Abstract
The paramedian pontine reticular formation contains the premotoneuronal cell groups that constitute the saccadic burst generator and control saccadic eye movements. Despite years of study and numerous investigations, the rostral portion of this area has received comparatively little attention, particularly the cell type known as long-lead burst neurons (LLBNs). Several hypotheses about the functional role of LLBNs in saccade generation have been proposed, although there is little information with which to assess them. To address this issue, I mapped and recorded LLBNs in the rostral pons to measure their discharge characteristics and correlate those characteristics with the metrics of the concurrent saccades. On the basis of their discharge and location, I identified three types of LLBNs in the rostral pons: excitatory (eLLBN), dorsal (dLLBN), and nucleus reticularis tegmenti pontis (nrtp) LLBNs. The eLLBNs, encountered throughout the pons, discharge for ipsilateral saccades in proportion to saccade amplitude, velocity, and duration. The dLLBNs, found at the pontomesencephalic junction, discharge maximally for ipsilateral saccades of a particular amplitude, usually <10°, and are not associated with a particular anatomical nucleus. The nrtp LLBNs, previously described as vector LLBNs, discharge for saccades of a particular direction and sometimes a particular amplitude. The discharge of the eLLBNs suggests they drive motor neurons. The anatomical projections of the nrtp LLBNs suggest that their involvement in saccade production is less direct. The discharge of dLLBNs is consistent with a role in providing the “trigger” signal that initiates saccades.
INTRODUCTION
Saccades are eye movements that step the eyes between fixation points to quickly point the high-acuity portion of the retina at objects of interest. The saccadic system is well understood (see Moschovakis et al. 1996 for detailed review; Scudder et al. 2002 for current perspectives) because its function (i.e., speed and accuracy) is known and it is mechanically simple, easy to monitor, and clinically significant. Furthermore, it is of broad general interest because it provides a convenient, accurate readout for investigations of higher-order central processes (e.g., attention) and structures (e.g., frontal cortex).
Saccades are generated by a pulse of force created by the muscles (Fig. 1, lateral rectus) to move the eye quickly against the viscous properties of the eye muscles and connective tissue and by a step of tension to hold the eye in its new position against the elastic restoring forces of the globe attachments. The pulse and step signals are attributed to separate neural sources that are relayed via the motoneurons (Fig. 1, Abducens) and transduced by the muscles (but see Scudder et al. 2002 for discussion of exceptions to this simplified scheme). For the horizontal system, the pulse is caused by a burst of action potentials in the excitatory burst neurons (EBNs, Fig. 1) that drives the burst in ipsilateral motoneurons. The step is thought to result from “integrator neurons” (Fig. 1, Neural Integrator) that mathematically integrate the burst from the burst neurons (BNs) into a tonic position signal (see Fukushima et al. 1992 for review). The EBNs are inhibited during intersaccadic intervals by omnipause neurons (OPN, Fig. 1) and OPNs, in turn, are inhibited during the saccades via an inhibitory interneuron (Latch, Fig. 1). Adding symmetry to the system, inhibitory burst neurons (IBNs; Hikosaka and Kawakami 1977) inhibit contralateral, antagonist motoneurons (not shown).
FIG. 1.
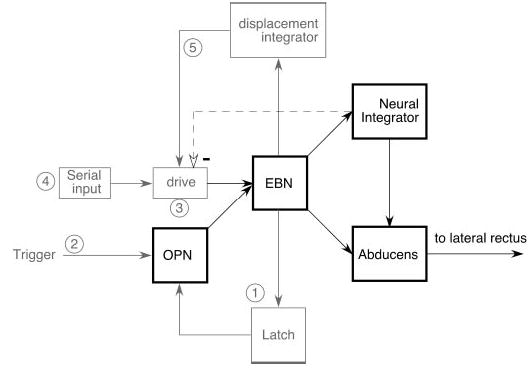
Schematic of the organization of the brain stem burst generator for horizontal saccades emphasizing potential functions of long-lead burst neurons (LLBNs). EBN, excitatory burst neurons; OPN, omnipause neurons.
Another group of neurons related to saccades are the long-lead burst neurons (LLBNs). Known to investigators since the earliest descriptions of saccade-related brain stem neurons (Luschei and Fuchs 1972), LLBNs are a heterogeneous category of neurons (for a detailed description, see Moschovakis et al. 1996). The LLBNs are most heavily concentrated in the rostral pons but their distribution overlaps that of the EBNs in cats (Kaneko et al. 1981) and in monkeys (Hepp and Henn 1983). LLBNs discharge like EBNs but the intense peri-saccadic burst is preceded by a low-level, unstructured prelude of activity. EBNs differ from one another only modestly in their directional tuning, their lead time preceding saccades, and their optimal activation direction (on-direction); on-directions vary only slightly (<approximately ±45°) from purely horizontal or purely vertical in cats (Kaneko and Fuchs 1981; Kaneko et al. 1981; Yoshida et al. 1982) and in rhesus macaques (Henn and Cohen 1976; Scudder et al. 1988), although they are more widely distributed in squirrel monkeys (Strassman et al. 1986a,b). In contrast, LLBNs show a wider range of directional tuning and a much greater range of lead-times, and their on-directions vary over a broader range that includes oblique directions (e.g., Scudder et al. 1988). In addition, LLBNs can also differ by displaying target-related discharge, saccade amplitude sensitivity, and nonvisual or motor discharge (see Moschovakis et al. 1996 for details).
At least five potential functions have been hypothesized for LLBNs (Fig. 1, ➀–➄). Two stem from the earliest hypothesis for saccade generation (Robinson 1975), which posited a “local feedback” circuit to control saccade amplitude (Fig. 1). In that model, local feedback (Fig. 1, light dashed line) of eye position from the proposed neural integrator was subtracted from a signal representing the desired eye position (Fig. 1, drive) to accurately control EBN discharge and thereby control saccade amplitude. In addition, the connections between OPNs and EBNs were suggested to be reciprocal and inhibitory, forming a “latch” circuit that allowed only “ON” or “OFF” states and prevented any unwanted activity of the high-gain burst generator during intersaccadic intervals. Thus one possible function of LLBNs might be to provide a portion of the latch (Fig. 1, ➀). The Robinson model also postulated an inhibitory “trigger” input (Fig. 1, trigger) to OPNs that was supposed to start the saccade. Since electrical stimulation of a more central saccade-related structure, the superior colliculus (SC), did not directly inhibit OPNs, Raybourn and Keller (1977) suggested that the trigger signal was relayed by LLBNs (Fig. 1, ➁). In cats, electrical stimulation of the LLBN area elicits monosynaptic inhibition in OPNs (Kamogawa et al. 1996), so those LLBNs may relay the saccadic trigger signal. The observation that EBN stimulation results in disynaptic OPN inhibition, presumably by the LLBNs (Kamogawa et al. 1996), is consistent with either latch or trigger functions. In addition, it was shown recently that OPN inhibition during a saccade is proportional to eye velocity (Yoshida et al. 1999). Since both EBN and some LLBN discharge rates are well correlated with saccade velocity, whereas a trigger input would not have to be related to saccade velocity, this finding is most consistent with the interpretation that burst neurons form a portion of the latch circuit. This is particularly true if the trigger originates in the SC, since those neurons do not show strong correlations between saccade velocity and their discharge under normal circumstances.
A less well-founded conjecture for the role of LLBNs in saccade production is that they substitute for collicular functions if the SC is damaged (Fig. 1, ➂). This suggestion was prompted by the finding that saccades still occur following complete ablation of the SC (e.g., Albano and Wurtz 1982; Schiller et al. 1980).
In the only previous study that specifically concentrated on LLBN function, Hepp and Henn (1983) suggested a fourth possibility. They hypothesized that LLBNs mediate the serial transformation from the eye displacement code of the SC into the temporal code used by the EBNs (Fig. 1, ➃). This idea was based on the anatomical distribution and discharge properties of LLBNs, which, they suggested, gradually transitions from types similar to those found in the SC to EBN-like discharge. Crandall and Keller (1985) confirmed that at least one class of LLBNs was actually visuomotor neurons, similar to those found in the SC, thus providing the first stage in the scheme proposed by Hepp and Henn (1983). Unfortunately, those neurons were located in the nucleus reticularis tegmenti pontis (nrtp), which projects to the cerebellum and not to the pontine saccade generator, so Crandall and Keller (1985) discounted a primary role for those neurons in saccade generation. Scudder et al. (1996) used intracellular staining in alert squirrel monkeys to show that LLBNs in the rostral pons form at least two groups: one, located in the nrtp, is precerebellar like those of Crandall and Keller (1985); the other, located more dorsally in the rostral pons, projects to EBN areas. While the second group might subserve the serial processing hypothesized by Hepp and Henn (1983), recent anatomical evidence (Moschovakis et al. 1998) does not support the notion of cell-to-cell processing. Instead, it suggests a differential organization of projections and terminal density (cf. Edwards and Henkel 1978) from the SC to the brain stem as the mechanism underlying the spatial-to-temporal transformation.
Finally, Scudder (1988) postulated that LLBNs are the locus of the resettable local feedback integrator (Becker and Jürgens 1979; Fig. 1, displacement integrator, ➄) that has come to replace the position feedback of the Robinson model (1975). Unfortunately, the technically difficult intracellular staining methods used by Scudder et al. (1996) did not produce sufficient examples of stained LLBNs to provide a morphological substrate for an integrative function (e.g., recurrent collaterals).
Thus although their existence has been known for quite some time, the exact function of LLBNs in saccade production is still controversial. To obtain quantitative data that might serve to distinguish between the five possible roles of LLBNs, I recorded the discharge of LLBNs in the rostral pons of alert, trained rhesus macaques. In particular, I examined their discharge to see whether these rostral pontine neurons could relay collicular saccade signals like a trigger, if they could act as a latch circuit to inhibit OPNs during saccades, or if they might constitute a portion of the resettable integrator.
METHODS
The methods used in this experiment are similar to those described elsewhere (Kaneko 1996, 1997, 1999; Kaneko and Fukushima 1998; Soetedjo et al. 2002a,b). All experiments were performed in strict compliance with the Guide for the Care and Use of Laboratory Animals (NRC 1997). Animal care exceeded the minimal recommendations of the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. Specific protocols were approved by the Animal Care and Use Committee of the University of Washington (ACC #2602– 01).
In brief, four juvenile (3–5 kg), male rhesus macaques (Macaca mulatta; monkeys O, M, H, and B) were anesthetized and implanted with a scleral search coil (Fuchs and Robinson 1966), a recording chamber, and head-stabilization lugs in aseptic surgery. The technique was that described by Judge et al. (1980) modified so that the lead wire exits though a hole drilled in the back orbit (cf. Kaneko et al. 1981). After they recovered, the monkeys were trained to track a moveable, back-projected laser spot for food reward. Then, in recording sessions while the head-immobilized animal tracked the target as it stepped randomly between points spaced at 5° intervals on a centered, 7 × 7 array (i.e., ±15° horizontally and vertically), I surveyed the rostral pons from the abducens to the mesencephalon, ±3.0 mm on each side of the midline and from the floor of the fourth ventricle ≥4.0 mm deep along trajectories as indicated in Fig. 2.
FIG. 2.
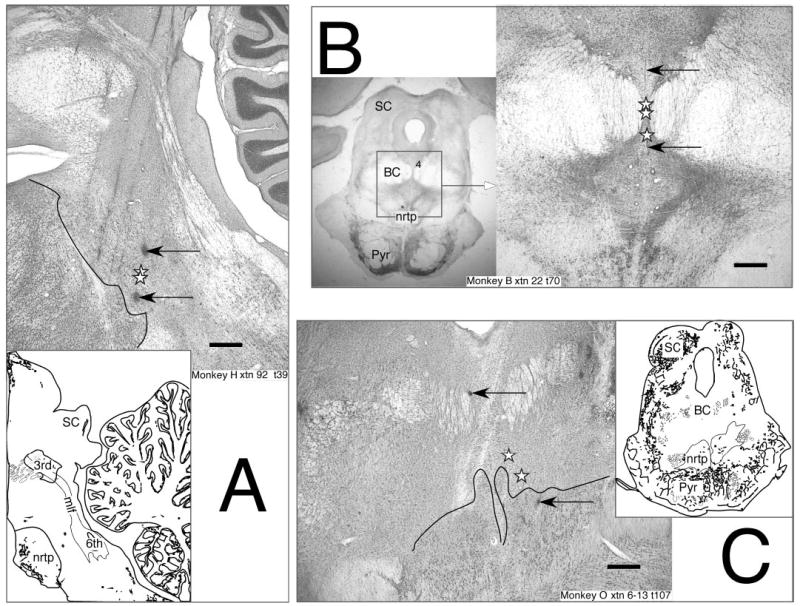
Reconstructed location of dorsal LLBNs (dLLBNs). Normal Nissl-stained parasagittal (left) and frontal (right) 50-μm sections from 3 animals showing marking lesions (arrows) and reconstructed location of dLLBNs (stars). Insets are ¼× magnification showing location of the section. Abbreviations: 3rd, oculomotor nucleus; 4, trochlear nucleus; 6th, abducens nucleus; BC, brachium conjunctivum; mlf, medial longitudinal fasciculus; nrtp, nucleus reticularis tegmenti pontis; Pyr, pyramidal tract; SC, superior colliculus. Calibration bars are 1.0 mm.
In the first animal (monkey O) I recorded uniformly over this entire area and used an approach angled 20° lateral from the sagittal plane (for monkey O, Fig. 2C and for monkey M, not shown). In the other three animals, I concentrated on rostral regions where amplitude-tuned LLBNs are known to be located (Fig. 2, insets) but approached the brain stem parasagittally from an angle of 15° posterior in monkeys H and B (Fig. 2, A and B). In all animals, when BNs were located, I examined on a storage oscilloscope the lead time and compactness of the burst preceding optimal (i.e., approximately ON-direction) saccades and classified the neurons according to the standard nomenclature (Kaneko et al. 1981; Luschei and Fuchs 1972; Scudder et al. 1988). If the BN showed a prelude of activity preceding a sharp, intense burst of action potentials associated with the saccade, I judged it to be a LLBN and assessed its optimal vector more precisely by restricting the target movement to whichever sectors of the visual field (±35° horizontal × ±20° vertical) it required (cf. Fig. 8) as described recently (Soetedjo et al. 2002b). Briefly, this involved approximating the optimal amplitude, testing the broadest angular range over which the neuron would respond to that amplitude step, and then reassessing the optimal amplitude more precisely (i.e., within a few degrees) along the angle that bisected the extremes of the previously assessed range. Once the “motor field” was mapped, if there was a component of the discharge associated with target presentation it was further assessed by requiring the animal to perform a delayed or remembered saccade task with the test spot located at the optimal movement vector. In the delayed task, the fixation spot remains on while a peripheral white test spot is presented via a second set of galvanometers and shutters. When the fixation laser spot is extinguished after a delay, the monkey must make a saccade to the test spot. In the remembered task, the test spot is flashed and the monkey must make a saccade to the remembered location of the test spot when the fixation spot is extinguished. This procedure temporally separates the visual response to the test spot from the motor response.
FIG. 8.
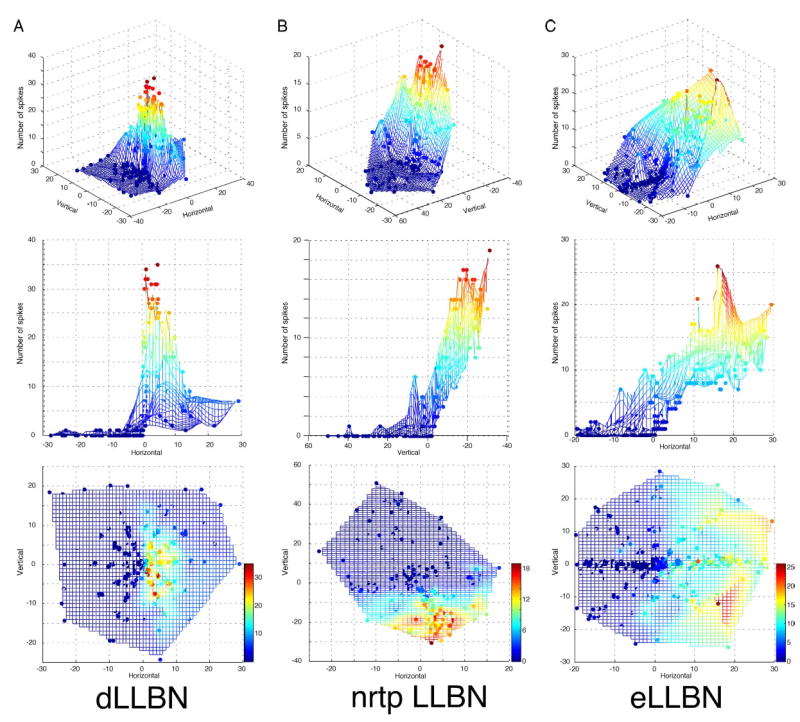
Three-dimensional plots of number of spikes in the burst as a function of horizontal and vertical saccade amplitude for the exemplary dLLBN (A), nrtp LLBN (B), and eLLBN (C) from Figs. 3–5. Top row: angle view from the OFF-direction toward the ON-direction. Note the narrow peak for dLLBNs compared with either nrtp LLBNs (B) or eLLBNs (C). Middle row: rotated to view from amplitude tuning. Note “closed field” and small-amplitude peak in A. Bottom row: top-down view. Color calibration bars in bottom row apply to each column.
Standard extracellular recording techniques were used. Home-made tungsten microelectrodes and electronics were used to isolate single BN activity as the monkey performed the tracking task. From tape recordings of the data, eye movements and BN discharge were digitized off-line at 1 kHz for eye, target and stimulation channels and with 10-μs resolution for the spike trace. Eye position data were low-pass filtered (500 Hz, −3dB) and velocity was calculated using digital differentiation with a 3-point, boxcar smooth. Digitized data were analyzed with custom interactive programs as described previously (Kaneko 1996, 1997, 1999; Kaneko and Fukushima 1998). The program identified the saccades on the basis of adjustable velocity criteria (usually 50°/s depending on the noise of the computer-calculated, 2-point differentiated, velocity trace). If the velocity of either the horizontal or vertical component exceeded the set criteria, it then searched for and marked the target and saccade onset and offset and saccade peak velocity separately for the horizontal and vertical position traces. Onset and offset were marked by marching backward and forward in time respectively, from the approximately 50°/s detection point using a criteria of 5–7°/s to define onset and offset. In general, LLBNs are more difficult to analyze than other saccade-related neurons because their prelude of activity makes demarcation of the intense burst somewhat problematic. The program searched for the occurrence of action potentials, forward and backward in time, from the beginning of the saccade and marked the burst onset and offset when the discharge fell below the criterion of 40 spikes/s. Thus if the first spike found preceded or followed the saccade onset by more than 25 ms, the discharge was not considered to constitute a burst related to the saccade. As long as the discharge remained above the 40 spikes/s criterion, all spikes were included in the marked burst. The automatic markings for each saccade and burst were then inspected individually and accepted, re-marked, or rejected. Saccade marks rarely had to be changed (<approximately 2%), whereas the burst was often re-marked for vector burst neurons (see following text) because the intense, saccadic portion of the burst had to be judged relative to the rest of the cell’s activity (the prelude discharge, e.g., Figs. 3–5). Once the saccades and bursts were accepted, the program calculated and stored all the saccade and discharge metrics for later analysis by other custom or commercial (Matlab, The MathWorks; Igor, Wave-metrics) software programs. The saved metrics included calculations for vector components (calculated by the Pythagorean theorem) as well as horizontal and vertical components. I analyzed all primary and secondary saccades associated with target steps and ignored spontaneous saccades, although the discharge associated with spontaneous saccades was not obviously different from that associated with targeting saccades of the same amplitude and direction.
FIG. 3.
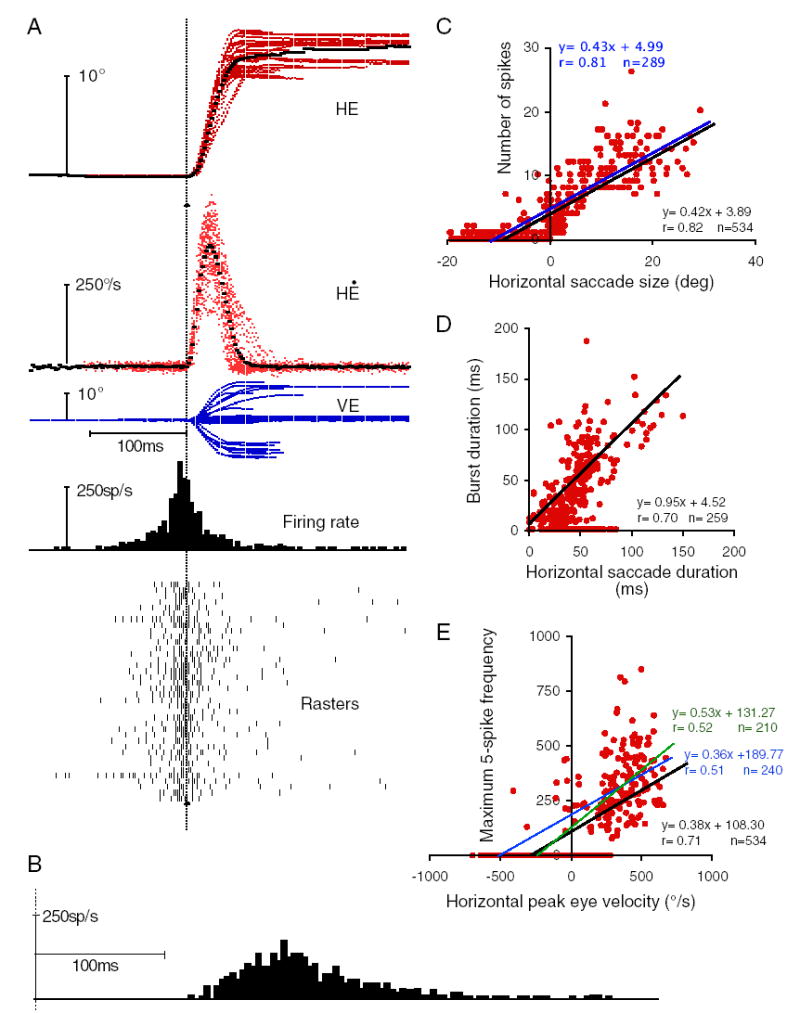
Typical excitatory LLBN (eLLBN) discharge. A: traces (top to bottom): horizontal eye position (HE), horizontal eye velocity (HĖ), vertical eye position (VE), histogram of the average unit discharge (firing rate), and rasters for 38 similarly sized (10 –15°) rightward (ip silateral) saccades. Heavy dotted line shows averages of position and velocity traces. B: same trials aligned on target onset (dotted vertical line). C: scatterplot of number of spikes for each saccade of the indicated horizontal component amplitude for all saccades elicited while recording this neuron that had a horizontal component. Line is the least-squares linear regression (inset). Blue line is linear regression for all points except those associated with zero number of spikes. Regression metrics in blue. D and E: as in C for burst duration and saccade duration (D) for all saccades accompanied by a burst or maximum frequency and peak velocity (E) for all saccades. E: blue line as in C with zero values removed. Green line is linear regression for ipsilateral points only. Regression metrics in green.
FIG. 5.
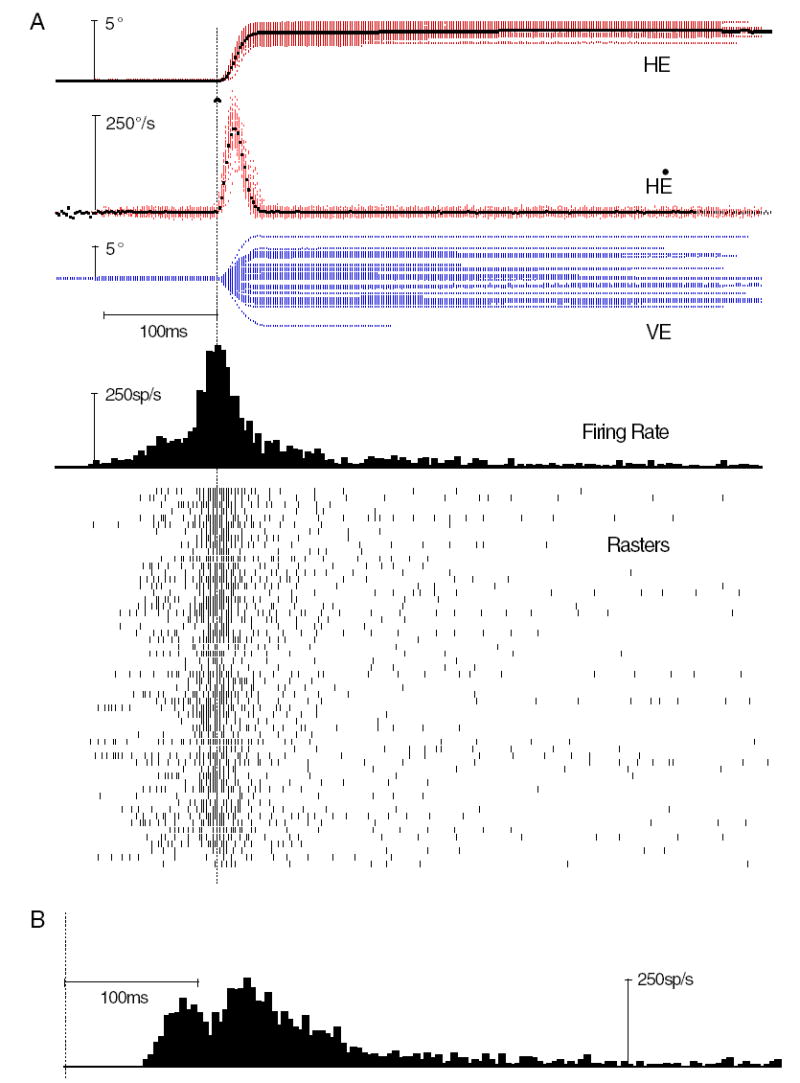
Typical dLLBN discharge. Conventions as in Fig. 3. A: 56 near optimal (4° ipsilateral, cf. amplitude Fig. 9 and direction Fig. 7) vector saccades. All saccades from 3 to 5° ipsilateral (rightward) saccades were included. B: histogram with rasters realigned on target step (vertical dotted line).
Although these traditional analyses worked well for all open field neurons (i.e., eLLBNs, and some nrtp LLBNs; see following text), the vector burst neurons were problematic. Because of the variability in bursts for nonoptimal saccades, the potential for inconsistency in marking them again if automatic marking failed, and concerns of reviewers, I repeatedly reanalyzed representative neurons using several more “objective” marking techniques to confirm the original methods. None of these “objective” methods (e.g., fixed burst firing rate threshold or replacing spikes with different continuous functions) gave consistent results or revealed improved correlations without further adjustments for differences between dLLBNs. This was because of their inability to handle the variability in the discharge, which I normally accounted for by manually adjusting the markings associated with nonoptimal saccades. I settled on a method similar to the one I used previously for superior colliculus neurons (Soetedjo et al. 2002a) because it gave more consistent results across neurons with the least amount of adjustment for each neuron. Briefly, we began by replacing each spike in the discharge with a Gaussian to smooth the discharge. These were added to produce a spike-density function and each spike-density function associated with each saccade was calculated. The peak of the spike density function was found and an arbitrary percentage of the peak amplitude was chosen (e.g., 50%). The times of the 50% rising and falling points were marked on the spike-density function. The program then searched the actual discharge from those time marks toward the peak for the occurrence of an actual spike, which is not the same as the Gaussian-convolved spike or the half-peak point. The first and last spikes thus found defined the burst. The width of the Gaussian and the percent of peak amplitude were varied from neuron to neuron to maximize the agreement (i.e., correlation and slope) between the blind method and the standard analysis.
Representative examples of dLLBN neurons (see following text) from each animal were re-analyzed using 10 –15 ms Gaussian width and 10 –50% of the peak (usually 10 or 15%). I then plotted the burst duration measured by the blind method against the original method. The average linear correlation between the blind method and the original method was 0.66 (±0.24 sd; n = 14) and the average slope of the regression line was 0.90 (±0.19 SD). Thus on average, the two methods resulted in equivalent marking of the burst. While the burst durations were similarly marked in the two methods, the correlation coefficients for peak saccade velocity versus peak burst frequency (measured as the maximum for five consecutive spikes) dropped by 0.33 (±0.32 SD), suggesting that the manual marking did a better job of capturing the features of the burst, so the results reported here are based on the older method.
Recording sites were confirmed histologically in normal Nissl-stained material. Areas of high concentration of particularly interesting LLBNs were marked by electrolytic lesions made by passing 30-μA anodal current through the recording electrode for ≥30 s.
RESULTS
Of the 149 BNs recorded during this survey, 105 were LLBNs. These LLBNs could be divided into three main groups on the basis of their discharge characteristics and anatomical location. One group (n = 29), scattered throughout the pons, I will call excitatory LLBNs (eLLBNs) because they resembled EBNs in discharging an intense burst for the horizontal component of all ipsilateral saccades. The two other types discharged optimally for particular amplitudes. One type (n = 9), which I will call nrtp LLBNs because they are located in the nrtp, have been described by Crandall and Keller (1985; see also Hepp and Henn 1983). Another type (n = 64), which I will call dorsal LLBNs (dLLBNs) because they were located more dorsorostrally, discharged for all ipsilateral saccades like eLLBNs but maximally for an optimal amplitude like nrtp LLBNs. Unlike nrtp LLBNs, dLLBNs usually had an optimal amplitude of <10°. Three LLBNs could not be placed into any of the above categories.
Because the discharge of LLBNs was optimal for certain directions and, in the case of nrtp LLBNs and dLLBNs, for certain amplitudes, LLBN discharge variability increased as saccade amplitude and direction differed from the optimal saccade. Discharge associated with optimal saccades was brisk (often exceeding 1,000 spikes/s), compact, and thus easily discernible. The further from the optimal saccade, the lower the peak discharge and the higher the temporal dispersion (see METHODS).
All types of LLBNs were recorded in patches with intervening areas void of any LLBN activity. The nrtp LLBNs were recorded within the borders of that nucleus as detailed below. I was unable to associate the distribution of dLLBNs with any known reticular nucleus. Consistent with the literature (see Scudder et al. 2002 for a recent review), eLLBNs were located throughout the pons.
eLLBNs
As implied by their name, eLLBNs are similar to the EBNs that provide the saccade-related synaptic drive to motoneurons (for reviews see Fuchs et al. 1985; Moschovakis et al. 1996; Scudder et al. 2002). The eLLBNs were found most often in the region around the abducens nuclei (i.e., dorsal and caudal to the marking lesions in Fig. 2A, arrows) but could be recorded as far rostrally as the dLLBNs and were often recorded in adjacent tracks or, occasionally, on the same track (cf. Fig. 2, stars) in each of the animals.
The burst metrics of eLLBNs also resemble those of EBNs. There is a high correlation between the metrics (e.g., number of spikes; Fig. 3C) of the intense portion of the burst and the metrics of the concomitant saccades (e.g., size of the ON-direction component of the saccade). Similar LLBNs have been recorded in both cats and monkeys (e.g., Kaneko et al. 1981; Luschei and Fuchs 1972; Scudder et al. 1988; Strassman et al. 1986a). To date, the only consistent criterion for distinguishing eLLBNs from short-lead BNs has been lead time and lack of prelude activity (Luschei and Fuchs 1972). Thus despite the lack of conclusive evidence that they provide a portion of the excitatory saccadic drive to abducens motoneurons, I will refer to them as eLLBNs.
The defining characteristics of a typical eLLBN are shown in Fig. 3A. For these rightward saccades (HE), irrespective of the vertical component (VE), the neuron discharged a brisk burst of action potentials (rasters and histogram). As previously described for EBNs, the discharge metrics of this eLLBN are well correlated with saccade metrics (Fig. 3, C–E). The number of spikes in the burst increases linearly with the size of the on-direction component of the saccade for all saccades (n = 534, bottom right inset) with horizontal components (Fig. 3C, r = 0.82, P < 0.01) and the duration of the burst is correlated with the duration of the rightward component of the saccade (Fig. 3D, r = 0.70, P < 0.01), with a slope of the linear regression near 1.0 (slope = 0.95). For the population of 29 eLLBNs, the average correlation coefficient (±SD) between number of spikes and saccade size was 0.69 (±0.16) and that between peak frequency (for five spikes) and peak velocity was 0.66 (±0.16). The average correlation between saccade duration and burst duration was 0.41 (±0.26), with an average slope of 0.76 (±0.62). All of these values are similar to those reported in previous studies (e.g., Luschei and Fuchs 1972; Scudder et al. 1988; Strassman et al. 1986a,b), but slightly lower for size and duration and slightly higher for velocity. For all but two of the 29 eLLBNs, the correlation coefficients between number of spikes and component amplitude and between peak frequency and peak velocity were highest for the on-direction component of the saccade. They were lower by about half for the vector metric (e.g., the vector amplitude or peak vector velocity) and even lower for the orthogonal component. The exception is the correlation between burst duration and saccade duration, which were similar for ON-direction component and total saccade duration. For 18/29 eLLBNs the correlation between burst duration and total saccade duration was as high as, or higher than, that between burst duration and component duration. The saccade direction associated with maximum discharge, as estimated by number of spikes, peak firing, or highest correlations (Henn and Cohen 1976), was near (<±30°) the horizontal (24/29) or vertical (5/29) meridian as in previous studies. The lead time from the beginning of the intense portion of the burst to the beginning of the saccade (i.e., the first component, either horizontal or vertical, to change) was 29 ms (±15 ms; n = 28; range 13–77 ms) for all saccades within ±30° of the optimal direction for each neuron.
To verify the inclusion of “zero” values (e.g., no spikes for large OFF-direction saccades), I eliminated those “zero” points and redid the linear regression (blue lines, Fig. 3, C and E). To assess the affects of including OFF-direction saccades in the analysis, I also checked linear regression of ipsilateral saccades only (green line, Fig. 3E). These manipulations had no consistent effect across eLLBNs, probably because of the small OFF-direction discharge, and did not reveal any unexpected relationships (cf. regression metrics in Fig. 3, C and E).
Although not noted in early studies (Henn and Cohen 1976; Luschei and Fuchs 1972), LLBNs sometimes discharge in response to target steps (cf. Crandall and Keller 1985) into their optimum movement field. As illustrated in Fig. 3B for the same rasters and saccades depicted in Fig. 3A but realigned on the target step (dotted line), there is no conclusive visual response in this example. The majority (20/29) of the eLLBNs did not show a time-locked discharge in response to the visual target (assessed by inspection of the target-aligned histograms). Nine of 29 eLLBNs recorded did show a consistent response to the target for optimal saccade vector steps in 15– 66% of the trials (cf. Fig. 5B). In addition, another five eLLBNs showed an occasional response (based on the analysis of individual saccades) on 2–10% of the target steps. Finally, the presence of visual responses in eLLBNs was quite variable across animals. Of the seven eLLBNs recorded in monkey B, five displayed visual responses, but none of the five eLLBNs recorded in monkey M did.
nrtp LLBNs
Because the nrtp has already been studied, and because tracks aimed at the nrtp could pass through the pontomesencephalic junction, thereby compromising dLLBN recordings, I did not sample the nrtp extensively. Instead, I sought only to confirm the previous findings regarding nrtp LLBNs after completing dLLBN recordings in monkeys H and B. Those preliminary recordings and analyses confirmed the presence of maximum discharge associated with a particular direction of movement for some medial nrtp LLBNs (Crandall and Keller 1985; Hepp and Henn 1983). The discharge of a representative nrtp LLBN neuron is shown in Fig. 4. This neuron discharged maximally for downward (283° polar angle; see following text) saccades with an amplitude of 23° and less vigorously as the saccade vector deviated from this optimum (Fig. 8B). The burst duration was weakly correlated with saccade duration (Fig. 4B) for all saccades associated with a burst while the neuron was being recorded (n = 130). The peak discharge was related to peak saccade velocity (Fig. 4C) but the peak velocity saturated at around 800°/s (n = 214). Neither eliminating zero values (blue) nor considering only ON-direction saccades (green) changed the correlations much. The discharge data for 50, 15–30° downward saccades show that there was considerably greater variability between the discharge for similar saccades than that displayed by eLLBNs (compare rasters and histogram Fig. 4A vs. Fig. 3A).
FIG. 4.
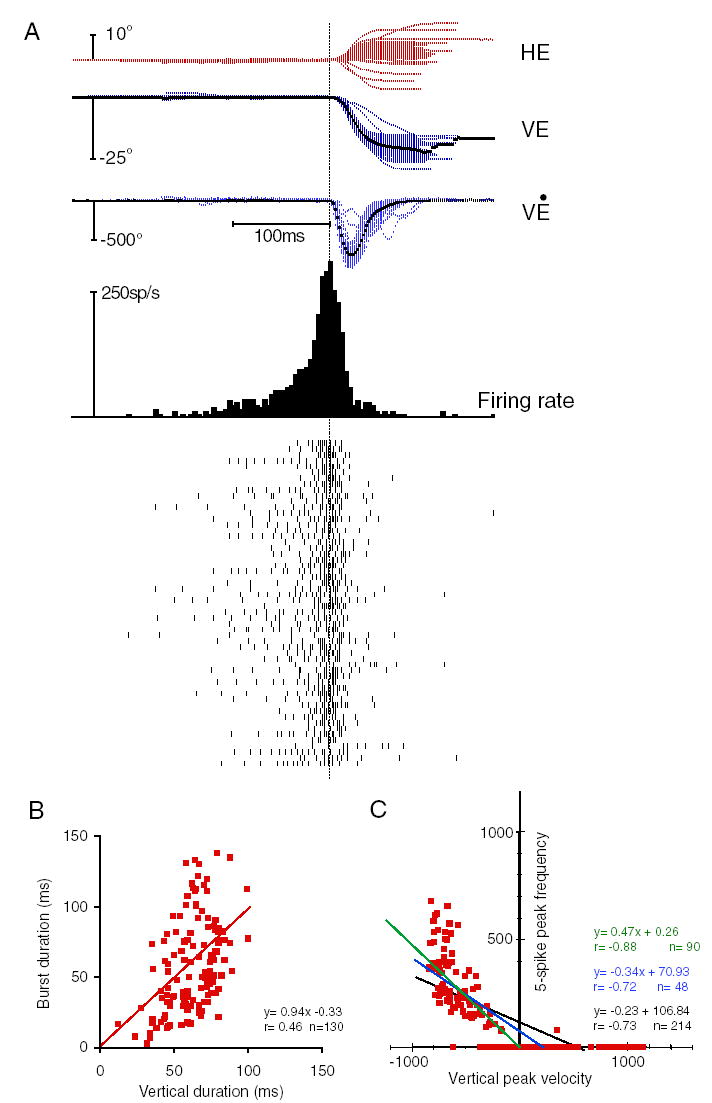
Typical nucleus reticularis tegmenti pontis (nrtp) LLBN discharge. Conventions as in Fig. 3. A: all 15 to 30° downward saccades were used so that the number of saccades is similar to that in Fig. 3A. All saccades were similarly associated with a brisk discharge and near the optimal direction. C: as in Fig. 3C.
In addition, this small sample (n = 9) suggests that there may be differences between these findings and those previously reported. One potential difference is that previous descriptions (Crandall and Keller 1985; Hepp and Henn 1983) of nrtp BNs have emphasized their differences from the more caudally located EBNs, but similar, short-lead BNs were sometimes recorded in the nrtp (e.g., Fig. 2C, arrow, bottom right). In fact, the lead-time of 3/9 vector LLBNs in the nrtp was short enough (6- to 11-ms lead) to categorize them as short-lead BNs. The lead time for all nrtp neurons was 16 ms (±7 ms; range 6 –25 ms) and 20 ms (±5 ms) for the six long-lead nrtp LLBNs for all saccades within ±30° of the optimal direction for each neuron. Crandall and Keller (1985) also emphasized the similarity to saccade-related burst neurons (SRBNs; Sparks 1978) in the SC. In contrast, this sample was mostly open field (i.e., linearly increasing discharge for larger amplitudes) and displayed significant correlations between burst and saccade metrics, whereas SRBNs typically do not show such correlations (cf. Soetedjo et al. 2002a). The average correlation between number of spikes and saccade size was 0.73 (±0.11, n = 9) and that between peak firing and peak velocity was 0.67 (±0.22). Because many nrtp neurons are vector LLBNs, their discharge diminished beyond optimal amplitude so the correlation between saccade and burst duration was poor (0.29 ± 0.27) and the slope was low (0.41 ± 0.47). Another potential difference is the location of nrtp LLBNs, which were recorded within the borders of the nrtp but were often found more laterally than in previous investigations. They were located along the dorsal margin of the nucleus as it extends laterally as well as deeper within the nucleus when tracks were located more medially. Finally, Crandall and Keller (1985) also emphasized the visual motor nature of nrtp LLBNs, but none of these nine nrtp LLBNs had a consistent visual response. Prompted by these potential differences, we have begun a more thorough investigation of the properties of nrtp neurons (Soetedjo et al. 2002c; Takeichi et al. 2005).
dLLBNs
Two criteria served to distinguish the dLLBNs from the other groups. First, they were located more dorsally than the nrtp LLBNs and more rostrally than most of the eLLBNs (Fig. 2, stars). Because these neurons may play a crucial role in saccade production and may correspond to neurons described in anatomical studies, I will describe their anatomical distribution in some detail. The dLLBNs recorded in this study were distributed in clusters and extended in a band from the pontomesencephalic border in a rostromedial (Fig. 2B) to caudolateral (Fig. 2, A and C) direction. The reconstructed location of dLLBNs in monkey H (Fig. 2A) was the most caudally recorded relative to other oculomotor structures of known location (i.e., the trochlear nucleus dorsally and the OPNs caudomedially) of any of the four animals studied. Thus the track and marking lesions shown in Fig. 2A represent the approximate caudal limit of the dLLBN distribution. The microelectrode tracks through the rostral pons are clearly visible in the photomicrograph of the Nissl-stained section. The two lesions were placed to bracket the location of the recording sites of two dLLBNs. Note that the location of the neurons (stars) is dorsal to the nrtp (thin black line, Fig. 2A). The mediolateral position of recording sites is illustrated in the photomicrograph and tracing of coronal sections from monkey O (Fig. 2C). Again, marking lesions (arrows) reveal the location of dLLBNs (stars) that are found dorsal to the nrtp (thin black line, also see inset; approximately ¼×). Similar distributions were found in monkeys M and B, but the dLLBNs were slightly more dorsal and rostral (e.g., Fig. 2B).
The second distinguishing characteristic of dLLBNs is their discharge. Qualitatively, the saccade-related discharge is distinguished by the prolonged activity both before and especially after the saccade and its associated compact burst (Fig. 5A; cf. Figs. 3A and A). This prolonged discharge served to identify dLLBNs and differentiate them from the other two groups, especially because it could greatly outlast the saccade (rasters in Fig. 5A). In contrast, the lead-time for the intense portion of the burst was similar to that for eLLBNs (average = 29 ± 22 ms; range 4 –103 ms) calculated in a similar fashion (i.e., for all saccades within ±30° of the optimal direction; see following text). Because the prolonged burst made it even more difficult to delimit the intense saccadic portion, the discharge of dLLBNs was often more difficult to analyze than discharge of the other types. This difficulty may account, in part, for the poor correlations between burst duration and saccade duration (see following text; Fig. 6A), but it does not account for the significant differences between the correlations calculated for dLLBNs compared with those for the other LLBNs.
FIG. 6.
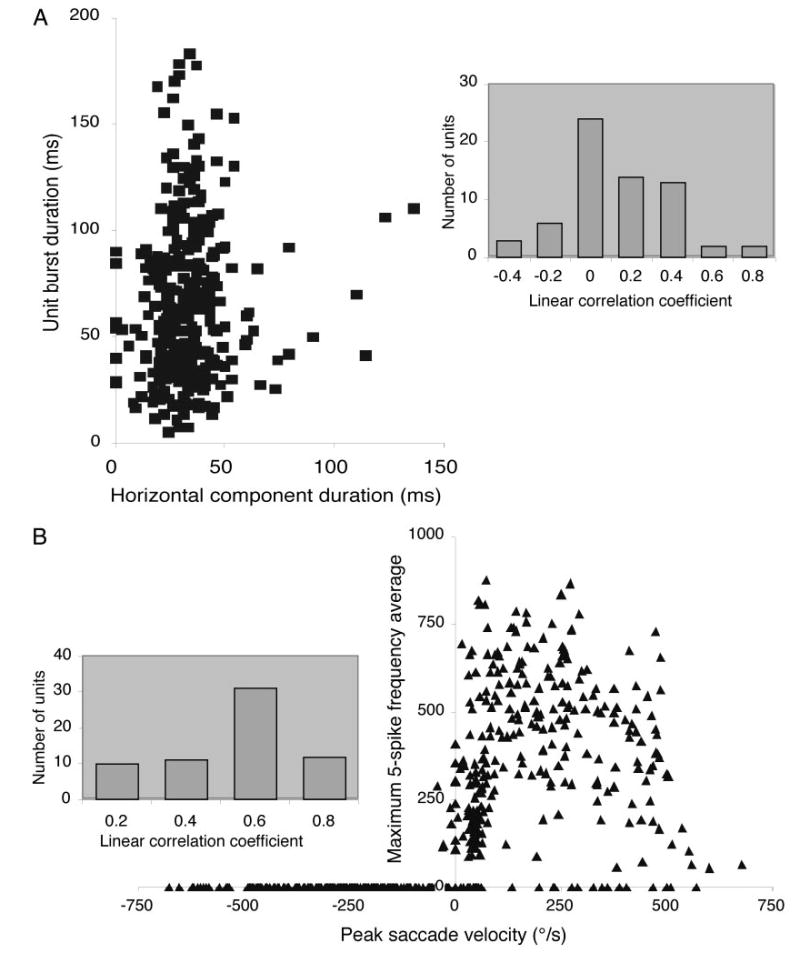
A: scatterplot of burst duration and saccade duration. B: scatterplot of burst frequency and saccade velocity. Insets: distribution of correlation coefficients for the population of dLLBNs.
Likewise, traditional linear regression did not effectively characterize the discharge of dLLBNs as it did for eLLBNs (Fig. 3) and, to a lesser extent, for nrtp LLBNs (Fig. 4). The distribution of correlation coefficients for dLLBNs is shown in Fig. 6 (insets). The distribution for the correlations between burst and saccade duration is centered near zero (average = 0.03 ± 0.25). The distribution of correlation coefficients between peak discharge and peak velocity is not as low (Fig. 6B, inset) but the scatterplot (Fig. 6B) shows clearly that the distribution is not linear. Thus I will propose a more appropriate analysis in the DISCUSSION (Fig. 9). The ON-direction was estimated using traditional methods (cf. Henn and Cohen 1976; Kaneko et al. 1981; King and Fuchs 1979). The number of spikes in the burst (Fig. 7, squares) or the peak firing frequency (Fig. 7, dots) was plotted as a function of saccade direction and the scatterplots were fit with a Gaussian of the form
FIG. 9.
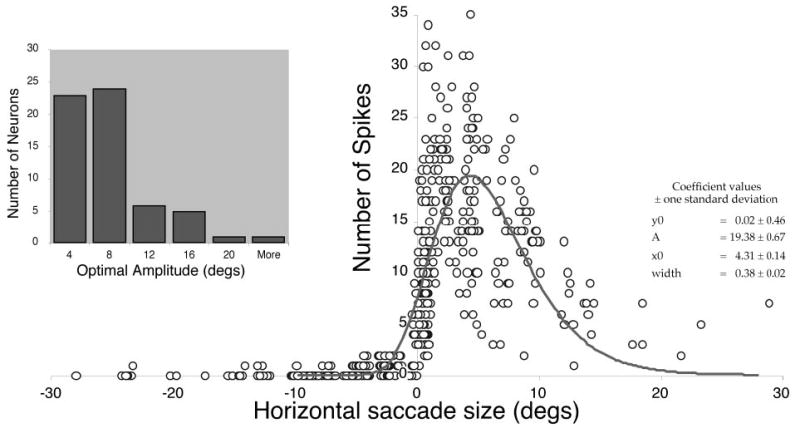
Amplitude tuning of dLLBNs. Plots of number of spikes as a function of horizontal saccade size were fit with log-normal function (inset) as described in the text. Frequency histogram plots the optimal amplitude thus estimated for the population of dLLBNs.
FIG. 7.
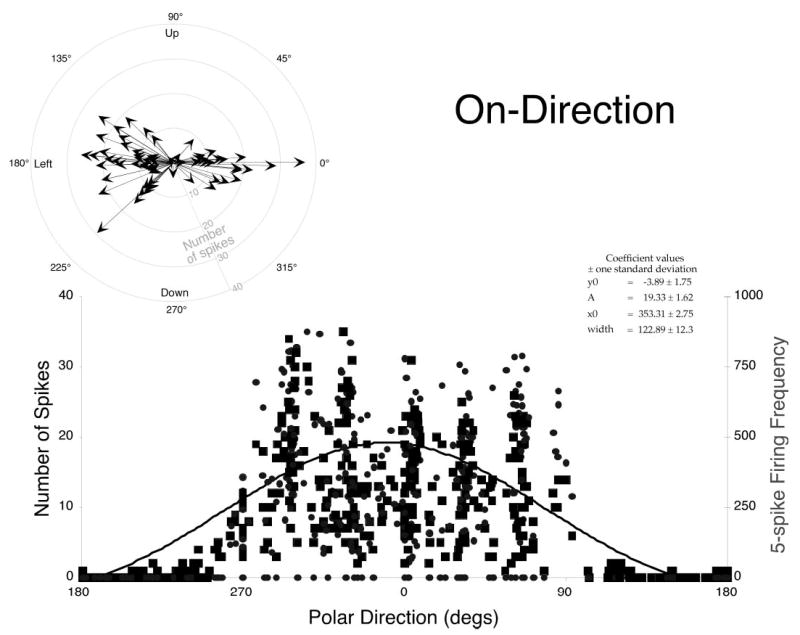
ON-Directions of dLLBNs. Plots of number of spikes (dark squares) or firing frequency (gray triangles) as a function of the polar direction of the associated saccade (0° = right, 90° = up, etc.) were fit with a Gaussian curve (inset) as described in text. Polar plot (top left) summarizes optimal direction (ON-direction) for each dLLBN. Length of arrow indicates peak discharge (gray scale). All dLLBNs had ipsilateral ON-directions (either left or right).
where y0 is the burst metric, x is the polar direction of the associated saccade, and x0 is the ipsilateral direction (0 = right and 180 = left). All saccades (i.e., all directions and amplitudes) were included in these fits.
The distribution illustrates two points. First, dLLBNs discharge for all ipsilateral saccades, and second, the center of the Gaussian is a reasonable estimate of the center of that distribution. The results of this process are summarized in a polar plot for the 64 dLLBNs (Fig. 7, inset). The direction is given as the polar angle and the amplitude represents the peak number of spikes as indicated by the peak of the Gaussian (note, these are not vector representations of the movement fields). Except for one downward, weakly discharging neuron, all have optimal directions within ±45° of the horizontal, just like EBNs and eLLBNs. The average width of the fit Gaussian curves was 96 ± 37°, confirming the expanse of their discharge sensitivity. This distribution is similar to that for short-lead BNs (Henn and Cohen 1976; Kaneko et al. 1981; Scudder et al. 1988; Strassman et al. 1986a,b). Such fits emphasize the fact that the BNs discharge for all saccades that have an ipsilateral (and even a small contralateral) component. However, the fits minimize the fact that the discharge is not constant for all ipsilateral saccades even if they are the same amplitude.
To visualize this decreased discharge as the saccade direction becomes more vertical, I also plotted the number of spikes three-dimensionally as a function of both horizontal and vertical amplitude (Fig. 8). Such plots reiterate the salient features of the discharge of dLLBNs. When viewed from the OFF-direction (top row), it is clear that the maximum dLLBN discharge is more narrow and peaked than either eLLBNs (Fig. 8C) or nrtp LLBNs (Fig. 8B). As shown in the top-down view (Fig. 8, bottom row), the discharge is maximal for small-amplitude (red dots between 0 and 10°) ipsilateral saccades (cf. Figs. 8A, middle, and 9). In contrast, eLLBNs that also discharge for ipsilateral saccades are more broadly tuned (compare yellow and red areas) and discharge increasingly for larger amplitudes (Figs. 2C and 8C, middle and bottom).
To quantify the amplitude tuning of dLLBNs, I examined their discharge in several other ways (not illustrated). No significant relations between burst duration and saccade duration could be gleaned from either rasters of the raw data, histograms, or smoothed discharge (e.g., spike density function). The peak discharge of dLLBNs, unlike that of nrtp LLBNs, was for ipsilateral saccades of very small amplitude, as noted in the preceding paragraph (Fig. 8, cf. A and B). For the dLLBN shown in Fig. 9, this is illustrated in a two-dimensional plot. The number of spikes in such scatterplots was fitted with a log-normal curve (Fig. 9, right), which provided a reasonable fit to the data but slightly overestimated the size of the optimal horizontal saccade. The distribution of peak amplitudes calculated in this manner is shown on the left. Of the 64 dLLBNs recorded in this study, 56 discharged maximally for saccades of <10° (Fig. 9, inset).
Visual responses
Finally, like most types of LLBNs, dLLBNs occasionally showed a visual response. The saccades plotted in Fig. 5A are realigned on target onset in Fig. 5B (vertical dotted line). A distinct initial discharge peaking at about 80 ms after the target step is clearly visible. Eighteen dLLBNs were tested to confirm the visual response using the delayed or remembered saccade paradigm. The visual response in both dLLBNs and eLLBNs assessed during normal tracking could be either more or less distinct than that assessed during remembered or delayed saccades. The average time to peak for 17 neurons was about 75 ms (range 65–90 ms), although three dLLBNs had bimodal responses with a second peak at about 115 ms and one dLLBN showed a distinct long-latency response at 120 ms.
DISCUSSION
The characteristics of LLBNs constrain their possible role in saccade generation. I will briefly consider the three types of LLBNs, then turn to which hypotheses for their saccadic function are best supported by the present data and end with a more general discussion of the structure of the saccadic burst generator.
This survey of the rostral pons revealed only three distinct groups of LLBNs. Rarely did I encounter LLBNs that did not fit in one of these categories, and those were usually of an intermediate type, possessing characteristics of dLLBNs and eLLBNs (e.g., peak discharge for small-amplitude ipsilateral saccades like dLLBNs but overall increasing discharge for larger saccades as well like eLLBNs; n = 2) or had poor and/or inconsistent saccade-related activity. The eLLBNs discharged for ipsilateral saccades and this discharge, like that of short-lead BNs, was correlated with the metrics of the saccade. The nrtp LLBNs had a variety of ON-directions, including oblique, and showed lower correlations. Finally, dLLBNs were ipsilateral, broadly directionally tuned, and not pan-directional like nrtp LLBNs; they usually were amplitude tuned for small amplitudes and showed a greatly prolonged discharge.
In general, the fact that the irregular prelude of presaccadic and intersaccadic discharge of LLBNs was never associated with an eye movement suggests that such discharge is ineffective in activating their target neurons. The lack of effectiveness may be due to low synaptic impact, antagonistic synaptic input to target neurons, or a combination of the two. I did not check for subthreshold LLBN effects as has been done in the SC (e.g., Glimcher and Sparks 1993) because nrtp LLBNs most likely project to the cerebellum (Scudder et al. 1996) and because the discharge of dLLBNs is not sensitive to small changes in saccade direction. Any such influences are likely to affect only threshold for activation because pontine eLLBNs and dLLBNs are not as directionally tuned as are neurons in the SC.
The role of LLBNs in saccade generation
I have assumed that eLLBNs may be EBNs that provide a portion of the excitatory drive to motoneurons that underlies the saccade. The evidence for this contention is circumstantial. Certainly there are LLBNs in both the IBN (Scudder et al. 1988; Strassman et al. 1986b) and EBN areas (Strassman et al. 1986a) that project to the abducens nuclei. In addition, neurons in the rostral pons are labeled following very small injections of horseradish peroxidase in the abducens nucleus (Langer et al. 1986) and intracellularly stained LLBNs project to the edges of the abducens nuclei (Scudder et al. 1996). Thus it seems that at least some of the recorded LLBNs probably project to the abducens. The most likely group are the eLLBNs whose discharge metrics are well related to saccade metrics.
Neurons in the nrtp have been found to project almost exclusively to the cerebellum, and this likely includes the nrtp LLBNs (Crandall and Keller 1985; Hepp and Henn 1983). At least a few nrtp projection neurons have been positively identified as LLBNs (Scudder et al. 1996). Their most likely role in saccade generation involves feedforward control of ongoing saccades (e.g., Lefèvre et al. 1998; see Scudder et al. 2002 for discussion), adaptive gain control of saccade amplitude (e.g., Vilis and Hore 1981), or both. Current data do not allow a choice between these alternatives. Thus we need more information to discern the function of nrtp LLBNs.
Only the dLLBNs are left to subserve the various saccadic functions previously attributed to LLBNs (see INTRODUCTION). The present data are most consistent with only one of these functions and specifically contradict several of those previously proposed. The lead time and variability of dLLBN discharge are consistent with their playing a role in triggering saccades. In alert cats, intracellular recording of OPNs (Yoshida et al. 1999) showed that half the OPNs have an initial slow, small hyperpolarization before saccades. All OPNs then show an abrupt, large decrease in membrane potential that decays in parallel with eye velocity. These two latter stages of OPN inhibition may correspond to, respectively, the actions of the inhibitory trigger that initiates saccades and the intrasaccadic inhibition that latches off the discharge of OPNs during the course of a saccade. The timing of the small and steep hyperpolarizations is compatible with the lead of dLLBNs. The abrupt hyperpolarization leads eye movement by nearly 24 ms, whereas the average lead of the intense burst in dLLBNs is 29 ± 22 ms. The initial slow, small hyperpolarization appeared in half the OPNs, and more than half of the dLLBNs (25/64) had a significantly extended prelude of activity. The slow portion led the steep hyperpolarization by 20 –30 ms. This is slightly less than the average lead of those dLLBNs that showed a prelude of activity of 34 ms longer than the overall average (i.e., 53 ± 18 ms). In addition, dLLBNs appear to project to the OPN region (Langer and Kaneko 1990; Scudder et al. 1996) and electrical stimulation at the site of LLBN recordings in cats results in inhibition of OPNs (Kamogawa et al. 1996).
Two aspects of dLLBN discharge are superficially inconsistent with their acting as trigger neurons. First, the discharge is tuned for small saccades, and second, it is prolonged beyond the end of the saccade. However, in his model of the saccade generator, Scudder (1988) proposed that saccades are initiated by a combination of excitatory drive to the EBNs and a weak trigger signal to inhibit OPNs. He found that the excitatory input to the burst generator was sufficient to initiate most saccades and that the use of a trigger signal to inhibit OPNs was necessary only to improve the trajectory of smaller saccades so that they appeared more normal. Thus the tuning of dLLBNs for small amplitudes is consistent with Scudder’s model, which uses a weak trigger for initiation of small saccades. As such, the prolonged discharge of dLLBNs would not be expected to have much of an effect on OPNs in the absence of strong excitation of EBNs. Consistent with this hypothesis, virtually every dLLBN showed instances in which the prolonged discharge lasted until the subsequent corrective saccade brought the eye on target, at which point they abruptly ceased discharging. Thus it seems that the prolonged discharge may facilitate successive corrective saccades. The prolonged dLLBN discharge may also underlie the lengthened initial interspike intervals seen in OPN discharge at the end of the saccade (Evinger et al. 1981).
For three reasons, dLLBNs probably do not play a role in a saccadic latch circuit. First, their discharge often appreciably outlasts the saccade; second, the duration of the saccadic burst is poorly correlated with the duration of the saccade (r = 0.03 on average); and third, the peak burst frequency is poorly correlated with saccade velocity. Yoshida et al. (1999) conjectured that their intracellularly recorded, hyperpolarization trajectory reflected the hypothesized latch circuit because BNs are known to have high correlations between discharge frequency and saccade velocity. The dLLBNs recorded in this study showed poor correlations between discharge rate and saccade velocity, and they discharged minimally for large saccades beyond their peak amplitude. Thus if the declining hyperpolarization of the OPN membrane does reflect the effects of the latch, dLLBNs cannot fulfill that function. Nonetheless, our data cannot completely rule out the possibility that dLLBNs inhibit OPNs between saccades if, for example, the latch is necessary only during the initial portions of the saccade or is effective only during the high-frequency burst.
The discharge of dLLBNs is not an appropriate substitute for collicular input to the saccadic burst generator. The poor correlations between dLLBN discharge and the concurrent saccade metrics, their prolonged discharge before and after the saccade, and their amplitude tuning are inappropriate for such a function. Instead, I speculate that the eLLBNs provide a portion of this function. The correlations between eLLBN activity and saccade metrics are higher and at least some of the eLLBNs are intermingled with dLLBNs. The known anatomy is consistent with eLLBNs subserving a relay function because LLBNs in their location (Fig. 2) project to both EBN and IBN areas (Scudder et al. 1996). In addition, eLLBNs were encountered in a patchlike distribution corresponding to the cortical input to this region, which is also patchlike (Yan et al. 1999), so they are appropriately distributed for relaying more central inputs.
The qualitatively different discharge patterns and the paucity of intermediate discharge types, as well as the overlapping spatial distribution, are inconsistent with the serial processing hypothesis of Hepp and Henn (1983). They distinguished a group of LLBNs, which they called V (for vector) neurons, whose discharge characteristics and distribution in the rostral pons were similar to those of dLLBNs. They proposed that these V neurons were distinct from more caudally located D (for directional) neurons and that both V and D neurons acted as sequential intermediary processors between the SC and short-lead BNs. I was unable to distinguish a population of D neurons, but I suspect that the D neurons described by Hepp and Henn may have been either nrtp LLBNs or incompletely characterized dLLBNs because the monkeys in those experiments were not trained and were not required to make a complete spectrum of saccade sizes and directions. In addition, I found that the majority of dLLBNs were tuned for small amplitudes, so it is unclear how they could mediate large saccades since they do not discharge for large amplitudes. A number of recent studies have confirmed the connections of the SC to medium-lead BNs and have found direct (Chimoto et al. 1996; Izawa et al. 1999) and disynaptic (Keller et al. 2000) connections, further diminishing the likelihood that serial processing is fundamental to saccade production.
Last, the quantitative analysis of dLLBN discharge is not consistent with their providing a local feedback signal or embodying the resettable integrator. The dLLBNs in this study did not discharge for the complete spectrum of saccade amplitudes (Fig. 7) and their discharge was poorly related to saccade metrics (Fig. 5). Also, intracellular staining of four potential dLLBNs (Scudder et al. 1996) did not reveal recurrent collaterals required by the feedback scheme (Scudder 1988). Nonetheless, dLLBNs may provide feedback of saccade-related discharge in a manner that is different from that conceived in most previous models of local feedback. We recently demonstrated that the SC receives feedback from the brain stem saccade generator (Soetedjo et al. 2002a). That feedback prolongs the saccade-related discharge of SC neurons during long-duration saccades. Although the prolonged discharge of dLLBNs is consistent with such a function, the poor correlation between burst and saccade duration is not. In addition, dLLBNs are involved in horizontal saccade production because they are all ipsilateral, although the feedback to the SC is active for vertical saccades as well (Soetedjo et al. 2002a).
The structure of the saccadic burst generator
The present findings and previous investigations of both the nucleus prepositus hypoglossi (nph) and burster-driving neurons (Kitama et al. 1992a,b, 1995) as possible sources of local feedback virtually eliminate all plausible brain stem sources for such a signal. Earlier I showed that the often-assumed source (e.g., Guitton et al. 1990), the nph, cannot supply eye-position feedback for saccade control (Kaneko 1997) and that horizontal burster-driving neurons do not exist in the monkey (Kaneko and Fukushima 1998). Scudder (1997) showed that feedback does not arise from the EBNs as originally hypothesized in the Robinson model. In a study that seems to contradict results reported by Scudder (1997), Barton et al. (2003) reversibly inactivated the rostrolateral pons, not the primary EBN area as Scudder did, and concluded that the (rostral) pons did participate in the feedback circuit. This conclusion is inconsistent with the current findings that LLBNs cannot support a feedback signal that controls the amplitude of the saccade as originally envisioned (Robinson 1975).
Results of behavioral experiments are inconsistent but several recent studies (see especially Schlag et al. 1995) strongly imply that the error signal that mediates on-line compensation (which demonstrates the existence of a feedback signal) can be present early in the process of saccade generation, such as in the lateral intraparietal area (Duhamel et al. 1992) or the frontal eye fields (Umeno and Goldberg 1997). It may then be relayed to the saccade generator to be summed with or to replace the initial error signal, in some cases inappropriately leading to inaccurate saccades. Although these and similar studies do not eliminate the possibility that local feedback is also used to control the saccade, they do seem to make it superfluous.
Perhaps as telling as the lack of anatomical substrate and the contradictory behavioral data is the fact that other studies have demonstrated parallel inputs to the saccade generator that are not within a local feedback loop. Behavioral studies (Keller et al. 1996; Stanford and Sparks 1994) have shown that the normal tight coupling between collicular discharge and saccades can be dissociated, thus implying other sources of saccade-related input that directly affect either the burst generator or the motoneurons. Scudder (1997) showed that the caudal fastigial nucleus projects monosynaptically to EBNs. In addition, the SC can project directly to EBNs (Chimoto et al. 1996), despite exerting their primary influence on EBNs at disynaptic latencies (Miyashita and Hikosaka 1996). The monosynaptic pathway bypasses LLBNs entirely, so it appears that some LLBNs act as only one of the parallel inputs.
These arguments notwithstanding, it is difficult to reject completely the local-feedback hypothesis of saccade amplitude control. This is because of the robustness of saccade accuracy in the face of on-line perturbation (e.g., Sparks and Mays 1983). Likewise, our recent findings have demonstrated a moment-to-moment feedback of ongoing saccade metrics to the SC (Soetedjo et al. 2002a), which requires the existence of at least a duration-related signal that could be used for feedback control. The source and pathway for this feedback remain a mystery. Thus as outlined in our recent review (Scudder et al. 2002), we now assume that saccades are largely ballistic but that their accuracy can be improved somewhat on-line. This improvement may be provided by multiple and recurrent pathways such as the cortical pathways mentioned above, as well as by feedback to the SC and, most likely, by cerebellar circuits involving the nrtp. These separate pathways may control different aspects of the saccade, such as its duration (Soetedjo et al. 2002a) or velocity (Edelman and Goldberg 2002), and thereby maintain saccade accuracy.
In conclusion, the different groups of LLBNs in the rostral pons described here seem to subserve separate saccadic functions. First, locally terminating dLLBNs probably mediate a trigger to suppress OPN discharge during small saccades. Second, nrtp LLBNs likely relay collicular-like activity to the cerebellum whose function is still unknown but may be involved in adaptive gain control (e.g., Fuchs et al. 1993), accuracy (e.g., Scudder et al. 2002), or both. They are unlikely to mediate feedback control of saccade amplitude (Lefèvre et al. 1998; Quaia et al. 1999) because the discharge of nrtp LLBNs is poorly correlated with saccade metrics (Fig. 3), although they may be important in adjusting final amplitude of largely ballistic saccades (Scudder et al. 2002). Finally, caudally projecting eLLBNs provide parallel inputs to EBNs along with, for example, the SC. Those inputs likely relay both cortical and collicular inputs.
Acknowledgments
The superb technical assistance of J. Balch and the editorial assistance of K. Elias are gratefully acknowledged. Drs. A. F. Fuchs, T. Knight, L. Ling, J. O. Phillips, F. R. Robinson, and R. Soetedjo made useful comments on an earlier version of the manuscript.
Footnotes
GRANTS
This research was funded by National Institutes of Health Grants EY-06558 and RR-00166.
References
- Albano E, Wurtz RH. Deficits in eye position following ablation of monkey Macaca mulatta superior colliculus pretectum and posterior medial thalamus. J Neurophysiol. 1982;48:318 –337. doi: 10.1152/jn.1982.48.2.318. [DOI] [PubMed] [Google Scholar]
- Barton EJ, Nelson JS, Gandhi NJ, Sparks DL. Effects of partial lidocaine inactivation of the paramedian pontine reticular formation on saccades of macaques. J Neurophysiol. 2003;90:372–386. doi: 10.1152/jn.01041.2002. [DOI] [PubMed] [Google Scholar]
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Chimoto S, Iwamoto I, Shimazu H, Yoshida K. Monosynaptic activation of medium-lead burst neurons from the superior colliculus in the alert cat. J Neurophysiol. 1996;75:2658 –2661. doi: 10.1152/jn.1996.75.6.2658. [DOI] [PubMed] [Google Scholar]
- Crandall WF, Keller EL. Visual and oculomotor signals in nucleus reticularis tegmenti pontis. J Neurophysiol. 1985;54:1326 –1344. doi: 10.1152/jn.1985.54.5.1326. [DOI] [PubMed] [Google Scholar]
- Duhamel J-R, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90 –92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Goldberg ME. Effect of short-term saccadic adaptation on saccades evoked by electrical stimulation in the primate superior colliculus. J Neurophysiol. 2002;87:1915–1923. doi: 10.1152/jn.00805.2000. [DOI] [PubMed] [Google Scholar]
- Edwards SB, Henkel CK. Superior colliculus connections with the extraocular motor nuclei in the cat. J Comp Neurol. 1978;179:451– 468. doi: 10.1002/cne.901790212. [DOI] [PubMed] [Google Scholar]
- Evinger C, Kaneko CRS, Fuchs AF. Oblique saccadic eye movements of the cat. Exp Brain Res. 1981;41:370 –379. doi: 10.1007/BF00238895. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kaneko CRS, Scudder CA. Brainstem control of saccadic eye movements. Annu Rev Neurosci. 1985;8:307–337. doi: 10.1146/annurev.ne.08.030185.001515. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068 –1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J Neurophysiol. 1993;70:1723–1740. doi: 10.1152/jn.1993.70.5.1723. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Kaneko CRS, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Prog Neurobiol. 1992;39:609 – 639. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Effects of low-frequency stimulation of the superior colliculus on spontaneous and visually guided saccades. J Neurophysiol. 1993;69:953–964. doi: 10.1152/jn.1993.69.3.953. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol. 1990;64:509 –531. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Henn V, Cohen B. Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res. 1976;108:307–325. doi: 10.1016/0006-8993(76)90188-8. [DOI] [PubMed] [Google Scholar]
- Hepp K, Henn V. Spatio-temporal recoding of rapid eye movement signals in the monkey paramedian pontine reticular formation (PPRF) Exp Brain Res. 1983;52:105–120. doi: 10.1007/BF00237155. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kawakami T. Inhibitory reticular neurons related to the quick phase of vestibular nystagmus—their location and projection. Exp Brain Res. 1977;27:377–396. doi: 10.1007/BF00235511. [DOI] [PubMed] [Google Scholar]
- Izawa Y, Sugiuchi Y, Shinoda Y. Neural organization from the superior colliculus to motoneurons in the horizontal oculomotor system of the cat. J Neurophysiol. 1999;81:2597–2611. doi: 10.1152/jn.1999.81.6.2597. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kamogawa H, Ohki Y, Shimazu H, Suzuki I, Yamashita M. Inhibitory input to pause neurons from pontine burst neuron area in the cat. Neurosci Lett. 1996;203:163–166. doi: 10.1016/0304-3940(95)12285-0. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS. The effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus monkeys. J Neurophysiol. 1996;75:2229 –2242. doi: 10.1152/jn.1996.75.6.2229. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. I. Saccades and fixation. J Neurophysiol. 1997;78:1753–1768. doi: 10.1152/jn.1997.78.4.1753. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys. II. Smooth pursuit, vestibular and optokinetic responses. J Neurophysiol. 1999;81:668 – 681. doi: 10.1152/jn.1999.81.2.668. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS, Evinger C, Fuchs AF. Role of cat pontine burst neurons in generation of saccadic eye movements. J Neurophysiol. 1981;46:387– 408. doi: 10.1152/jn.1981.46.3.387. [DOI] [PubMed] [Google Scholar]
- Kaneko CRS, Fuchs AF. Inhibitory burst neurons in alert, trained cats: comparison with excitatory burst neurons and functional implications. In: Progress in Oculomotor Research, edited by Fuchs AF and Becker W. New York: Elsevier. Dev Neurosci. 1981;12:63–70. [Google Scholar]
- Kaneko CRS, Fukushima K. Discharge characteristics of vestibular saccade neurons in alert monkeys. J Neurophysiol. 1998;79:835– 847. doi: 10.1152/jn.1998.79.2.835. [DOI] [PubMed] [Google Scholar]
- Keller EL, Gandhi NJ, Weir PT. Discharge of superior collicular neurons during saccades made to moving targets. J Neurophysiol. 1996;76:3573–3577. doi: 10.1152/jn.1996.76.5.3573. [DOI] [PubMed] [Google Scholar]
- Keller EL, McPeek RM, Salz T. Evidence against direct connections to PPRF EBNs from SC in the monkey. J Neurophysiol. 2000;84:1303–1313. doi: 10.1152/jn.2000.84.3.1303. [DOI] [PubMed] [Google Scholar]
- King WM, Fuchs AF. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol. 1979;42:861– 876. doi: 10.1152/jn.1979.42.3.861. [DOI] [PubMed] [Google Scholar]
- Kitama T, Ohki Y, Shimazu H, Tanaka M, Yoshida K. Site of interaction between saccade signals and vestibular signals induced by head rotation in the alert cat: functional properties and afferent organization of burster-driving neurons. J Neurophysiol. 1995;74:273–287. doi: 10.1152/jn.1995.74.1.273. [DOI] [PubMed] [Google Scholar]
- Kitama T, Ohki Y, Shimazu H, Yoshida K. Characteristics of medullary neurons that drive bursters for horizontal rapid eye movements in the alert cat. In: Shimazu H, Shinoda Y, editors; Karger S, editor. Vestibular and Brain Stem Control of Eye, Head and Body Movements. Tokyo: Japan Scientific Societies Press/Basel; 1992a. pp. 147–155. [Google Scholar]
- Kitama T, Shimazu H, Tanaka M, Yoshida K. Vestibular and visual interaction in generation of rapid eye movements. Ann NY Acad Sci. 1992b;656:396 – 407. doi: 10.1111/j.1749-6632.1992.tb25224.x. [DOI] [PubMed] [Google Scholar]
- Langer TP, Kaneko CRS. Brainstem afferents to the oculomotor omni-pause neurons in monkey. J Comp Neurol. 1990;295:413– 427. doi: 10.1002/cne.902950306. [DOI] [PubMed] [Google Scholar]
- Langer TP, Kaneko CRS, Scudder CA, Fuchs AF. Afferents to the abducens nucleus in the monkey and cat. J Comp Neurol. 1986;245:379 – 400. doi: 10.1002/cne.902450307. [DOI] [PubMed] [Google Scholar]
- Lefèvre P, Quaia C, Optican LM. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Networks. 1998;11:1175–1190. doi: 10.1016/s0893-6080(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972;35:445– 461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Miyashita N, Hikosaka O. Minimal synaptic delay in the saccadic output pathway of the superior colliculus studied in awake monkey. Exp Brain Res. 1996;112:187–196. doi: 10.1007/BF00227637. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Kitama T, Dalezios Y, Petit J, Brandi AM, Grantyn AA. An anatomical substrate for the spatiotemporal transformation. J Neurosci. 1998;18:10219 –10229. doi: 10.1523/JNEUROSCI.18-23-10219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1997. [Google Scholar]
- Quaia C, Lefèvre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol. 1999;82:999 –1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Raybourn MS, Keller EL. Colliculoreticular organization in primate oculomotor system. J Neurophysiol. 1977;40:861– 878. doi: 10.1152/jn.1977.40.4.861. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implication. Oxford, UK: Pergamon; 1975. pp. 337–374. [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye field and superior colliculus ablations. J Neurophysiol. 1980;44:1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Illusory localization of stimuli flashed in the dark before saccades. Vision Res. 1995;35:2347–2357. doi: 10.1016/0042-6989(95)00021-q. [DOI] [PubMed] [Google Scholar]
- Scudder CA. A new local feedback model of the saccadic burst generator. J Neurophysiol. 1988;59:1455–1475. doi: 10.1152/jn.1988.59.5.1455. [DOI] [PubMed] [Google Scholar]
- Scudder CA. Reduction of excitatory burst neuron (EBN) discharges reduces saccade size: implications for models of saccade generation. Soc Neurosci Abstr. 1997;23:2368. [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol. 1988;59:1430 –1454. doi: 10.1152/jn.1988.59.5.1430. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CRS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142:439 – 462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey. II. Pontine neurons. J Neurophysiol. 1996;76:353–370. doi: 10.1152/jn.1996.76.1.353. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF, Kaneko CRS. Saccade-related neurons in the alert monkey nucleus reticularis tegmenti pontis. Soc Neurosci Abstr. 2002c;28:463.9. [Google Scholar]
- Soetedjo R, Kaneko CRS, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol. 2002a;87:679 – 695. doi: 10.1152/jn.00886.2000. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko CRS, Fuchs AF. Saccade generation by the superior colliculus: evidence against a moving hill of activity. J Neurophysiol. 2002b;87:2778 –2789. doi: 10.1152/jn.2002.87.6.2778. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Functional properties of neurons in the monkey superior colliculus: coupling of neuronal activity and saccade onset. Brain Res. 1978;156:1–16. doi: 10.1016/0006-8993(78)90075-6. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol. 1983;49:45– 63. doi: 10.1152/jn.1983.49.1.45. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Nelson JS. Reversible inactivation of neurons in the paramedian pontine reticular formation (PPRF): effects on latency, velocity, duration and amplitude of horizontal, vertical and oblique saccades. Soc Neurosci Abstr. 1997;23:7. [Google Scholar]
- Stanford TR, Sparks DL. Systematic errors for saccades to remembered targets: evidence for a dissociation between saccade metrics and activity in the superior colliculus. Vision Res. 1994;34:93–106. doi: 10.1016/0042-6989(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986a;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol. 1986b;249:358 –380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- Takeichi N, Kaneko CRS, Fuchs AF. Discharge of monkey nucleus reticularis tegmenti pontis (NRTP) neurons changes during saccade adaptation. J Neurophysiol. 2005;94:1938 –1951. doi: 10.1152/jn.00113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Vilis T, Hore J. Characteristics of saccadic dysmetria in monkeys during reversible lesions of medial nuclei. J Neurophysiol. 1981;46:828 – 838. doi: 10.1152/jn.1981.46.4.828. [DOI] [PubMed] [Google Scholar]
- Yan Y-J, Cui D-M, Lynch JC. Efferent targets of the pursuit subregion of the frontal eye field in cebus monkey include the superior colliculus, pontine nuclei, and caudate nucleus. Soc Neurosci Abstr. 1999;25:1397. [Google Scholar]
- Yoshida K, Iwamoto Y, Chimoto S, Shimazu H. Saccade-related inhibitory input to pontine omnipause neurons: an intracellular study in alert cats. J Neurophysiol. 1999;82:1198 –1208. doi: 10.1152/jn.1999.82.3.1198. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCrea R, Berthoz A, Vidal PP. Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. J Neurophysiol. 1982;48:761–784. doi: 10.1152/jn.1982.48.3.761. [DOI] [PubMed] [Google Scholar]


