Abstract
The stop-signal or countermanding task probes the ability to control action by requiring subjects to withhold a planned movement in response to an infrequent stop signal which they do with variable success depending on the delay of the stop signal. We investigated whether performance of humans and macaque monkeys in a saccade countermanding task was influenced by stimulus and performance history. In spite of idiosyncrasies across subjects several trends were evident in both humans and monkeys. Response time decreased after successive trials with no stop signal. Response time increased after successive trials with a stop signal. However, post error slowing was not observed. Increased response time was observed mainly or only after cancelled (signal inhibit) trials and not after noncancelled (signal respond) trials. These global trends were based on rapid adjustments of response time in response to momentary fluctuations in the fraction of stop signal trials. The effects of trial sequence on the probability of responding were weaker and more idiosyncratic across subjects when stop signal fraction was fixed. However, both response time and probability of responding were influenced strongly by variations in the fraction of stop signal trials. These results indicate that the race model of countermanding performance requires extension to account for these sequential dependencies and provide a basis for physiological studies of executive control of countermanding saccade performance.
Keywords: Serial order, sequential effect, stop signal task, executive control, race model, reaction time, saccade latency
INTRODUCTION
The stop signal or countermanding paradigm, which includes both a task design and a theoretical construct, was developed to investigate the control of action (see Logan, 1994). In addition to examining movement preparation and inhibition, the stop signal task has been used to examine inhibitory control in other contexts, such as inhibition of return (Taylor & Ivanoff, 2003), Stroop and Eriksen flanker tasks (Verbruggen, Liefooghe & Vandierendonck, 2004), and task switching (Verbruggen, Liefooghe, Szmalec & Vandierendonck, 2005). Many investigators have used an oculomotor version of the countermanding task that requires a subject to cancel a planned saccade at various degrees of preparation when presented with an imperative stop signal (Armstrong & Munoz, 2003; Asrress & Carpenter, 2001; Cabel, Armstrong, Reingold & Munoz, 2000; Curtis, Cole, Rao & D’Esposito, 2004; Hanes & Carpenter, 1999; Hanes, Patterson & Schall, 1998; Hanes & Schall, 1995; Kornylo, Dill, Saenz & Krauzlis, 2003; Logan & Irwin, 2000; Paré & Hanes, 2003; Stuphorn, Taylor & Schall, 2000). It is commonly observed across experimental conditions and response modalities that subjects’ response times tend to increase in the context of the countermanding task relative to that in simple response time tasks (e.g., Logan 1981; Logan & Burkell, 1986; van den Wildenberg, van Boxtel, & van der Molen, 2003; Mirabella, Pani, Pare, & Ferraina, 2006; but see Öyzurt, Colonius, & Arndt, 2003).
Based on a race between a GO and a STOP process with independent stochastic finish times, Logan & Cowan (1984) demonstrated that the time needed to cancel a movement, the stop signal reaction time (SSRT), can be estimated from the distribution of response times when no stop signal is presented and the probability of responding given that a stop signal occurred. This race model has been implemented in a linear rise to threshold model framework (Hanes et al., 1999) and in a network of interacting units with delayed potent inhibition (Boucher, Palmeri, Logan & Schall, 2006).
The race model of countermanding performance makes no assumptions regarding the effect of stimulus and performance history on the outcome of subsequent trials. However, a number of studies have shown that the probability of responding and response times vary according to recent trial history in speeded response tasks requiring saccades (Carpenter, 2001; Dorris, Paré & Munoz, 2000; Dorris, Taylor, Klein & Munoz, 1999; Juttner & Wolf, 1992; Kornylo et al., 2003; Paré & Munoz, 1996). Furthermore, post-error slowing in choice tasks has been regarded as evidence of executive control (e.g., Rabbitt, 1966a; Rabbit & Phillips, 1967; Lamming, 1979). In addition to these trial-to-trial variations in response time, human subjects increase response times with increases in the global fraction of stop signal trials, and these changes in response times are accompanied by changes in the probability of responding (Logan, 1981; Logan & Burkell, 1986; Ramautar, Kok, & Ridderinkhof, 2004). Some performance adjustments according to trial history in the stop signal task have been reported for saccades (Cabel et al., 2000; Kornylo et al., 2003; Özyurt, Colonius & Arndt, 2003; Curtis, Cole, Rao & D’Esposito 2005) and for manual responses (Rieger & Gauggel, 1999; Schachar, Chen, Logan, Ornstein, Crosbie, Ickowicz & Pakulak, 2004; Li, Krystal & Mathalon, 2005), but a systematic analysis of sequential effects during saccade countermanding has not been performed.
The purpose of the present study was to determine if and characterize how adjustments in response times in the countermanding task are affected by stimulus (stop signal versus no signal) and performance history (correct versus errant saccades) and if these adjustments lead to a decreased probability of responding on stop signal trials. The results indicate that shifts in the probability of responding are the result of shifts in response time, which are influenced by both recent and long-term trial history. Some of these results have been presented in abstract form (Emeric, Stuphorn & Schall, 2004, 2005; Schall & Taylor 1998).
Methods
Macaque data collection
Data were collected from 4 male rhesus monkeys (Macaca mulatta; 7–12 kg) and 2 male bonnet monkeys (Macaca radiata: 8–10 kg) in two laboratories that were cared for in accordance with USDA and Public Health Service Policy on the humane care and use of laboratory animals. All surgical procedures and electrophysiological techniques have been described previously (Hanes et al., 1998; Paré & Hanes, 2003).
The experiments were under computer control to present stimuli, record eye movements, and deliver reinforcement. Detailed descriptions of the behavioral training and tasks and the methods used to collect these data have been described in detail (Hanes et al., 1995; Hanes et al., 1998; Paré & Hanes, 2003). Eye position was monitored while monkeys were head-restrained and seated in an enclosed chair within a magnetic field via a scleral search coil. The fixation spot subtended 0.25 – 0.30° of visual angle, and the target stimuli subtended between 0.25 and 3.00° of visual angle, depending on their eccentricity and had a luminance of 2,10, or 30 cd/m2 on a <0.1 or 1 cd/m2 background. Each animal was tested for approximately 4 hours a day, 5 days a week. During testing, water or fruit juice was given as positive reinforcement. Access to water in the home cage was controlled and monitored. Fluids were supplemented as needed.
The countermanding task is illustrated in Figure 1. All trials began when the monkey fixated a centrally located target for a variable interval (500–800 ms). Simultaneously, the fixation stimulus was extinguished and a peripheral target was presented at one of two diametrically opposed locations in opposite hemifields, cuing the monkey to make a single saccade to the target. In no stop signal trials, the monkey was reinforced for making a saccade within 500–700 ms to the target and fixating the target for 200–400 ms. On stop signal trials, the central fixation target reappeared after a delay, referred to as the stop signal delay, instructing the monkey to inhibit saccade initiation. This happened on 10–70% of the trials, depending on the block condition. Two outcomes were possible on stop signal trials; the monkey could either make a saccade (known as a noncancelled, or signal-respond, trial) or not (known as cancelled, or signal-inhibit, trials). Monkeys were reinforced for maintaining fixation on the stop signal for 600–700 ms after the stop signal appeared. A saccade to the target on a stop signal trial was incorrect, not reinforced, and resulted in a 1500 ms timeout. Stop signal delays ranged from 25 to 450 ms and were constant within an individual session. Behavioral and neurophysiolgical data from these monkeys has appeared in previous publications (Hanes et al., 1998; Ito, Stuphorn, Brown & Schall, 2003; Paré & Hanes, 2003; Stuphorn et al., 2000). In addition to examining the effects of the local trial history on performance, we systematically manipulated the global proportion of stop signal trials. Behavioral data were obtained from monkey N performing a saccade countermanding when the proportion of stop signal trials was varied between 0.1 and 0.7 from session to session.
Figure 1.
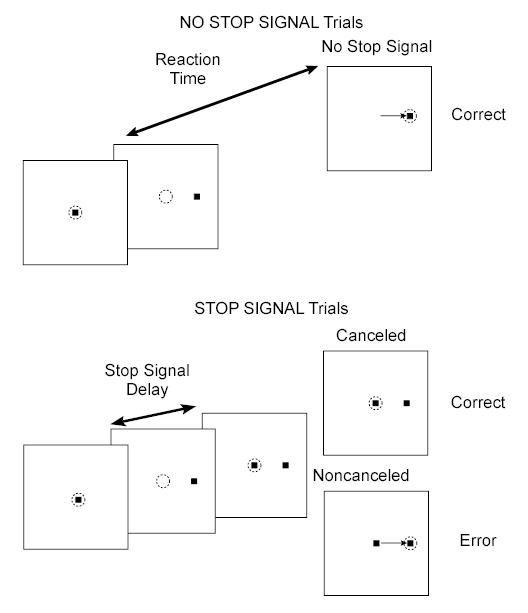
Trial displays for the countermanding task. Dotted circle indicates the focus of gaze at each interval; arrow, the saccade. All trials began with the presentation of a central fixation spot. After fixation of this spot for a variable interval, it disappeared. Simultaneously, a peripheral target appeared. During the trials in which the stop signal was not presented (no stop signal trials), producing a single saccade to the peripheral target is the correct response. During stop-signal trials, after a variable delay, the fixation spot reappears, which is the cue to inhibit/cancel movement initiation. During cancelled trials, fixation was maintained on the central spot for 700 ms. During noncancelled trials, a saccade to the peripheral target is produced.
Human data collection
Human data were collected in two different laboratories from 7 subjects using similar paradigms. Two subjects were from Cambridge University and 5 were from Vanderbilt University. Each subject participated in a minimum of 8 (maximum of 11) sessions. All subjects reported having normal or corrected-to-normal vision. Informed consent was obtained before the experiment began. The Cambridge University Institutional Review Board and the Vanderbilt University Institutional Review Board approved the experimental procedures. The volume of data from 2 of the subjects was insufficient to provide sufficient statistical power for the comparisons examined below and could not contribute to all of the analyses.
Eye position was monitored using either the EyeLink II eye tracker (SR Research, Canada) at a sampling rate of 250 Hz with average gaze position error <0.5°, noise limited to <0.01° RMS with pupil tracking, or an infrared scleral reflection oculometer (for details see Reddi & Carpenter, 2000), sampled at 10msec intervals by a computer system, SPIC (Carpenter, 1994) that also controlled stimulus presentations. Saccades were detected online using conventional velocity and acceleration criteria. For the subjects tested at Vanderbilt University, the fixation and targets subtended 1.0° and were light gray (34 cd/m2) on a darker gray (18 cd/m2) background and the stop signal targets subtended 1.0° and were blue (34 cd/m2), yellow (34 cd/m2), or red (34 cd/m2). For the subjects tested at Cambridge University, the fixation, targets, and stop signal targets subtended 0.22° and were yellow LEDs of luminance 160 cd/m2 on a uniform background of 3 cd/m2. The saccade stimuli were positioned in a horizontal row at a spacing of 4.5 deg on each side of the mid-line; the LEDs were optically superimposed on a uniform background of 3 cd/m2, and were therefore of very high contrast.
All countermanding trials began with the presentation of a central fixation target which was accompanied by a warning tone for two subjects. After a random delay (500–1000 ms) the fixation stimulus went off and an eccentric target appeared at one of 4 random locations (45º from the cardinal positions) equidistant (8.5°) from the central fixation. Subjects were instructed to respond as quickly as possible to the appearance of the target. The remaining 30% of trials were stop signal trials during which the fixation point re-illuminated after a variable delay and indicated to the subject that the response they were instructed to make needed to be inhibited. Subjects were instructed that they would be unable to inhibit approximately half of the stop signal trials. The stop signal delays ranged from 25 to 275 ms in 50 ms steps or 50 to 120 ms in 10 ms steps. Each delay occurred with equal probability.
Primary data analysis
Behavioral data from the countermanding task include the distribution of response times on trials with no stop signal, the distribution of response times on noncancelled trials, and the probability of responding as a function of stop signal delay (SSD) (Logan, Cowan & Davis, 1984). The inhibition function plots the probability of responding as a function of SSD; at the shortest SSD almost all saccades are cancelled, and at the longest SSD almost all saccades are not cancelled. To extract measures of the inhibition function, it was fit with a cumulative Weibull function of the form, W (t) = γ − (γ − δ) · exp ( − ( t / α)β), where t is the time after target presentation, α is the time at which the inhibition function reaches 64% of its maximum value, β is the slope, and γ and δ are the maximum and minimum of the inhibition function, respectively.
Saccades were detected using an algorithm that detects the first significantly elevated velocity (>30º/s) using digital differentiation. Saccade initiation and termination were defined as the beginning and end of monotonic change in eye position before and after the high velocity gaze shift. Trials during which saccades were initiated after the target was presented while the monkey was fixating the central target and terminated on the target were classified as valid trials. For each valid trial, response time was the interval from target presentation to saccade initiation. The mean response time for each subject is the mean of session means and the standard error is the mean of the standard errors across sessions.
For each behavioral session, an estimate of SSRT was determined from the distribution of response times on no stop signal trials and the inhibition function. SSRT can be estimated in at least two ways (Logan et al., 1984). The first method of estimating the SSRT assumes that it is a random variable. Logan and Cowan (1984) showed that the mean SSRT is equal to the difference between the mean reaction time during no stop signal trials and the mean value of the inhibition function. The second method of estimating the SSRT assumes that it is constant. By this method, the SSRT is estimated by integrating the no stop signal saccade response time distribution, beginning at the time of target presentation, until the integral equals the proportion of noncancelled trials at that SSD. Detailed descriptions of these methods have appeared previously (Hanes et al., 1995; Logan & Cowan, 1984; Band van der Molen & Logan, 2003). In practice, these two methods rarely give identical values of SSRT because of noise and unavoidable measurement error. However, if enough trials are collected, then there is no reason to weight one method more than another (Band et al., 2003). Therefore, we identified a single estimate of SSRT from the behavioral data collected during each physiological recording session by averaging the SSRT estimates derived from both methods (see Hanes et al., 1998; Kornylo et al., 2003).
Trial history analysis
Saccadic response times on no stop signal trials were sorted based on the trial history of stimuli and performance and were examined as a function of (1) the number of preceding no stop signal trials, (2) the number of preceding stop signal trials, and (3) whether the preceding stop signal trial was cancelled or noncancelled or was a no stop signal trial. Stop signal trials were sorted according to the same criteria and inhibition functions were derived for each subset of trials. Specifically, stop signal trials were first grouped as either (1) a function of the number of preceding stop signal trials (e.g., preceded by 1, 2, or 3 or more no stop trials) or (2) the type of preceding trial (i.e. cancelled, noncancelled, or no stop signal). Next, each subset of stop signal trials was then grouped by stop signal delay. Finally, inhibition functions were produced for each data subset by determining the proportion of noncancelled trials produced at each stop signal delay. A significant shift in the probability of responding was identified using maximum-likelihood fits of two nested general logistic regression models and by examining the significance of each factor through log-likelihood ratio statistics (Dobson, 1990). Each inhibition function was fitted independently with a logistic regression function with stop signal delay and recent trial history as factors,
where P is the probability that a noncancelled saccade is produced on a stop signal trial, SSD is the value of stop signal delay, NNo-stop trials and NStop trials are the number of preceding no stop signal and stop signal trials respectively. SST is a binary index where 1 or 0 represent the presence or absence of a stop signal on the preceding trial, respectively. Correct is a binary index where 1 and 0 represent if the preceding trial was correct or incorrect. For example, cancelled stop signal trials and no stop signal trials were assigned a value of 1, whereas noncancelled stop signal trials were assigned a value of 0. Finally, b0, b1, and b2 are coefficient estimates of the logistical fit. The residual sum of squares for each of the above model fits was compared to a logistic regression function with only stop signal delay as a factor,
If the residual sum of squares of the model fit without the b2 and b3 coefficients was significantly greater when compared with a chi-square distribution then the amplitude of shift was determined to be significantly different (p < 0.05).
Results
Data consisted of multiple saccade countermanding sessions performed by 6 monkeys and 7 human subjects. Five of the human subjects provided sufficient data for all analyses mentioned below; 2 subjects only contributed to some analyses.
Overall countermanding performance
The probability of responding on a stop signal trial for each monkey (Figure 2A) and human subject (Figure 2B), regardless of the preceding trial events, was an increasing function of the stop signal delay. These inhibition functions are characteristic of performance in this task and demonstrate that all of the subjects were sensitive to the delivery of the stop signal.
Figure 2.
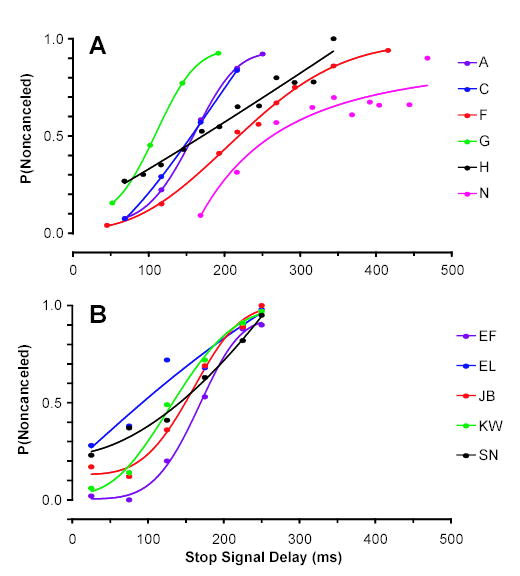
Overall inhibition functions from all stop signal trials across all sessions (A) for all monkeys and (B) for all human subjects. Data points are the probability of responding at each stop signal delay. The data from each individual is fit with a cumulative Weibull function.
Across all 7 human subjects, the mean no stop signal response time was 256 ± 2 ms and ranged from 232 ms to 270 ms (Figure 3, Table 2). The mean noncancelled response time on stop signal trials was 241 ± 5 and ranged from 191 to 293 ms. Across the 5 human subjects from whom we had sufficient data, noncancelled stop signal response times were significantly shorter than response time on no stop signal trials (t(4) = −3.80; p = 0.01). Likewise, across monkeys, the mean no stop signal reaction time was 273 ± 19 ms and ranged from 208 ms to 318 ms (Figure 3, Table 1). The mean noncancelled response time on stop signal trials was 241 ± 15 ms and ranged from 183 to 293 ms. Across monkeys, noncancelled stop signal response times were significantly shorter than response time on no stop signal trials (t(5) = −9.48; p < 0.01). The orderly quality of the inhibition functions and shorter latency noncancelled response time compared to no stop signal response time indicates that both humans and monkeys were performing the task appropriately and justifies further analysis using the race model (Hanes et al., 1999; Hanes et al., 1995; Logan et al., 1984).
Figure 3.
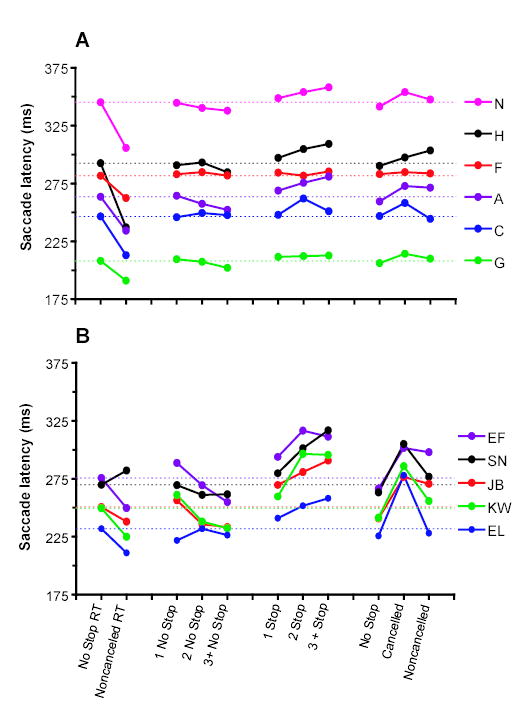
The influence of recent trial history on response time on no stop signal trials. The first columns represent the mean no stop signal response time and the mean noncancelled response time. All other columns represent the mean no stop signal reaction time for trials with the sequences of preceding trials indicated on the abscissa. The mean no stop signal reaction time for each subject is represented by the horizontal dotted line.
Table 2.
Response times of no stop signal trials, noncancelled trials, percent of stop signal trials that were noncancelled, and stop signal reaction time (SSRT) for human subjects.
| Subject | No Stop Signal | Noncancelled | SSRTint | SSRTmean |
|---|---|---|---|---|
| SN | 270 ± 2 | 282 ± 6 | 142 | 103 |
| JB | 251 ± 2 | 238 ± 4 | 150 | 82 |
| KW | 250 ± 2 | 225 ± 4 | 123 | 113 |
| EF | 276 ± 2 | 249 ± 5 | 120 | 114 |
| EL | 232 ± 3 | 211 ± 5 | 124 | 92 |
Values are means ± SE. SSRTint, stop signal reaction time determined using the method of integration. SSRTmean stop signal reaction time determined using the difference between the mean of the inhibition function and the mean of the response time distribution.
Table 1.
Response times of no stop signal trials, noncancelled trials, and stop signal reaction time (SSRT) for each monkey.
| Monkey | No Stop Signal | Noncancelled | SSRTint | SSRTmean |
|---|---|---|---|---|
| A | 256 ± 5 | 229 ± 7 | 94 ± 3 | 95 ± 2 |
| C | 246 ± 3 | 217 ± 6 | 98 ± 6 | 106 ± 5 |
| F | 282 ± 5 | 264 ± 7 | 103 ± 5 | 78 ± 5 |
| G | 208 ± 2 | 191 ± 3 | 95 ± 3 | 96 ± 2 |
| H | 252 ± 4 | 210 ± 8 | 114 ± 8 | 88 ± 4 |
| N | 318 ± 3 | 293 ± 6 | 98 ± 3 | 81 ± 1 |
Values are means ± SE. SSRTint, stop signal reaction time determined using the method of integration. SSRTmean stop signal reaction time determined using the difference between the mean of the inhibition function and the mean of the response time distribution.
The effect of trial history on saccade latency on no stop signal trials
We measured the influence of preceding no stop signal trials on saccade latencies produced in trials with no stop signal (Figure 3). Trials were sorted into groups preceded by one, by two, or by three or more successive trials with no stop signal. Of the five human subjects with sufficient data, four demonstrated a decrease in no stop signal response time as the number of preceding no stop signal trials increased. Across these subjects, there was a significant effect of the number of preceding no stop trials on response time (F (2,4) = 5.30; p = 0.03). Similarly, four of six monkeys demonstrated a decrease in no stop signal response time as the number of preceding no stop signal trials increased. Across all monkeys, there was a significant effect of the number of preceding no stop trials on response time (F(2,5) = 5.59; p = 0.02).
We next measured the influence of preceding stop signal trials on the response time of trials with no stop signal (Figure 3). For five human subjects, no stop signal response time increased as the number of preceding stop signal trials increased. There was a significant effect of the number of preceding stop trials on response time (F(2,4) = 19.49; p < 0.001). For three of six monkeys, there was an increase in the no stop signal response time as the number of preceding stop signal trials increased. Across all monkeys, there was a significant effect of the number of preceding stop signal trials on response time (F(2,5) = 4.27; p = 0.05).
We next measured the influence of the preceding performance history on the response time of trials with no stop signal. Stop signal trials could result in either correct cancelled (signal inhibit) or error noncancelled (signal respond) responses. No stop signal trials were sorted into groups preceded by no stop signal, by a stop signal that resulted in a cancelled saccade, and by a stop signal that resulted in a noncancelled saccade. Figure 3 displays the response time on no stop signal trials as a function of previous trial type.
Recall that we obtained sufficient data from 5 of 7 of the human subjects tested for statistical analysis. It’s worth noting, however, that several trends were apparent in all seven subjects. First, no stop signal response time tended to be greater if immediately preceded by a cancelled stop trial than if preceded by a no stop trial. Second, response time on no stop signal trials were shorter if immediately preceded by a noncancelled stop signal trial than those preceded by a cancelled trial. Third, response times on no stop signal trials were greater following noncancelled trials compared to response time on no stop signal trials following no stop signal trials.
We next performed statistical analyses in the form of t-tests on the data from the five human subjects that we obtained sufficient data. We used a bonferroni corrected alpha level of 0.02 to determine significance. No stop signal response time was significantly greater following cancelled trials compared to no stop signal response time following no stop signal trials (t(4) = −14.04; p<0.01). There was no significant difference in no stop signal response time between trials preceded by a noncancelled trial or a no stop signal trial (t(4) = −3.34; p = 0.03) or between trials preceded by a noncancelled trial or a canceled trial (t(4) = 2.78; p = 0.05).
Data obtained from the six monkeys in this task provided comparable results to the human data. No stop signal response time was significantly greater following cancelled trial compared to no stop signal response time following no stop signal trials (t(5) = −5.05; p < 0.01). There were no significant differences in no stop signal response times between trials preceded by a cancelled trial or a no stop signal trial, (t(5) = 1.20; p = 0.29) or between trials preceded by a noncancelled trial or a canceled trial (t(5) = −2.20; p = 0.08).
Time course of the effect of trial history
The large volume of data collected from the monkeys allowed for an analysis of the time course of the effect of trial history on response times. The moving average of response time was calculated as the mean no stop signal response time for rewarded trials in the preceding 40 trials for each of 516 sessions (Figure 4). Likewise, the proportion of stop signal trials was determined from the fraction of stop signal trials in the same 40-trial window. Previous studies have demonstrated that neuronal activity and behavior in recent trials are weighted more than earlier trials (e.g., Cho, Nystrom, Brown, Jones, Braver, Holmes & Cohen, 2002; Hasegawa, Blitz, Geller & Goldberg, 2000; Sugrue, Corrado & Newsome, 2004). Weighted moving averages were calculated using an exponentially decaying function with time constants of 5 and 20 trials. The cross-correlation sequence of the normalized response time on no stop signal trials and the normalized recent fraction of stop signal trials was determined. For example, if the peak of the cross-correlation sequence occurs at a lag of −10 trials, this implies a response time correlation with stop signal trials that occurs 10 trials in the past. A significant peak correlation was defined as a correlation that exceeded the 99% confidence interval (Chatfield, 1975) that occurred in the trial interval between −15 and +5 trials. This is because correlations outside this window are most likely due to statistical fluctuations in the data.
Figure 4.
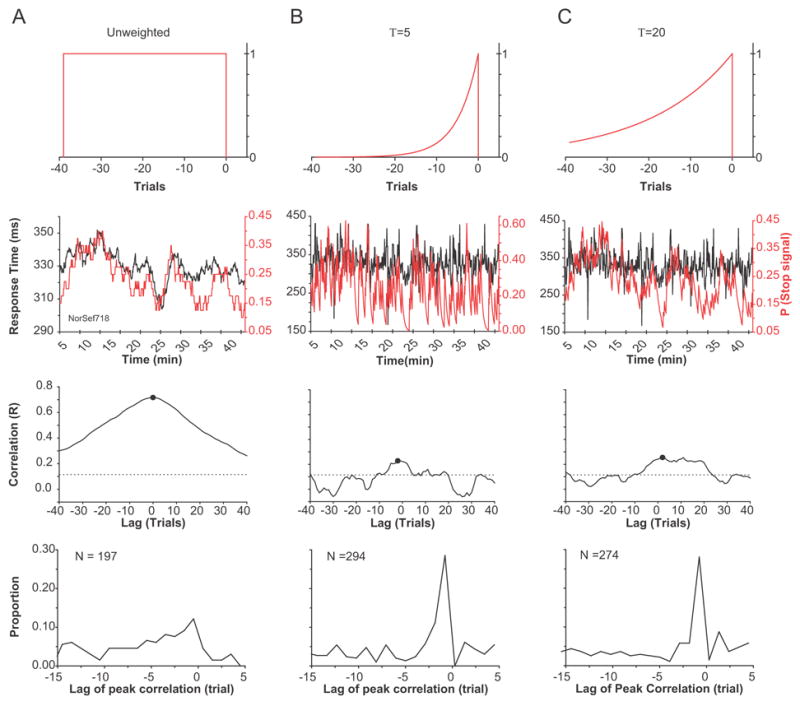
Cross-correlation between moving averages of the fraction of stop signal trials and response time in the preceding 40 trials. (A) Example of temporal correlation between local fraction of stop signal trials and response time. The top row of figures are schematics of the functions used to convolve the response times and stop fractions. The first, second, and third columns represent unweighted and weighted means with time constants of 5 and 20 trials, respectively. (B) Correlation coefficient of stop fraction with response time shifted the number of trials at that point on the ordinate. The circle is the maximum correlation coefficient. The dashed line defines the two-tailed 99% confidence limit. (C). Distribution of the lags at which the cross correlation between moving averages of response time and the fraction of stop signal trials was maximized.
In 197 sessions of the 516 sessions examined, the unweighted moving averages of no stop signal response time correlated significantly with the moving averages of the fraction of stop signals. For sessions in which the moving averages were weighted with a time constant of 5 trials, the response time correlated significantly with the fraction of stop signal trials in 294 sessions. For sessions in which the moving averages were weighted with a time constant of 20 trials, the response time correlated significantly with the fraction of stop signal trials in 274 sessions. The mean significant correlation coefficient for the entire data set, unweighted and weighted with time constants of 5 and 20 trials, was 0.30, 0.15, 0.15, respectively. The maximum correlation for all three methods of computing the moving averages occurred at a shift of −1 trial. Regardless of whether distantly preceding trials were weighed more (unweighted or weighted with a time constant of 20 trials) or less (weighted with a time constant of 5 trials), response time on no stop signal trials was affected most by the immediately preceding trial.
The effect of trial history on canceling
To determine if the recent fraction of stop signal trials influences the probability of responding, a logistic regression with factors stop signal delay and the recent trial history was performed. The results are plotted in Figure 5. The probability of responding significantly increased for two of six monkeys (monkeys A and G) as the number of preceding no stop signal trials increased. The probability of responding significantly decreased for two of six monkeys (monkeys G and F) as the number of preceding stop signal trials increased. For three of six monkeys (monkeys A, G, and N), the probability of responding was greatest if preceded by a no stop signal trial, less if preceded by a noncancelled trial, and least if stop signal trials were preceded by cancelled trials.
Figure 5.
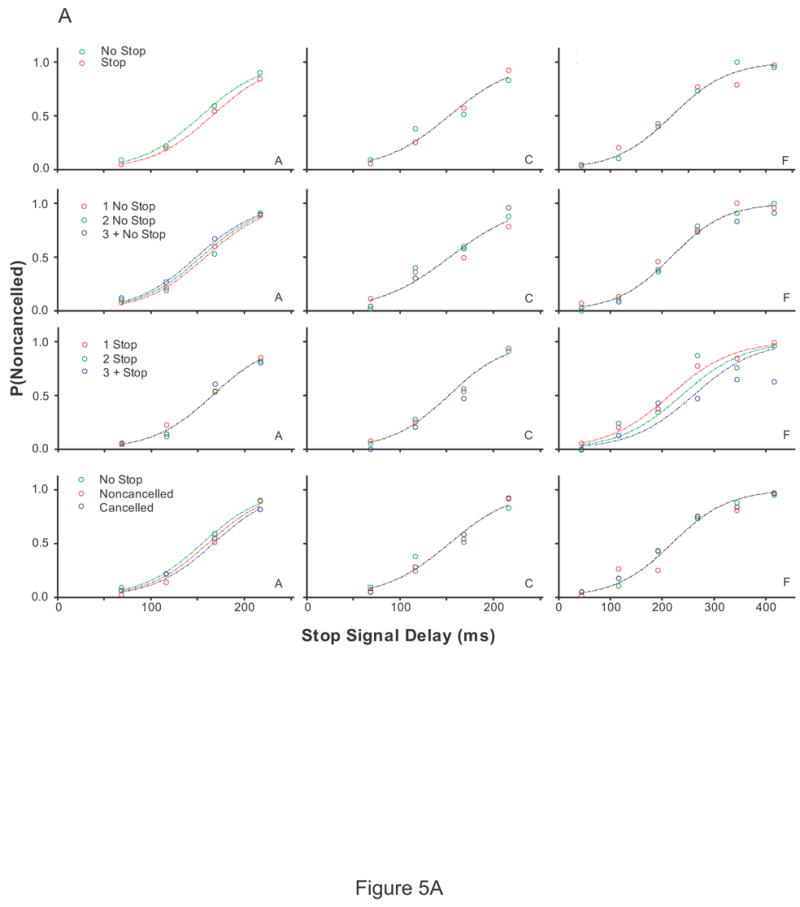
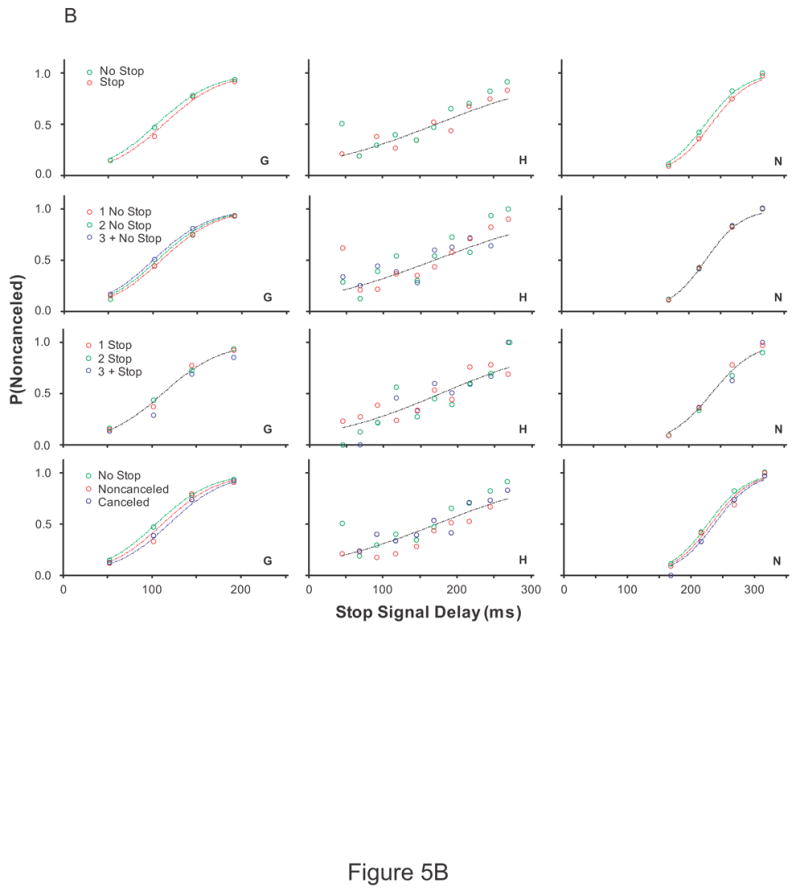
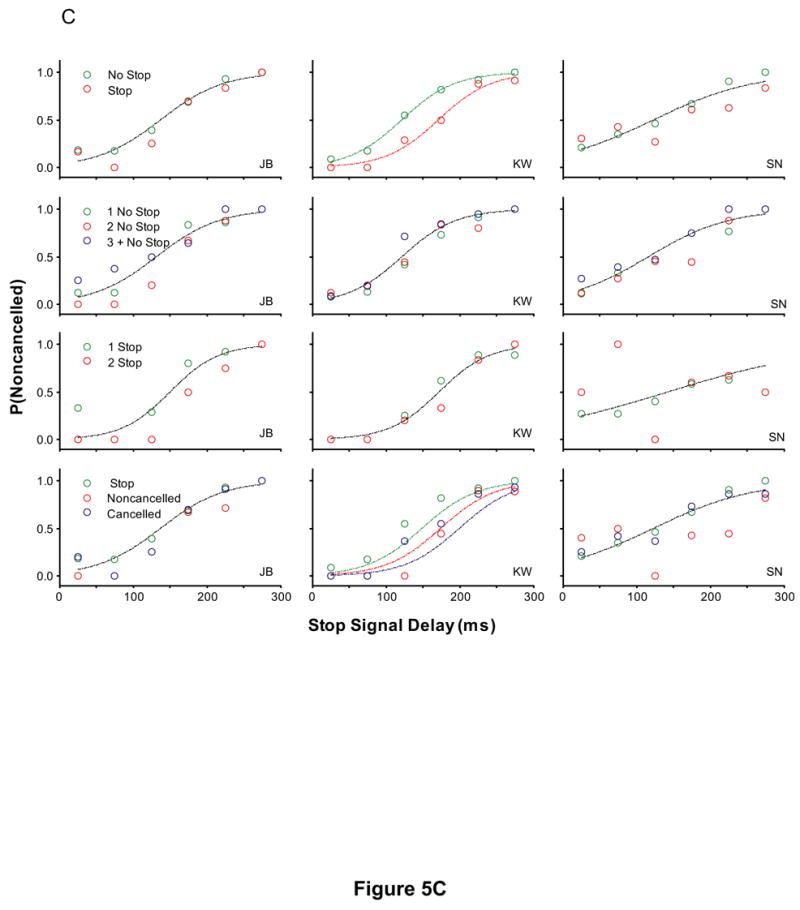
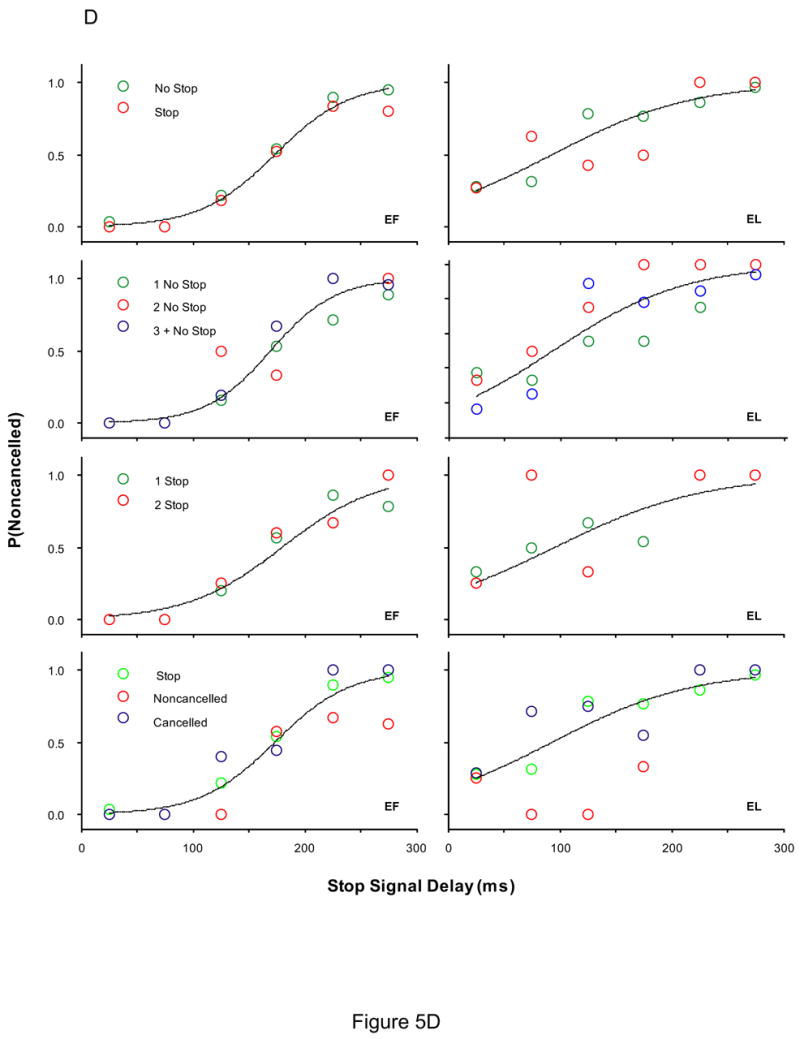
The effect of recent trial history on the probability of responding. Each column is the data from a single subject. (A) Monkeys A, C, and F (B) Monkeys G, H, and N (C) Human subjects EF and EL (D) Human subjects JB, KW, and SN. Inhibition functions from stop signal trials preceded by specific sequences of trials fit with logistical models with stop signal delays and the local trial history as factors, log [P/(1-P)] = b0 + b1*SSD + b2 * TRIAL HISTORY and only stop signal delay as a factor, log [P/(1-P)] = b0 + b1*SSD. A significant effect of trial history is indicated by a fit plotted for each inhibition function. No effect of trial history is indicated by a single fit. A leftward shift in the fit indicates a lower probability of responding. Each row of plots is the probability of responding when stop signal trials were immediately preceded by a no stop signal trial versus a stop signal trial (1st row), preceded by 1, 2, or 3 or more no stop signal trials (2nd row), preceded by 1, 2, or 3 or more stop signal trials (3rd row), immediately precede by a no stop signal trial, a cancelled stop signal trial, or a noncancelled stop signal trial (3rd row).
Similar to the analysis on the data obtained from monkeys, stop signal trials for human subjects were sorted based on whether the immediately preceding trial was a no stop signal trial or a stop signal trial. For one of five human subjects (subject KW), the probability of responding on stop signal trials was less if stop signal trials were immediately preceded by a stop signal trial compared to stop signal trials immediately preceded by no stop signal trials. In addition, the probability of responding for subject KW was greatest if preceded by a no stop signal trial, less if preceded by a noncancelled trial, and least if stop signal trials were preceded cancelled trials. There was no discernable pattern in the inhibition functions for the remaining four human subjects.
The effect of the global stop signal probability on countermanding performance
In addition to local trial history, variation in stimulus and response history on a longer time scale have also been demonstrated to affect countermanding performance (Logan, 1981; Logan & Burkell, 1986; Ramautar et al., 2004). To determine if the global proportion of stop signal trials affects both the response time and the probability of responding, behavioral data were obtained from one monkey while systematically varying the fraction of stop signal trials between 0.1 and 0.7 between sessions for each day of testing. Monkey N performed 22 sessions of saccade countermanding over the course of 7 days. Significant shifts in response time on no stop signal trials in response to changes in the global stop fraction occurred on all 7 days (Kruskal-Wallis test p < 0.05) (Figure 6B). In 5 of 7 days, the probability of responding decreased significantly with increasing stop signal fraction (p < 0.05) (Figure 6A). A linear regression of the change in response time on the change in stop fraction from session to session revealed a significant correlation (R2 = 0.43, p < 0.05).
Figure 6.
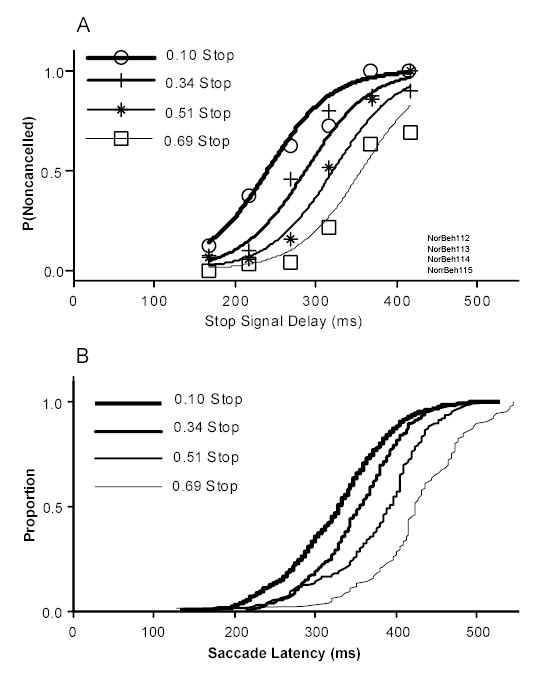
The effect of varying the global probability of a stop signal trials on the probability of responding and response time on no stop signal trials for monkey N. (A) The probability of responding was fit with logistical models with stop signal delays and the global stop ratio as factors, log [P/(1-P)] = b0 + b1*SSD + b2 * STOP RATIO, and with only the stop signal delay as a factor, log [P/(1-P)] = b0 + b1*SSD. A significant effect of trial history is indicated by a fit plotted for each inhibition function. Leftward shifts in the curves indicate a lower probability of responding. (B) Cumulative density functions of no stop signal reaction times as a function of stop ratio. The distributions are significantly different (Kruskal-Wallais test, p<0.05).
Discussion
Summary of results and relation to previous results
The results of the present analysis of trial and performance history in humans and macaque monkeys performing a saccade countermanding task revealed significant, systematic shifts in response times and smaller idiosyncratic changes in the probability of responding on trials with a stop signal. Overall, response times on trials with no stop signal decreased significantly with the number of preceding no stop signal trials. Conversely, a significant increase in response time on no stop signal trials with the number of preceding stop trials was observed for the human subjects, but not for the monkeys. Both human subjects and monkeys produced longer saccade latencies on no stop signal trials following correct cancelled trials, but not following error noncancelled trials. In other words, we found no post-error slowing in the saccade countermanding task for humans or monkeys. The response time adjustments on no stop signal trials were driven mainly by the immediately preceding stop signal trial. In contrast to these adjustments of response times, the probability of responding on stop signal trials was only weakly affected by trial history unless large changes in the fraction of stop trials occurred within a session.
Our results replicate and extend those from previous countermanding studies. Specifically, the overall delay of response times following stop signal trials has been reported for saccades (Cabel et al., 2000; Kornylo et al., 2003) and manual responses (Rieger et al., 1999; Schachar et al., 2004). Furthermore, the findings that saccade latencies on trials with no stop signal are shorter following no stop signal trials than following stop signal trials and that saccade latencies on trials with no stop signa are elevated more following cancelled trials than following noncancelled trials replicates previous reports (Cabel et al. 2000; Kornylo et al. 2003). However, Curtis et al (2005) report the opposite -- saccade latencies following stop signal trials awere shorter than response timessaccade latencies following no stop trials, and saccade latencies were shorter following cancelled trials than saccade latencies following noncancelled trials. However, a major difference in this the Curtis study was the inclusion of catch trials in which no saccade target was presented and the subjects were only required to maintain fixation on the central target for the duration of the trial. Therefore a direct comparison between these data may not be valid.
The absence of elevated saccade latencies following noncancelled errors in the saccade countermanding task is inconsistent with previous observations of delayed responses following errors in choice response time tasks (e.g., Rabbitt 1966a,b; Rabbitt & Phillips, 1967; Laming 1979; for review see Rabbitt & Rogers, 1977). The absence of post-error slowing in our data can be explained a number of ways. First, we may not have obtained enough data to reveal the effect. This is unlikely, though, because we analyzed a large quantity of data in this study from six monkeys and seven human subjects across three laboratories. This amount of data should have revealed a post-error slowing effect if such an effect was present. Second, countermanding errors may have a different salience or valence than errors produced in choice response time experiments. For the monkeys, a noncancelled saccade to the target resulted in the omission of reinforcement and sometimes a prolonged intertrial interval. We believe that these conditions are clear, unambiguous cues regarding the outcome of the trial. For the human subjects, the difference in instructions for choice response time tasks versus countermanding may also explain the absence of post-error slowing. In response time tasks, subjects are typically instructed not to make errors, thus errors might be perceived as a significant event. Conversely, human countermanding subjects were instructed that they would be unable to inhibit approximately half of the stop signal trials and not to worry if they were unable to successfully inhibit responses. In addition, there was no error feedback at the conclusion of each trial. Thus, human subjects were dependent on internal performance monitoring to detect whether errors had been produced and, because of the instructions, may have been less inclined to monitor and correct errors. In summary, by design, errors in the stop signal task are common and so may not engage executive control to delay responding as much as might errors in other tasks. Third, a difference between monitoring saccadic and manual errors may result in a difference in how and when the error signal is used to adapt the behavior. In fact, delayed manual response times following noncancelled and cancelled stop signal trials have been reported in choice tasks (Rieger et al., 1999; Schachar et al., 2004) and reaching movements (Mirabella et. al., 2006). Preliminary work from this lab indicates an absence of post-error slowing for noncanceled manual joystick movements as well (Boucher, Stuphorn, Logan, Schall, & Palmeri, Unpublished observation). Clearly, further work is required to determine if monitoring manual and saccade countermanding errors differ.
The response conflict monitoring hypothesis may provide a parsimonious explanation for the increase in response times following cancelled trials and the absence of post-error slowing (Botvinick, Braver, Barch, Carter & Cohen, 2001). In this model, conflict is defined as the coactivation of mutually incompatible response processes. The countermanding task creates an incompatibility between the process that initiates the movement (GO process) and the process that inhibits the movement (STOP process). Several lines of evidence indicate that for saccade production, the GO process can be identified with the activity of presaccadic movement neurons in the frontal eye field and superior colliculus; while the STOP process can be identified with the activity of fixation neurons (reviewed by Schall 2004; see also Boucher et al. 2006). Neurophysiological recordings in monkeys performing the saccade stop signal task have demonstrated that movement and fixation neurons are maximally coactive during cancelled trials but are not coactive in noncancelled trials or no stop signal trials (Hanes et al., 1998; Paré & Hanes, 2003). According to the proposition that conflict monitoring serves to translate the occurrence of conflict into compensatory adjustments in control, the greater coactivation on cancelled trials should result in greater slowing of saccades on the subsequent trials which is just what we observed.
Response time adjustments are not unique to the countermanding task. Previous studies, using other tasks, have also found that macaque monkeys are sensitive to sequential dependencies (Dorris, et al., 1999, 2000; Procyk et al. 2000; see also Bichot & Schall 1999; reviewed by Fecteau & Munoz 2003). In this data set, the sensitivity of response time to stimulus history was revealed further through the strong correlation observed between a running average of response latency and a running average of the fluctuating fraction of stop signal trials. However, the time scale of this relation appears to be relatively short. We found that the correlation between response time and the fraction of stop signal trials was largest for the immediately preceding trial, and the correlation was absent across entire sessions. These adjustments in response time as a result of preceding trial coincided with subtle and variable effects of stimulus or performance history on the probability of responding. Macaque monkeys and humans subjects were sensitive to both stimulus history (stop signal trial versus no stop signal trial) and performance history (cancelled saccade versus noncancelled saccade).
Sequential effects and the race model
The trial history effects reported here and in previous studies cannot be explained by the original race model of stop signal performance (Logan & Cowan, 1984). As originally conceived, the race model accounts for the outcome of an individual trial by drawing a GO process finish time and a STOP process finish time from stochastically independent distributions and determining which process finished first. Thus, the original formulation of the race model has no memory. Accordingly, some have suggested that the occasional occurrence of longer latency responses on short stop signal delay trials constitutes a violation of the assumption that the GO and STOP processes are independent (e.g., Özyurt et al., 2003; Colonius, Özyurt, & Arndt, 2001). However, independence within and across trials must be distinguished. It seems clear that when stop signal trials occur, subjects adopt a more cautious strategy by slowing responses on subsequent trials. However, such deliberate slowing does not necessarily violate the fundamental premise of the race model that the GO and STOP finish times are stochastically independent. In fact, when subjects do not delay responses systematically, then their performance does not conform to the predictions of the race model (Özyurt et al., 2003).
It is not hard to conceive of how the original race model could be extended to account for sequential effects. According to the race model, response time adjustments and changes in the probability of responding must be produced via a modification in the finishing times of the GO and STOP processes. For instance, decreasing the finish times of the GO process on successive trials biases the outcome of the race toward producing a movement. Therefore, following a sequence of no stop signal trials when saccade latency is reduced, the probability of canceling the movement is reduced on subsequent stop signal trials. Conversely, increasing the finish times of the GO process on successive trials biases the outcome of the race toward inhibiting a movement. Therefore, following a sequence of stop signal trials when saccade latency is increased, the probability of canceling the movement is increased on subsequent stop signal trials.
What mechanisms could be the basis for these effects? Two non-exclusive alternatives will be considered here. On the one hand, the adjustments in performance could come about through processes intrinsic to the mechanism that produces the movement. On the other hand, the adjustments could require intervention of a process extrinsic to the mechanism that produces the movement.
Intrinsic adjustment mechanism
It is possible that the adjustments of performance due to trial history occur through changes in the mechanisms that produce the response. For example, adjustments of response time according to stimulus history can be accounted for within the framework of the LATER model (Carpenter & Williams 1995; Reddi & Carpenter 2000; Carpenter 2001). According to this model, movements are initiated when an accumulating signal reaches a fixed criterion or threshold. Because the threshold does not vary, the stochastic variability in response time originates in randomness of the rate of growth or the starting level of the processes. However, changes in the probability of responding and response time can also occur through changes in the starting level or criterion of the accumulator (Carpenter & Williams 1995; Reddi & Carpenter 2000; Carpenter 2001). In other words, the starting levels for the racing signals - their handicaps, in effect - may be influenced by prior likelihoods. This was examined, and confirmed by Carpenter and Williams (1995) for a simple reaction time task in which no stop signals were presented. Alterations in expectation induced by changes in the prior probabilities of the targets resulted in changes in mean latencies and in the distribution of latencies that could be quantitatively predicted by the LATER model. Recently, studies have demonstrated the effect of the immediately prior stimulus history in a way that can be explained by the effects of stimulus history on target expectations (Carpenter 2001). It is not difficult to imagine a similar mechanism at work in the countermanding task. A local increase in the frequency of stop trials may result in an elevated starting level for the STOP process. This would lead to a decreased probability of responding and a reduced SSRT. However, this could not explain the observed increased response times on no stop signal trials when preceded by a run of stop signal trials. It may be that another factor is at work in addition to prior probability information, namely a change in the criterion level at which the racing signals trigger a response. In simple saccadic response time tasks, instructions to the subject to make fewer errors appear to result in an elevation of this criterion or threshold level (Reddi & Carpenter 2000). Thus, it seems clear that the presence of both cancelled and noncancelled stop signal trials could result in a more cautious setting for the criterion level.
The neural mechanisms that control the initiation of saccadic eye movements can also offer some insights (Schall et al. 1999; Munoz et al. 2000; Stuphorn & Schall 2001). The architecture of a stochastic growth to a fixed threshold corresponds to the pattern of neural activity in the frontal eye field and superior colliculus that produces saccades (Hanes & Schall 1996; see also Sparks 1976; Dorris, Paré, & Munoz, 1997; Dorris & Munoz 1998). However, the absolute level of the triggering threshold might vary with the context of the task (Everling, Dorris, Klein, & Munoz 1999; Everling & Munoz 2000). Nevertheless, the activity of presaccadic movement and fixation neurons in the frontal eye field and superior colliculus modulate in a manner sufficient to control whether or not saccades are produced in the countermanding task (Hanes et al. 1998; Paré & Hanes 2003). Furthermore, a new interactive race model shows that the GO and the STOP processes of the race model can be instantiated by units with properties corresponding to movement and fixation neurons (Boucher, Palmeri, Logan, & Schall, 2006). Further evidence that the adjustments of performance observed in this study may be mediated by these neurons is derived from observations of the covariation of movement and fixation neuron activity in the superior colliculus with changes in saccade probability and latency (Dorris et al., 1998; Dorris et al., 1997; 2000). For example, the level of activation of movement neurons before a stimulus appears is correlated with the latency of the saccade to the stimulus. Thus, these data indicate that changes in the processes that produce saccades can account for changes in the probability and latency of the movement.
One drawback of considering data related to the intrinsic mechanisms of response time adjustments is that these data do not reveal how such changes in activity come about. We turn our attention next to extrinsic adjustment mechanisms, which may provide such an explanation.
Extrinsic adjustment mechanism
Many have suggested that executive control over the perception, selection, and production systems is a central component of human cognition (e.g. Logan, 1985; Norman & Shallice, 1986; Allport et al., 1994; Baddeley & Della Salla, 1996; Logan & Gordon, 2001; Repovs & Baddeley, 2006). When the environment is ambiguous or presents competing demands, or the mapping of stimulus onto response is complex or contrary to habit—thereby making performance prone to errors—this executive control system is called into action. The original behavioral evidence for an executive control system included adjustments in response time following errors (e.g. Rabbitt, 1966a,b; Rabbitt & Phillips, 1967; Laming, 1979).
Physiological evidence for a monitoring system in the medial frontal lobe has also been obtained. Event-related potential and neuroimaging studies have shown that activation in the medial frontal lobe, centered in anterior cingulate cortex (ACC) is associated with registering the production of errors or conflicting processes, and the need for adjusted control of behavior (reviewed by van Veen & Carter 2002; Nieuwenhuis et al. 2004; Ridderinkhof et al. 2004). Evidence consistent with this general hypothesis has been obtained in neurophysiological recordings from the supplementary eye field (SEF) and ACC in monkeys performing the countermanding task (Stuphorn et al. 2001; Ito et al. 2003).
Consistent with the ERP and neuroimaging literature, neurons in SEF do not generate signals sufficient to control gaze according to the logic of the countermanding paradigm (see Schall et al., 2002). Instead, distinct groups of neurons in SEF and ACC are active either after errors, after successful withholding of a partially prepared movement, or in association with reinforcement (Stuphorn et al. 2001; Ito et al., 2003). In addition, Curtis et al. (2005) observed SEF activation that covaried with response time adjustments. Thus, a part of the brain that is not directly responsible for producing movements of the eyes, appears to produce signals that are the basis of models of self-monitoring and control. Altogether, the evidence indicates that SEF activity reflects performance monitoring, but does it play a role in response time adjustments? Recent evidence indicates that subthreshold, intracortical electrical stimulation of SEF reduces the probability of countermanding errors by increasing saccade latency (Stuphorn & Schall 2006). Thus, these signals are capable of exerting influence on behavior.
Conclusions
The neural basis of the self-control of eye movements has been investigated with increasing precision due in large part to improved behavioral testing procedures and theoretical perspectives. We have examined the relationship between such control and predispositions derived from the responses produced on previous trials. The purpose of this retrospective analysis was to determine whether such contextual effects were present in human and macaque monkey subjects performing the countermanding task, and if so, to verify if current models of stop signal performance could explain such behavioral adjustments. The results provide strong evidence that performance in a saccade countermanding task is influenced by trial history and indicate that the Logan and Cowan (1984) race model of countermanding will need to be extended to explain these results. Preliminary results demonstrate that history-dependent modulation of the finish time of the GO process can account for these effects (Boucher, Logan, Palmeri, & Schall 2006).
Acknowledgments
Supported by NIH T32-MH065782 (EE), F32-EY016679 (LB), R01-MH55806 (JS), NSF BCS0218507 (GL, JS), McKnight Endowment Fund for Neuroscience (JS), Wellcome Trust (RC), Canadian Institutes of Health Research, the EJLB Foundation (MP), NSERC (TT) as well as P30-EY08126, P30-HD015052 and Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience.
References
- Allport DA, Styles E, Hsieh S. Shifting intentional set: Exploring the dynamics of control of tasks. In: Umilta C, Moscovitch M, editors. Attention and Performance XV: Conscious and Nonconscious Information Processing. Cambridge, MA: MIT Press; 1994. –421.pp. 452 [Google Scholar]
- Armstrong IT, Munoz DP. Inhibitory control of eye movements during oculomotor countermanding in adults with attention-deficit hyperactivity disorder. Exp Brain Res. 2003;152:444–452. doi: 10.1007/s00221-003-1569-3. [DOI] [PubMed] [Google Scholar]
- Asrress KN, Carpenter RHS. Saccadic countermanding: a comparison of central and peripheral stop signals. Vision Res. 2001;41:2645–2651. doi: 10.1016/s0042-6989(01)00107-9. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psych Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Interactive race model of countermanding saccades. 2004 doi: 10.1037/0033-295X.114.2.376. (Under Review) [DOI] [PubMed] [Google Scholar]
- Boucher L, Stuphorn V, Logan GD, Schall JD, Palmeri JT. Independent Stopping of Eye and Hand Movements. 2005 doi: 10.3758/bf03193779. (Under Review) [DOI] [PubMed] [Google Scholar]
- Cabel DW, Armstrong IT, Reingold E, Munoz DP. Control of saccade initiation in a countermanding task using visual and auditory stop signals. Exp Brain Res. 2000;133:431–441. doi: 10.1007/s002210000440. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. SPIC: a PC-based system fro rapid measurement of saccadic responses. Journal of Physiology (Proceedings) 1994;480:4P. [Google Scholar]
- Carpenter RHS. Express saccades: is bimodality a result of the order of stimulus presentation? Vision Res. 2001;41:1145–1151. doi: 10.1016/s0042-6989(01)00007-4. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Chatfield C. The analysis of time series: Theory and practice. London: Chapman and Hall; 1975. p. 263. [Google Scholar]
- Cho RY, Nystrom LE, Brown ET, Jones AD, Braver TS, Holmes PJ, Cohen JD. Mechanisms underlying dependencies of performance on stimulus history in a two-alternative forced-choice task. Cogn Affect Behav Neurosci. 2002;2:283–299. doi: 10.3758/cabn.2.4.283. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Colonius H, Özyurt J, Arndt PA. Countermanding saccades with auditory stop signals: testing the race model. Vision Res. 2001;41:1951–1968. doi: 10.1016/s0042-6989(01)00084-0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D’Esposito M. Canceling Planned Action: An fMRI Study of Countermanding Saccades. Cereb Cortex. 2004;9:1281–9. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- Dobson AJ. An Introduction to Generalized Linear Models. Vol. 174. Chapman and Hall; London: 1990. [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Immediate neural plasticity shapes motor performance. J Neurosci. 2000;20:RC52. doi: 10.1523/JNEUROSCI.20-01-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Taylor TL, Klein RM, Munoz DP. Influence of previous visual stimulus or saccade on saccadic reaction times in monkey. J Neurophysiol. 1999;81:2429–2436. doi: 10.1152/jn.1999.81.5.2429. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Stuphorn V, Schall JD.2004Evidence for supervisory control of countermanding saccades Program No. 211.112004 Abstract Viewer/Itinerary Planner San Diego, CA: Society for Neuroscience. [Google Scholar]
- Emeric EE, Stuphorn V, Brown JW, Schall JD. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Absence of post - error slowing of countermanding saccades. Program No. 412.3. [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Exploring the consequences of the previous trial. Nat Rev Neurosci. 2003;4:435–443. doi: 10.1038/nrn1114. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Carpenter RHS. Countermanding saccades in humans. Vision Res. 1999;39:2777–2791. doi: 10.1016/s0042-6989(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79 (2):817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hasegawa RP, Blitz AM, Geller NL, Goldberg ME. Neurons in monkey prefrontal cortex that track past or predict future performance. Science. 2000;290:1786–1789. doi: 10.1126/science.290.5497.1786. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Juttner M, Wolf W. Occurrence of human express saccades depends on stimulus uncertainty and stimulus sequence. Exp Brain Res. 1992;89:678–681. doi: 10.1007/BF00229892. [DOI] [PubMed] [Google Scholar]
- Kornylo K, Dill N, Saenz M, Krauzlis RJ. Cancelling of pursuit and saccadic eye movements in humans and monkeys. J Neurophysiol. 2003;89:2984–2999. doi: 10.1152/jn.00859.2002. [DOI] [PubMed] [Google Scholar]
- Laming D. Autocorrelation of choice-reaction times. Acta Psychol (Amst) 1979;43:381–412. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Lappin JS, Eriksen CW. Use of a delayed signal to stop a visual reaction-time response. Journal of Experimental Psychology. 1967;72:805–811. [Google Scholar]
- Li CS, Krystal JH, Mathalon DH. Fore-period effect and stop-signal reaction time. Exp Brain Res. 2005;167:305–309. doi: 10.1007/s00221-005-0110-2. [DOI] [PubMed] [Google Scholar]
- Logan GD. Attention, automaticity, and the ability to stop a speeded choice response. In: Long J, Baddeley AD, editors. Attention and Performance IX. Hillsdale, NJ: Erlbaum; 1981. [Google Scholar]
- Logan GD. Executive control of thought and action. Acta Phychol. 1985;60:193–210. [Google Scholar]
- Logan GD. Dagenbach, Carr T, editors. On the abilty to inhibit thought and action: A user’s guide to the stop signal paradigm. Inhibitory Processes in Attention, Memory, and Language 1994 [Google Scholar]
- Logan GD, Burkell J. Dependence and independence in responding to double stimulation: A comparison of stop, change, and dual-task paradigms. J Exp Psychol Hum Percept Perform. 1986;12:549–563. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit simple and choice reaction time responses: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Logan GD, Irwin DE. Don’t look! Don’t touch! Inhibitory control of eye and hand movements. Psychon Bull Rev. 2000;7:107–112. doi: 10.3758/bf03210728. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. J Neurosci. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Pani P, Pare M, Ferraina S. Inhibitory control of reaching movements in humans. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0456-0. (In press) [DOI] [PubMed] [Google Scholar]
- Munoz DP, Dorris MC, Paré M, Everling S. On your mark, get set: brainstem circuitry underlying saccadic initiation. Can J Physiol Pharmacol. 2000;78:934–944. [PubMed] [Google Scholar]
- Norman DA, Sallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro, editors. Conscioudness and Self- Regulation (Vol. 4): Advances in Research and Theory. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Ollman RT. Attention and performance, IV. New York: Academic Press; 1973. Simple reactions with random countermanding of the “go”-signal; pp. 571–581. [Google Scholar]
- Özyurt J, Colonius H, Arndt PA. Countermanding saccades: evidence against independent processing of go and stop signals. Percept Psychophys. 2003;65:420–428. doi: 10.3758/bf03194573. [DOI] [PubMed] [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- Procyk E, Ford Dominey P, Amiez C, Joseph JP. The effects of sequence structure and reward schedule on serial reaction time learning in the monkey. Brain Res Cogn Brain Res. 2000;9:239–248. doi: 10.1016/s0926-6410(00)00002-1. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Error correction time without external error signals. Nature. 1966a;212:438. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966b;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM, Phillips S. Error-detection and correction latencies as a function of S-R compatibilty. J Exp Psychol. 1967;19:37–42. doi: 10.1080/14640746708400065. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM, Rodgers B. What does a man do after he makes an error – Analysis of response programming. Q J Exp Psychol. 1977;29:727–743. [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn. 2004;56:234–52. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Reddi BA, Carpenter RH. The influence of urgency on decision time. Nat Neurosci. 2000;3(8):827–830. doi: 10.1038/77739. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S. Inhibitory after-effects in the stop signal paradigm. British Journal of Psychology. 1999;90:509–518. [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schall JD, Taylor TL. Sequential effects in countermanding performance of macaque monkeys. Society for Neuroscience Abstracts. 1998;24:172. [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36(2):309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Functional properties of neurons in the monkey superior colliculus: coupling of neuronal activity and saccade onset. Brain Res. 1976;156:1–16. doi: 10.1016/0006-8993(78)90075-6. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Neuronal control and monitoring of initiation of movements. Muscle Nerve. 2002;26:326–339. doi: 10.1002/mus.10158. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- Taylor TL, Ivanoff J. The interplay of stop signal inhibition and inhibition of return. Q J Exp Psychol A. 2003;56:1349–1371. doi: 10.1080/02724980343000099. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Szmalec A, Vandierendonck A. Inhibiting responses when switching: Does it matter? Exp Psychol. 2005;52:125–130. doi: 10.1027/1618-3169.52.2.125. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Vandierendonck A. The interaction between stop signal inhibition and distractor interference in the flanker and Stroop task. Acta Psychol (Amst) 2004;116:21–37. doi: 10.1016/j.actpsy.2003.12.011. [DOI] [PubMed] [Google Scholar]


