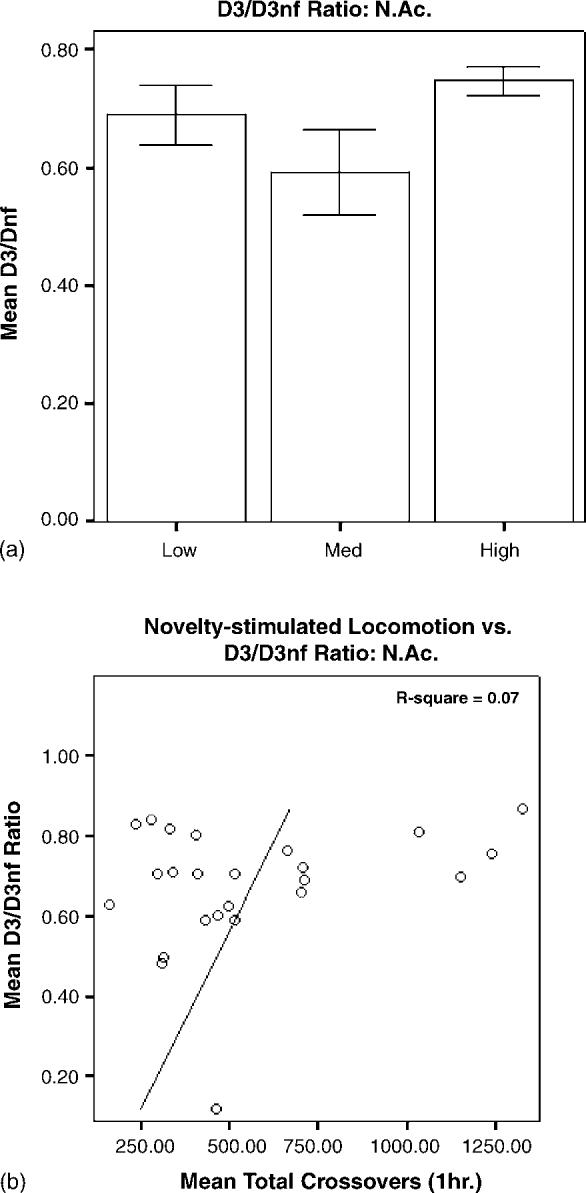
(a) Relative quantities of D3/D3nf mRNA in N.Acc. are expressed as mean ± S.E.M. of the ratio of D3 (normalized to GAPDH)/D3nf (normalized to GAPDH). There was no effect of group on D3/D3nf ratio in N.Acc. (n = 8/group; F2,23 = 2.174; p = 0.139). (b) There was no significant correlation between novelty-stimulated locomotor activity and D3/D3nf ratios for individual animals (R 2 = 0.07, p = 0.198).
Fig. 4.
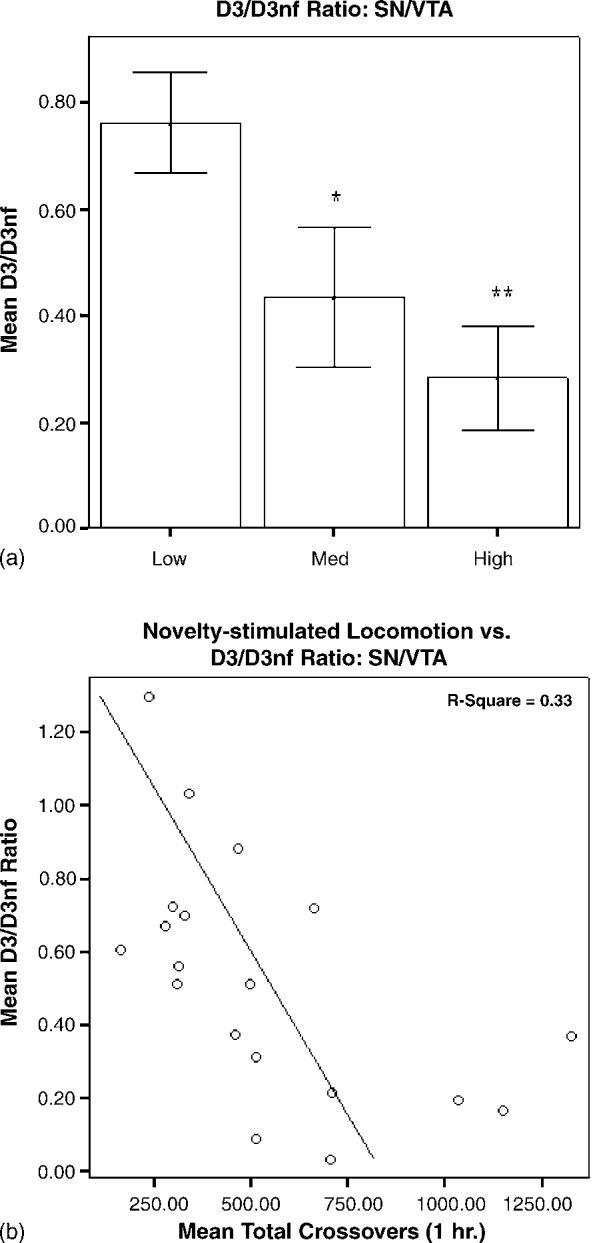
(a) Relative quantities of D3/D3nf mRNA in SN/VTA are expressed as mean ± S.E.M. of the ratio of D3 (normalized to GAPDH)/D3nf (normalized to GAPDH). The D3/D3nf ratio in SN/VTA for LRs (n = 8) was significantly higher than for HRs (n =6, **p < 0.01) and MRs (n =5, *p < 0.05). (b) Novelty-stimulated locomotor activity and D3/D3nf ratios for individual animals were negatively correlated (R 2 = 0.33, p = 0.01).
3.3. Linear regression analysis of novelty-induced locomotion and mRNA levels
Linear regression analysis revealed a significant negative correlation between novelty-stimulated locomotion and PFC D3/D3nf ratio, with 30% of the individual variance shared between the two variables (R2 = 0.30, p = 0.01; Fig. 3b). A similar negative correlation was observed for SN/VTA (R2 = 0.33, p = 0.01; Fig. 4b); individual variation in D3/D3nf ratios in this region explained approximately 33% of the variance in novelty-stimulated locomotion. No significant correlation was observed for N.Acc. (R2 = 0.07, p = 0.198; Fig. 5b).
4. Discussion
The relationship between individual differences in locomotor response to novelty and drug self-administration was first described by Piazza et al., who observed increased propensity to self-administer amphetamine in high responders to novelty [27]. The neural substrates underlying such individual differences are therefore of significant interest, as they may represent targets for prevention or treatment of substance abuse. Activity of the mesolimbic DA system has been implicated in individual differences between HRs and LRs. HRs have higher and longer-lasting stress-induced dopamine release in N.Acc., and this group difference is eliminated by blockade of stress-induced corticosterone release [16,34]. The increase in N.Acc. extracellular dopamine in response to cocaine is also greater in HRs than in LRs [12]. Additionally, basal dopamine metabolite levels are reduced in prefrontal cortex and increased in N.Acc. of HRs relative to LRs [28]. Thus, individual differences in modulation of mesolimbic DA system activity may result in part from enhanced HPA axis activation in HRs during novelty exposure. While differences in mesolimbic dopamine system activity have been characterized in HR/LRs, the roles of individual dopamine receptor subtypes in this model have not been defined. Radioligand binding studies have indicated increased D1-family and decreased D2-family dopamine receptors in N.Acc. of HRs relative to LRs [13]; however, there are few other studies evaluating individual dopamine receptor subtypes in the HR/LR model. There is, however, considerable evidence for involvement of the D3 receptor in behavioral responses to novelty. D3 receptor activation inhibits locomotor response to a novel testing environment [29], and two separate groups observed hyperactivity in D3 receptor mutant mice during initial exposure to a novel environment [1,40] (but also see [2,39]). The D3 receptor also plays an important role in modulating locomotor response to psychostimulants. D3 receptor mutant mice exhibit an enhanced locomotor response to cocaine [40] and amphetamine (unpublished observations, submitted for publication). D3 receptor agonists attenuate, while antagonists enhance hyperlocomotion induced by cocaine [6] and amphetamine [37]. Additionally, there is an inverse correlation between individual differences in locomotor activation by amphetamine and locomotor inhibition by D3 receptor agonists [33]. Based on these observations, our aim was to identify the role of the D3 receptor in the HR/LR behavioral model. Because D3 receptor function may be regulated by D3nf expression in a dominant-negative fashion [7,19,32], we tested rats for behavioral response to a novel spatial environment and quantified mRNA for D3 and D3nf expression from three brain regions comprising the mesolimbic dopamine system.
Consistent with our working hypothesis, the ratio of D3/D3nf mRNA levels in SN/VTA is greater in LRs than in HRs. D3 receptors are expressed in cell bodies of midbrain dopamine neurons within the ventral tegmentum [5], suggesting that presynaptic D3/D3nf expression could contribute to group differences in nucleus accumbens dopamine levels observed in HR/LR rats [12,34]. The decreased D3/D3nf ratio observed in our study would decrease D3 receptor availability for D3-mediated autoreceptor function, and the resulting attenuation of D3-mediated inhibition of midbrain dopamine neurons would therefore contribute to increased responsiveness of the mesolimbic dopamine system in HRs. Increased basal firing rate and decreased sensitivity to quinpirole-mediated inhibition in midbrain DA neurons of HRs suggest that these animals have reduced autoreceptor function relative to LRs [23]. Our data therefore add to studies supporting an autoreceptor role for midbrain D3 receptors [15,41]; however, the results must also be interpreted in the context of another study which did not observe differences in autoreceptor function between wild-type and D3 receptor knockout mice [20].
Elevated D3/D3nf ratios observed in PFC of LRs could similarly contribute to individual differences in locomotor response to novelty. D2/D3-family receptors in PFC gate excitatory amino acid projections from PFC to N.Acc. and VTA, and are inhibitory to locomotion [17,18]. Extracellular dopamine is increased in rat PFC during exposure to a novel environment [30], and PFC dopamine turnover is reduced in HRs, relative to LRs, as evidenced by lower DOPAC/DA ratios [28], suggesting a PFC DA hypofunction in HRs. The decreased PFC D3/D3nf ratio would impair D3 receptor-mediated inhibition of glutamatergic afferents to the N.Acc. and VTA, which, in turn, modulate dopamine release from nerve terminals in N.Acc. [22,38]. Thus, the observed differences in D3 receptor isoform expression in PFC would contribute to individual differences in the neuro-chemical and behavioral response to novelty and psychostimulants [4,8,30].
While it is possible that differences in D3/D3nf mRNA ratios observed in this study were produced by differing behavioral or neurochemical responses to overnight housing in the RACs prior to sacrifice, this explanation is unlikely, based on similar locomotor activity between groups during the first hour of the overnight housing in the RACs (Fig. 2b). The time-point for sacrifice (24 h after novelty exposure) was specifically chosen to avoid potential changes in mRNA levels induced by group differences in the locomotor response to novelty.
Because the efficiencies of PCR reactions for D3 and D3nf assays differed in our studies, we are unable to make accurate quantitative comparisons between the two different splice variants. The two splice variants were quantified using separate standard curves, providing between group comparisons (LR versus MR versus HR), but not allowing comparison between splice variants. However, based on the threshold cycle (Ct) for each splice variant (cycle number at which probe fluorescence reached 10 times baseline), it is likely that D3nf mRNA is significantly less abundant than the full-length transcript in all regions examined. Optimization of reaction conditions to achieve equal efficiencies would be necessary to estimate a direct quantitative comparison between D3 and D3nf mRNA.
We have used a quantitative RT-PCR assay to examine expression of mRNAs for D3 dopamine receptor and the alternatively spliced isoform D3nf in the HR/LR model. The ratio of D3/D3nf mRNA in prefrontal cortex and substantia nigra/ventral tegmentum account for approximately one-third of the individual variation in novelty-stimulated locomotion in this study, suggesting that individual differences in D3 receptor splicing and expression in these regions could contribute to differences in locomotor response to novelty. These findings are also consistent with the hypothesis that alternative splicing may contribute to regulation of D3 dopamine receptor function. Whether the observed differences in mRNA ratios are a primary feature of the HR or LR phenotypes, or a consequence of pre-existing differences in dopaminergic activity remains to be determined. Further examination of individual differences in D3 and D3nf protein expression will be of interest to extend this finding.
Acknowledgements
This work was supported by Medical Research Service, Department of Veterans Affairs (NMR), DA 016778-01 (NMR), and a Scottish Rite Schizophrenia Research Fellowship (LMP).
References
- 1.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betancur C, Lepee-Lorgeoux I, Cazillis M, Accili D, Fuchs S, Rostene W. Neurotensin gene expression and behavioral responses following administration of psychostimulants and antipsychotic drugs in dopamine D(3) receptor deficient mice. Neuropsychopharmacology. 2001;24:170–182. doi: 10.1016/S0893-133X(00)00179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1999;38:555–565. doi: 10.1016/s0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 4.Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123(Pt 5):927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- 5.Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J. Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellinwood EH, King GR, Davidson C, Lee TH. The dopamine D2/D3 antagonist DS121 potentiates the effect of cocaine on locomotion and reduces tolerance in cocaine tolerant rats. Behav. Brain Res. 2000;116:169–175. doi: 10.1016/s0166-4328(00)00270-9. [DOI] [PubMed] [Google Scholar]
- 7.Elmhurst JL, Xie Z, O'Dowd BF, George SR. The splice variant D3nf reduces ligand binding to the D3 dopamine receptor: evidence for heterooligomerization. Brain Res. Mol. Brain Res. 2000;80:63–74. doi: 10.1016/s0169-328x(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 8.Feenstra MG, Botterblom MH, van Uum JF. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: inhibition by diazepam. Neurosci. Lett. 1995;189:81–84. doi: 10.1016/0304-3940(95)11456-7. [DOI] [PubMed] [Google Scholar]
- 9.Fishburn CS, Belleli D, David C, Carmon S, Fuchs S. A novel short isoform of the D3 dopamine receptor generated by alternative splicing in the third cytoplasmic loop. J. Biol. Chem. 1993;268:5872–5878. [PubMed] [Google Scholar]
- 10.Fu D, Skryabin BV, Brosius J, Robakis NK. Molecular cloning and characterization of the mouse dopamine D3 receptor gene: an additional intron and an mRNA variant. DNA Cell Biol. 1995;14:485–492. doi: 10.1089/dna.1995.14.485. [DOI] [PubMed] [Google Scholar]
- 11.Hondo H, Spitzer RH, Grinius B, Richtand NM. Quantification of dopamine D3 receptor mRNA level associated with the development of amphetamine-induced behavioral sensitization in the rat brain. Neurosci. Lett. 1999;264:69–72. doi: 10.1016/s0304-3940(99)00163-9. [DOI] [PubMed] [Google Scholar]
- 12.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- 13.Hooks MS, Juncos JL, Justice JB, Jr., Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J. Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp. Clin. Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- 15.Joseph JD, Wang Y-M, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Caron MG, Wightman RM. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D3 receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 16.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J. Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine and GABA in the frontal cortex of mice in modulating a motor-stimulant effect of amphetamine and cocaine. Pharmacol. Biochem. Behav. 1998;60:237–244. doi: 10.1016/s0091-3057(97)00581-9. [DOI] [PubMed] [Google Scholar]
- 18.Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine in the mouse frontal cortex: a new hypothesis of behavioral sensitization to amphetamine and cocaine. Pharmacol. Biochem. Behav. 1998;61:435–443. doi: 10.1016/s0091-3057(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 19.Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3–D3nf interaction causes mislocalization of D3 receptors. Mol. Pharmacol. 2000;58:677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- 20.Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J. Neurosci. 1998;18:2231–2238. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Bergson C, Levenson R, Schmauss C. On the origin of mRNA encoding the truncated dopamine D3-type receptor D3nf and detection of D3nf-like immunoreactivity in human brain. J. Biol. Chem. 1994;269:29220–29226. [PubMed] [Google Scholar]
- 22.Louilot A, Le Moal M, Simon H. Opposite influences of dopaminergic pathways to the prefrontal cortex or the septum on the dopaminergic transmission in the nucleus accumbens. An in vivo voltammetric study. Neuroscience. 1989;29:45–56. doi: 10.1016/0306-4522(89)90331-x. [DOI] [PubMed] [Google Scholar]
- 23.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J. Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J. Neurosci. Meth. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- 25.Nagai Y, Ueno S, Saeki Y, Soga F, Yanagihara T. Expression of the D3 dopamine receptor gene and a novel variant transcript generated by alternative splicing in human peripheral blood lymphocytes. Biochem. Biophys. Res. Commun. 1993;194:368–374. doi: 10.1006/bbrc.1993.1829. [DOI] [PubMed] [Google Scholar]
- 26.Nimchinsky EA, Hof PR, Janssen WGM, Morrison JH, Schmauss C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J. Biol. Chem. 1997;272:29229–29237. doi: 10.1074/jbc.272.46.29229. [DOI] [PubMed] [Google Scholar]
- 27.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 28.Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J, Berger SP, Richtand NM. 7-OH-DPAT and PD 128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology. 2003;28:100–107. doi: 10.1038/sj.npp.1300018. [DOI] [PubMed] [Google Scholar]
- 30.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 31.Richtand NM. Laboratory analysis of behavioral effects of drugs of abuse in rodents. Meth. Mol. Med. 2003;79:475–480. doi: 10.1385/1-59259-358-5:475. [DOI] [PubMed] [Google Scholar]
- 32.Richtand NM. Behavioral sensitization, alternative splicing and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301163. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richtand NM, Welge JA, Levant B, Logue AD, Hayes S, Pritchard LM, Geracioti TD, Coolen LM, Berger SP. Altered behavioral response to dopamine D3 receptor agonists 7-OH-DPAT and PD 128907 following repetitive amphetamine administration. Neuropsychopharmacology. 2003;28:1422–1432. doi: 10.1038/sj.npp.1300182. [DOI] [PubMed] [Google Scholar]
- 34.Rouge-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- 35.Sax KW, Strakowski SM. Enhanced behavioral response to repeated d-amphetamine and personality traits in humans. Biol. Psychiatry. 1998;44:1192–1195. doi: 10.1016/s0006-3223(98)00168-1. [DOI] [PubMed] [Google Scholar]
- 36.Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorn L, Ashmeade TE, Storey VJ, Routledge C, Reavill C. Evidence to suggest that agonist modulation of hyperlocomotion is via post-synaptic dopamine D2 or D3 receptors. Neuropharmacology. 1997;36:787–792. doi: 10.1016/s0028-3908(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 38.Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog. Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 39.Waddington JL, Clifford JJ, McNamara FN, Tomiyama K, Koshikawa N, Croke DT. The psychopharmacology–molecular biology interface: exploring the behavioural roles of dopamine receptor subtypes using targeted gene deletion (‘knockout’) Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:925–964. doi: 10.1016/s0278-5846(01)00152-x. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 41.Zapata A, Witkin JM, Shippenberg TS. Selective D3 receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology. 2001;41:351–359. doi: 10.1016/s0028-3908(01)00069-7. [DOI] [PubMed] [Google Scholar]


