Abstract
Since the prevailing form of hormone replacement therapy is associated with the development of cancer in breast and endometrial tissues, alternatives are needed for the management of menopausal symptoms. Formulations of Trifolium pratense L. (red clover) are being used to alleviate menopause-associated hot flashes but have shown mixed results in clinical trials. The strobiles of Humulus lupulus L. (hops) have been reported to contain the prenylflavanone, 8-prenylnaringenin (8-PN) as the most estrogenic constituent, and this was confirmed using an estrogen receptor ligand screening assay utilizing ultrafiltration mass spectrometry. Extracts of hops and red clover and their individual constituents including 8-PN, 6-prenylnaringenin (6-PN), isoxanthohumol (IX), and xanthohumol (XN) from hops, and daidzein, formononetin, biochanin A, and genistein from red clover, were compared using a variety of in vitro estrogenic assays. The IC50 values for the estrogen receptor α and β binding assays were 15 and 27 μg/mL, respectively, for hops and 18.0 and 2.0 μg/mL, respectively, for the red clover extract. Both of the extracts, genistein, and 8-PN activated the estrogen response element (ERE) in Ishikawa cells while the extracts, biochanin A, genistein, and 8-PN significantly induced ERE-luciferase expression in MCF-7 cells. Hop and red clover extracts, as well as 8-PN upregulated progesterone receptor (PR) mRNA in the Ishikawa cell line. In the MCF-7 cell line, PR mRNA was significantly upregulated by the extracts, biochanin A, genistein, 8-PN, and IX. The two extracts had EC50 values of 1.1 and 1.9 μg/mL, respectively, in the alkaline phosphatase induction assay. Based on these data, hops and red clover could be attractive for development as herbal dietary supplements to alleviate menopause-associated symptoms.
Keywords: alkaline phosphatase, estrogen receptor, hops, Humulus lupulus, menopause, progesterone receptor, red clover, Trifolium pratense
ABBREVIATIONS USED: 6-PN, 6-prenylnaringenin; 8-PN, 8-prenylnaringenin; AP, alkaline phosphatase; CBS, calf bovine serum; DMEM/F12, Dulbecco’s Modified Eagle/F12 medium; E2,17β-estradiol; EDTA, ethylenediaminetetraacetic acid; ER, estrogen receptor; ERE, estrogen-responsive element; FBS, fetal bovine serum; HAPS, hydroxyapatite slurry; IX, isoxanthohumol; MEME, minimum essential medium; NEAA, non-essential amino acids; PR, progesterone receptor; SRB, sulforhodamine B; TCA, trichloroacetic acid; WHI, Women’s Health Initiative; XN, xanthohumol
INTRODUCTION
Many menopausal and post menopausal women suffer from a variety of complaints including depression, mood swings, sleep disturbances, vaginal dryness, and hot flashes (1–3). Hormone replacement therapy (HRT) has been used as an effective method of treating various menopause-related symptoms (4). Many women have been turning to herbal remedies (5–7) even prior to several large studies of HRT. The effects of HRT on women’s health have been evaluated in the Million Women Study (8), (Etude Epidémilogique de femmes de la Muteulle Générale de l’Education Nationale -- European Prospective Investigation into Cancer and Nutrition) E3N-EPIC (9), and the Women’s Health Initiative (WHI) (10–12), all of which showed an increased risk of breast cancer. Considering the growing use of herbal alternatives, it is important to fully understand their mechanism(s) of action to avoid potential adverse effects and to establish their efficacy.
The strobiles of Humulus lupulus L. (hops) are primarily used to flavor beer, although it has been studied since 1953 for a potential estrogenic mechanism of action (13–15). In the past, the flavonoids, 8-prenylnaringenin (8-PN), 6-prenylnaringenin (6-PN), and the chalcone, isoxanthohumol (IX), have been isolated and reported to be estrogenic in vitro and/or in vivo (16,17). The most potent estrogen in hops is 8-PN, which is an artifact formed through isomerization of the precursor chalcone, desmethylxanthohumol (18,19). There are several reports on 8-PN in various biological models (20–22). On the other hand, Trifolium pratense L. (red clover) contains the estrogenic isoflavones, daidzein, formononetin, biochanin A, and genistein (23,24). Metabolism and clinical pharmacokinetics studies (25) have shown that formononetin and biochanin A, the same isoflavones found in Glycine max Merrill (soy) (26), can be converted by cytochrome P450 to their more active estrogenic metabolites, daidzein and genistein, respectively (Figure. 1B). Red clover has been tested in clinical trials as a menopause therapy (27) with formulations based on the active metabolites and their metabolic precursors (13). In the present study we confirmed the presence of 8-PN as the most potent estrogen in hops using a mass spectrometry-based screening assay developed in our laboratory (28). In vivo, additional 8-PN can be formed as a metabolite of the more abundant compounds IX and xanthohumol (XN) (29). This is similar to red clover in that both plants have weak or inactive precursors that can be converted to more potent estrogens.
Figure 1.
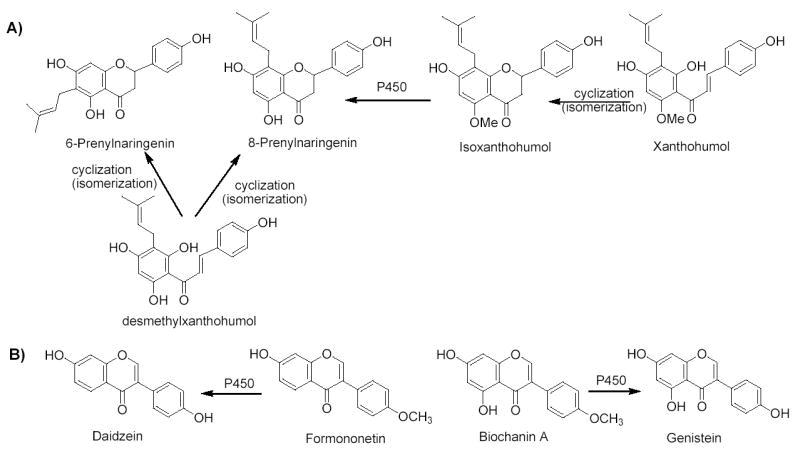
Formation of A) 8-PN from precursors from hops and B) genistein and daidzein from precursors from red clover.
While estrogenic activities have been reported in the literature for both hops and red clover, few studies have directly compared the estrogenicities of the extracts (23,30,31), and to our knowledge, none of the previous reports have compared the isolated compounds responsible for estrogenic activity. Extracts of hops and red clover and their individual constituents including daidzein, formononetin, biochanin A, and genistein, from red clover, and 8-PN, 6-prenylnaringenin (6-PN), isoxanthohumol (IX), and xanthohumol (XN) from hops (Figure 1) were compared using a variety of in vitro estrogenic assays. These data suggest that hops and red clover have the potential for development as herbal dietary supplements to alleviate symptoms associated with menopause.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals and reagents were purchased from Fisher (Hanover Park, IL) or Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. All media for cell culture and human recombinant ERα and ERβ were purchased from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, GA). Genistein, daidzein, biochanin A, and formononetin were purchased from Indofine Chemical Co. (Belle Mead, NJ). 8-PN, 6-PN, IX, and XN, were isolated from Humulus lupulus L. cv. Nugget as described previously (19).
Plant material, extraction, and fractionation
The chloroform partition of a methanol extract from a previously CO2-extracted nugget cultivar of Humulus lupulus L. (Cannabaceae) (provided by Yakima Chief, Inc., Sunnyside WA) was used as previously described (19). The Trifolium pratense L. (Fabaceae) extract (provided by PureWorld, Hackensack, NJ) was an enzymatically treated ethanolic extract, in which glycosidic isoflavones were converted to aglycones such that it contained 30% isoflavones as described previously (32).
Cell culture conditions
The Ishikawa cell line was provided by Dr. R. B. Hochberg (Yale University, New Haven, CT) and were maintained in Dulbecco’s Modified Eagle medium (DMEM/F12) containing 1% sodium pyruvate, 1% non-essential amino acids (NEAA), 1% glutamax-1, 0.05% insulin, and 10% heat-inactivated FBS. A day prior to treating the cells, the medium was replaced with phenol red-free DMEM/F12 medium containing charcoal/dextran-stripped FBS and supplements. The MCF-7 cell line was purchased from American Tissue Culture Collection (Manassas, VA). MCF-7 cells were grown in RPMI 1640 media containing 1% glutamax-1, 1% NEAA, 0.05% insulin, and 5% heat-inactivated FBS. A day prior to treating the cells, the medium was replaced with phenol red-free RPMI 1640 medium containing charcoal/dextran-stripped FBS and supplements. Stripped FBS was prepared by incubating FBS with acetone-washed activated charcoal (100 mg/mL) at 4 °C for 30 min, and centrifuged at 4,000 RPM for 15 min at 4 °C. This step was repeated in triplicate. The concentration of extracts and compounds did not cause any significant cell death under these experimental conditions. DMSO concentrations for all cell culture assays were below 0.1%.
ERα and ERβ competitive binding assays
A screening assay based on ultrafiltration mass spectrometry (28) was used to identify the estrogenic compounds in the hops and red clover extracts. Briefly, 13.3 μg/mL of a DMSO stock extract was incubated for 2 h at room temperature with 100 pmol ERα or ERβ in binding buffer consisting of 50 mM Tris-HCl (pH 7.5), 10% glycerol, 50 mM KCl, and 1 mM EDTA, in a total volume of 150 μL. Identical control incubations in which denatured ER was substituted for active ER was used to correct for nonspecific binding of estrogens to the ultrafiltration membrane and holder. After incubation each mixture was filtered through a Microcon (Millipore, Bedford, MA) YM-30 centrifugal filter containing a regenerated cellulose ultrafiltration membrane with a 30,000 MW cutoff and washed three times with 150 μL aliquots of ammonium acetate buffer (pH 7.5) at 4 °C to remove the unbound compounds. The bound ligands were released by adding 400 μL of methanol/water (90:10; v/v) followed by centrifugation at 10,000 g for 10 min. The ultrafiltrates were dried in vacuo, and the ligands were reconstituted in 60 μL of methanol/water (50:50; v/v). Aliquots (30 μL) were analyzed using LC-MS, which consisted of a reverse phase separation on a Waters (Milford, MA) Xterra MS C18 (3.5 μm, 2.1 × 100 mm) HPLC column and mass spectrometric analysis on a Micromass (Manchester, UK) Quattro II electrospray triple quadrupole mass spectrometer. The mobile phase consisted of a 15 min linear gradient from 15–50% acetonitrile in water, and negative ion electrospray was used for mass spectrometric detection of the ligands.
After ER ligands were identified in the hops extract using mass spectrometry, competitive ERα and ERβ binding assays were used with tritiated estradiol based on the method of Obourn et al. (33) with minor modifications (23) to determine in vitro binding affinities of the substrates with the receptors. The reaction mixture consisted of 5 μL of extract in DMSO, 5 μL pure human recombinant diluted ERα and ERβ (0.5 pmol) in ER binding buffer, 5 μL of “Hot Mix” [400 nM, prepared fresh using 95 Ci/mmol [3H] estradiol, diluted in 1:1 ethanol:ER binding buffer; obtained from NEN Life Science Products (Boston, MA)], and 85 μL of ER binding buffer. The incubation was carried out at room temperature for 2 h before 100 μL of 50% HAPS was added. The tubes were incubated on ice for 15 min with vortexing every 5 min. The appropriate ER wash buffer was added (1 mL), and the tubes were vortexed before centrifuging at 10,000 × g for 1 min. The supernatant was discarded, and this wash step was repeated three times. The HAPS pellet containing the ligand-receptor complex was resuspended in 200 μL of ethanol and transferred to scintillation vials. An additional 200 μL of ethanol was used to rinse the centrifuge tube. Cytoscint [4 mL/vial; ICN (Costa Mesa, CA)] was added, and the radioactivity was counted using a Beckman LS 5801 liquid scintillation counter (Schaumburg, IL). The percent inhibition of [3H] estradiol binding to each ER was determined using equation 1.
| Eq 1 |
The binding capability (percent) of the sample was calculated in comparison with that of estradiol (50 nM, 90%).
Measure of ERE activation
The Dual-Luciferase Reporter Assay System from Promega (Madison, WI) was used to evaluate the functional formation of the ER-ERE complex and luciferase protein expression. Briefly, Ishikawa cells, grown overnight in phenol-free medium, were plated (1.0 × 105 cells/well) in 6-well dishes. The following day the cells were cotransfected using Effectene (Qiagen, Valencia, CA) according to the company’s protocol with ERE plasmid (0.4 μg/mL), pRL-TK plasmid (0.2 μg/mL), and the respective ratios of reagents. MCF-7 cells, grown overnight in phenol-free medium, were trypsinized and resuspended at 1 × 107 cells/mL of serum-free medium. Cells (5 × 106) were incubated with the ERE plasmid (3 μg/mL) obtained from Dr. V.C. Jordan, Northwestern University (34) and pRL-TK plasmid (Promega) (1 μg/mL) in a 4 mm gap cuvette for 5 min at room temperature before electroporation at 950 μF and 250 V using the GenePulser Xcell (BioRad Laboratories, Hercules, CA). Transfected cells were diluted in serum containing medium and plated in 6-well dishes (8.0 × 105 cells/well). Following a 24 h recovery period, the cells were washed with PBS and treated with 2 μg/mL of extract or 100 nM of compounds, including the E2 positive control, for an additional 24 h. Cell lysates (20 μL) were placed in 96 well plates. Luciferase Assay Reagent II (100 μL) was injected followed by a 12 s read by a FLUOstar OPTIMA (BMG LabTech, Offenburg, Germany). Stop & Glo® (100 μL) was added followed by a 12 s read; thus, allowing the termination of the firefly luciferase expression and the activation of the renilla expression. The sample results were normalized to pRL-TK, to account for transfection efficiency, by dividing the sum of the luciferase activity by the sum of the renilla activity. Samples were converted into fold induction such that the DMSO fold induction was 1.
Measurement of PR mRNA expression levels
Quantitative real-time PCR was used to examine the modulation of the progesterone receptor (PR) following treatment of Ishikawa and MCF-7 cells with the herbal extracts and test compounds. Experiments were performed three independent times in triplicate. Using the method described previously (35), Ishikawa and MCF-7 cells (2 × 106) were preincubated overnight in estrogen-free medium. Cells were treated with extracts (2 μg/mL) and test compounds (100 nM) in DMSO for 4 days. mRNA was isolated using the RNAqueous® kit (Ambion Inc., Austin, Texas). Following isolation, mRNA was quantitated by UV analysis at 260 nm. cDNA synthesis was performed in a total volume of 10 μL, containing 1 x TaqMan® RT buffer, 5.5 μM MgCl2, 2 mM dNTPs mixture, 2.5 μM random hexamers, 4 U RNase inhibitor, 12.5 U MultiScribe® RT (Perkin Elmer/Applied Biosystems, Foster City, CA), and 0.2 μg of RNA. The reaction was carried out for 10 min at 25 °C, followed by 48 °C for 30 min and a 5 min incubation step at 95 °C. The PCR and subsequent analyses were performed using the GeneAmp 5700 Sequence Detection System (Applied Biosystems). Quantitation was performed using the TaqMan® technology of Applied Biosystems. PR was evaluated using a pre-developed gene expression primer/probe set (Applied Biosystems’ Assay on Demand). The reaction mixtures were first incubated at 50 °C for 2 min, followed by 10 min at 95 °C. PCR reactions were performed in triplicate and consisted of 40 cycles with 15 s at 95 °C and 1 min at 60 °C each. The fluorescence signal was measured during the last 30 s of the annealing/extension phase. Following analysis, a fluorescence threshold value was set and threshold cycle (Ct) values were determined, i.e., the fractional cycle at which the fluorescence signal reached this threshold. These values were used for further calculations. 18 S (TaqMan PDAR control, Applied Biosystems) was used as an endogenous reference to correct for any differences in the amount of total RNA used for a reaction and to compensate for different levels of transcription during reverse transcription of RNA into cDNA. PR expression was related to a standard curve derived from a serial dilution of their estradiol-treated cDNA. Subsequently, normalization was achieved by dividing the expression level of PR by its respective 18 S expression level. Finally, results were expressed as a fold induction, where the levels of PR observed in the DMSO-treated samples were defined as 1.
Induction of alkaline phosphatase in cultured Ishikawa cells
The procedure of Pisha et al. was used as described previously (36). Ishikawa cells (1.5 × 104 cells/190 μL/well) were preincubated in 96-well plates overnight in estrogen-free medium. Test samples (10 μL at varying concentrations in DMSO) were added to determine EC50 values, and the cells in a total volume of 200 μL media/well were incubated at 37 °C for 4 days. For the determination of antiestrogenic activity, 2 × 10−8 M estradiol was added to the media. The induction plates were processed by washing the plates with PBS and adding 50 μL of 0.01% Triton x 100 in 0.1 M Tris buffer (pH 9.8). An aliquot (150 μL) of 24 mM p-nitrophenyl phosphate (phosphatase substrate) was added to each well. The enzyme activity was measured by reading the release of p-nitrophenyl phosphate at 405 nm every 15 s with a 10 s shake between readings for 16 readings using a Power Wave 200 microplate scanning spectrophotometer (Bio-Tek Instruments, Winooski, VT). The maximal slopes of the lines generated by the kinetic readings were calculated. For estrogenic determination, the percent induction as compared with the estradiol control was calculated using equation 2. For antiestrogenic determination, the percent induction as compared with the background induction control was calculated using equation 3.
| Eq 2 |
| Eq 3: |
Statistics
Statistical analyses were performed using the SAS® statistical package (SAS Institute, Cary, NC). One-way ANOVA was used to analyze the ERE-luciferase data, followed by Dunnett test for pair-wise comparison between the DMSO control and each of the other compounds. A general linear model with two main effects and their interaction was used to analyze the real time RT-PCR data, followed by post hoc comparison between the control versus each of the other compounds. All the tests were two-sided, and results were considered significant when p < 0.05.
RESULTS
Relative affinity of extracts and compounds for ERα and ERβ
Ultrafiltration LC-MS screening was used to identify ligands for ERα and ERβ in the complex botanical extracts. In addition, competitition of botanical ligands with tritiated estradiol was used to confirm that binding occurred at the estradiol site of ER and to measure the relative affinities of these compounds based on their IC50 values. As shown in the ultrafiltration LC-MS chromatograms (Figure. 2), two ligands for ER were detected in the hop extract. One of the ligands was identified as the known hop estrogen, 8-PN, by comparison of its HPLC retention time and mass spectrum with a standard that had previously been isolated over the course of several months using traditional ER binding assay-directed fractionation. Aided by the knowledge of the HPLC retention time and mass spectrometric data gained through ultrafiltration mass spectrometry, the second ligand was isolated from a crude extract and identified as IX by its mass and NMR spectra in only 5 days. Both compounds showed affinity for ERα as well as ERβ. Although isomeric with 8-PN, 6-PN did not show any specific binding to ER (Figure 2). Also the abundant prenylated hops constituent XN did not show affinity for ER during ultrafiltration LC-MS. Screening of the red clover extract using ultrafiltration LC-MS has been reported previously (23) and resulted in the identification of daidzein, formononetin, biochanin A, and genistein as the most estrogenic constituents.
Figure 2.
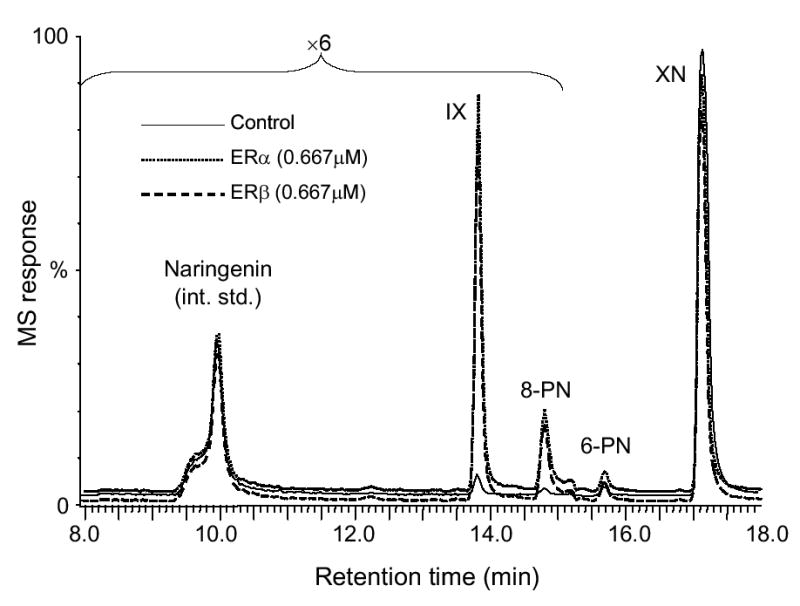
Negative ion electrospray LC-MS chromatograms showing the ultrafiltration mass spectrometric screening of a hop extract for ligands to ERα and ERβ. Denatured ER was used as a control for non-specific binding and specific binding is indicated by increases in the chromatographic peak areas. IX and 8-PN were detected as the highest affinity ligands to both ERα and ERβ.
Tested as complex botanical extracts, hops and red clover showed similar ERα activity with IC50 values of 15 and 18 μg/mL, respectively (Table 1). However, these extracts had different ERβ IC50 values of 27 and 2 μg/mL, respectively. For the isolated compounds the relative binding affinities for ERα were as follows; genistein ≈ 8-PN > daidzein > biochanin A > formononetin > IX, while the relative binding affinities for ERβ were genistein > 8-PN ≈ daidzein > biochanin A > formononetin ≈ IX (Table 1). Genistein and 8-PN had similar affinities for ERα with IC50 values of 0.51 and 0.3 μM, respectively, whereas genistein was more selective for ERβ as indicated by the IC50 values of 0.020 and 1.7 μM, respectively.
Table 1.
ER Binding, ERE-luciferase, PR mRNA Expression, and AP Induction of Hops, Red Clover, and Their Respective Compounds a.
| ERαIC50b (n=6) | ERβ IC50b (n=6) | ERE-luciferase Ishikawa Fold Inductionc,d (n=2) | ERE-luciferase MCF-7 Fold Inductionc,d (n=2) | PR Ishikawa mRNA Fold Inductionc (n=9) | PR MCF-7 mRNA Fold Inductionc (n=9) | AP Induction EC50b (n=9) | |
|---|---|---|---|---|---|---|---|
| E2 | 0.021 ± 0.003 | 0.015 ± 0.002 | 3.5 ± 0.7 g | 4.3 ± 0.2 g | 39 ± 12 g | 10 ± 6 g | 0.00014 ± 0.00003 |
| Hops | 15 ± 3 | 27 ± 3 | 3 ± 0.3 g | 4.2 ± 0.2 g | 19 ± 2 e,g | 5.6 ± 2.4 g | 1.1 ± 0.8 |
| Red Clover | 18 ± 5 | 2.0 ± 0.8 | 7.2 ± 2.8 g | 6.3 ± 0.3 g | 22 ± 11 g | 5.9 ± 2.4 g | 1.9 ± 0.5 |
| Daidzein | 17 ± 3 | 1.20 ± 0.01 | 1.1 ± 0.1 | 1.6 ± 0.3 | 2.0 ± 1 e | 1.5 ± 1.3 | 0.5 ± 0.1 |
| Formononetin | 104 ± 8 | 60 ± 7 | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.9 ± 0.3 | 1.5 ± 0.8 | N/A |
| Biochanin A | 35 ± 1 | 4.1 ± 0.8 | 0.8 ± 0.2 | 4.0 ± 0.4 g | 1.4 ± 0.3 e | 3.4 ± 2.0 g | 4.6 ± 0.8 |
| Genistein | 0.30 ± 0.01 | 0.020 ± 0.002 | 3.5 ± 1.2 g | 4.0 ± 0.5 g | 2.8 ± 0.3 | 4.0 ± 2.2 g | 0.3 ± 0.1 |
| 8-PN | 0.51 ± 0.07 | 1.7 ± 0.1 | 3.5 ± 0.1 g | 4.2 ± 0.3 g | 46 ± 15 g | 8.6 ± 2.9 g | 0.013 ± 0.002 |
| 6-PN | N/Ae | N/A | 0.9 ± 0.4 | 0.7 ± 0.1 | 1.3 ± 0.3 | 1.1 ± 0.6 | N/A |
| IX | 266 ± 56 | 56 ± 13 | 0.8 ± 0.1 | 1.8 ± 0.3 h | 1.8 ± 0.7 | 2.5 ± 2.2 g | 1.1 ± 0.8 |
| XN | N/A | N/A | 0.7 ± 0.3 | 0.7 ± 0.2 | 2.0 ± 0.7 | 0.8 ± 0.4 e | N/A |
Values are expressed as the mean ± SD of n determinations. Experimental details are described in Materials and Methods. Values were significantly different from the control as determined by one-way ANOVA with follow-up Dunnett test (p < 0.05).
Values are expressed in μg/mL for extracts and μM for pure compounds.
Fold inductions tested at 2 μg/mL for extracts, and 100 nM concentrations for compounds where DMSO is equal to 1. Results were normalized relative to 18S.
Ratio of the sum of the firefly and renilla luminescences.
n = 6.
N/A, not active.
Statistically significant fold induction compared with DMSO (p < 0.05).
Marginally significant increased fold induction compared with DMSO (0.1 > p > 0.05).
ERE luciferase activity
Ishikawa and MCF-7 cells that had been transiently co-transfected with the ERE-luciferase and the pRL-TK control plasmid were used to measure the formation of the functional ER-ERE complex in response to treatment with the botanical extracts or individual compounds. Samples were normalized to the control transfection and then expressed as a fold induction compared with DMSO treated cells (Table 1). In Ishikawa cells (Figure 3) the hop and red clover extracts had significant fold inductions, 3 and 7.2, respectively. Genistein and 8-PN had significant fold inductions each of 3.5. None of the other compounds had significant activity. In MCF-7 cells (Table 1, Figure 3), hop and red clover extracts both activated ERE similarly (4.2 and 6.3, respectively). Consistent with Joung et al. (37), biochanin A and genistein had significant activity (4.0 and 4.0, respectively), 8-PN was statistically active (4.2), while IX had a 1.8 fold induction and was considered marginally active (p-value = 0.0978).
Figure 3.
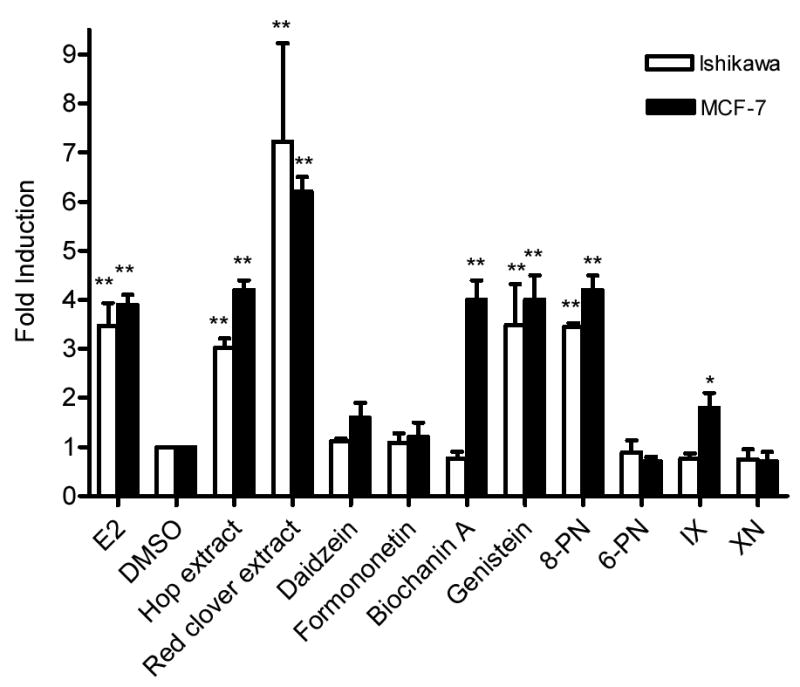
ERE-Luciferase induction in Ishikawa and MCF-7 cells by hop and red clover extracts, and their respective compounds. Cells were treated with either extracts (2 μg/mL) or pure compounds (100 nM) for 24 h, and then analyzed for chemiluminescence. Results were normalized for tranfection efficiency and they are shown as a fold induction relative to the level observed in cells treated with solvent only. Results are the mean of two determinations ± SD. Significant different from the control value was determined by one-way ANOVA with follow-up Dunnett test * 0.1 > p > 0.05. ** p < 0.05.
PR mRNA expression
The estrogen-inducible PR mRNA expression was studied by using real time RT-PCR in the Ishikawa and MCF-7 cell lines (Table 1, Figures 4, 5). In the Ishikawa cell line, PR mRNA was similarly and significantly upregulated in both the hop and red clover extracts (19 and 22, respectively). This was consistent with previous published results obtained in our laboratory using semi-quantitative RT-PCR (23). The upregulation induced by 8-PN was equivalent to E2 (46 and 39, respectively). The remaining compounds did not upregulate the PR mRNA. In the MCF-7 cell line, PR mRNA was significantly upregulated in both the hop and red clover extracts (5.9 and 5.6). Biochanin A, genistein, 8-PN, and IX all significantly upregulated the PR gene (3.4, 4.0, 8.6, and 2.5, respectively).
Figure 4.
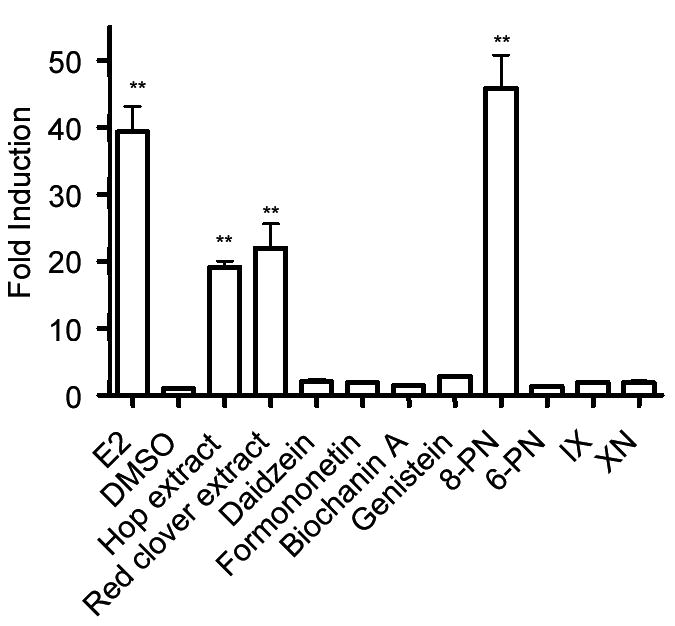
PR mRNA expression levels in the Ishikawa cell line by hops, red clover, and their respective compounds. Ishikawa cells were treated with either extracts (2 μg/mL) or pure compounds (100 nM) for 96 h, and then analyzed for PR mRNA. Results are shown as a fold induction relative to the level observed in cells treated with solvent only. Results are the mean of nine determinations ± SD with the exception of the hop extract, daidzein, and biochanin A where n = 6. * 0.1 > p > 0.05. ** p < 0.05.
Figure 5.
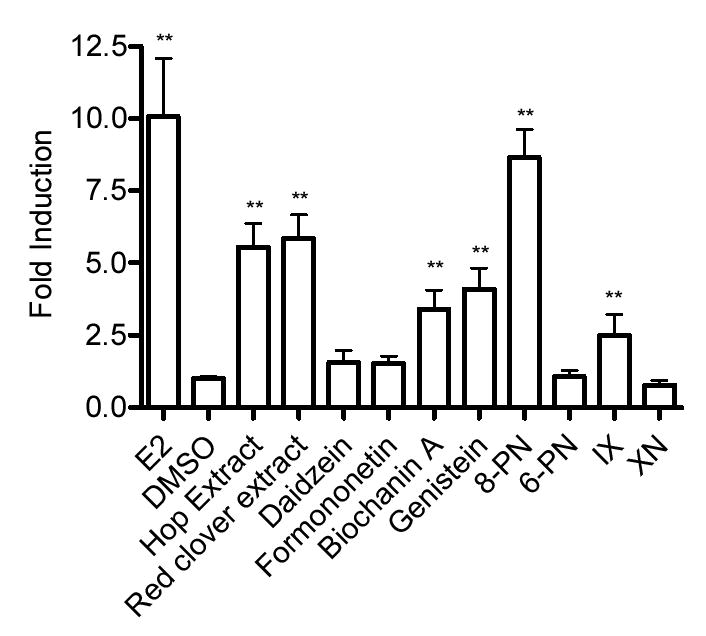
PR mRNA expression levels in the MCF-7 cell line by hop and red clover extracts, and their respective compounds. MCF-7 cells were treated with either extracts (20 μg/mL) or pure compounds (100 nM) for 24 h, and then analyzed for PR mRNA. Results are shown as a fold induction relative to the level observed in cells treated with solvent only. Results are the mean of nine determinations ± SD with the exception of xanthohumol where n=6. * 0.1 > p > 0.05. ** p < 0.05.
AP induction in Ishikawa cells
The Ishikawa cell line is a well-differentiated ERα positive endometrial adenocarcinoma cell line that responds to estrogens and antiestrogens at near physiological levels (38). Induction of alkaline phosphatase (AP) is indicative of an estrogenic response, while competition with estrogen supplemented media and prevention of AP expression indicates an antiestrogenic response (38). In this assay, the EC50 values for the hop and red clover extracts were comparable; 1.1 and 1.9 μg/mL, respectively. The EC50 values for the compounds found in the red clover extract, namely, genistein, daidzein, formononetin, and biochanin A, were consistent with previous results obtained in our laboratory (23). Furthermore, EC50 values for genistein and daidzein, as well as compounds isolated from the hop extract, namely 8-PN, IX, and XN (Table 1), were consistent with values reported by Milligan et al. (16). The isolated compound 6-PN was not active in this assay even when tested at 20 μg/mL. The relative EC50 ranking for all 8 compounds were; 8-PN >> genistein ≈ daidzein > IX > biochanin A (Table 1). None of the samples had antiestrogenic activity (data not shown). All of the samples were tested well below their LC50 concentrations for the Ishikawa cell line (data not shown).
DISCUSSION
In this study the estrogenicity of hops and red clover extracts and eight isolated compounds, identified as ligands for ERα and ERβ based on the ultrafiltration LC-MS prescreening or as a precursor of an active compound, were evaluated using several in vitro estrogenic assays. We investigated different mechanisms to evaluate the estrogenic response of the sample since one endpoint in one tissue of interest may not predict clinical pharmacology (39). The competitive binding of the isolated estrogen receptor, induction of a transiently transfected ERE-luciferase plasmid and the upregulation of the mRNA transcripts of the estrogen sensitive gene, PR, in both MCF-7 and Ishikawa cell lines, and the induction of the endogenous AP enzyme in the Ishikawa cell line were used. Based on these data, the activities of the two extracts and eight compounds were directly compared and were found to be consistent with the ultrafiltration LC-MS screening results and previous reports of activity for specific samples.
The competitive binding of ERα and ERβ provides direct information on ligand-receptor binding in a cell-free environment. Based on the ER distribution, ERβ binding is generally considered beneficial based on the relative tissue distributions of the two receptors (40,41). The red clover extract preferentially bound to the ERβ receptor nine-times greater than to ERα. The hop extract had nearly a two-fold preference for ERα compared with ERβ. Since all of these studies were carried out using cell-based assays with ERα positive cell lines, it is important to note that the hop and red clover extracts had equivalent ERα activity.
Daidzein, formononetin, biochanin A, and genistein from red clover, and 8-PN and IX from hops, bound to ERα. Genistein and 8-PN had equivalent IC50 values for ERα, 0.3 and 0.51 μM, respectively, followed by daidzein and biochanin A, which had weaker IC50 values of 17 and 35 μM, respectively. Formononetin and IX had much weaker IC50 values of 104 and 266 μM, respectively. Daidzein, formononetin, biochanin A, and genistein, as well as 8-PN and IX bound ERβ. Genistein bound the strongest with an IC50 of 0.02 μM. Daidzein, biochanin A, and 8-PN had similar activity; 4.1, 1.2, and 1.7 μM, respectively. Formononetin and IX also had similarly weak activity; 60 and 56 μM, respectively. The relative binding affinities observed for the compounds are in agreement with the ultrafiltration LC-MS screening and previously published data (42,43).
The activation of a transiently transfected ERE-luciferase construct was designed to test the binding of ERα with the ERE using both the Ishikawa endometrial cell line and the MCF-7 breast cancer cell line. Studies of the DNA response element by Paech et al. (44) provide evidence of a potential transcription control mechanism for estrogen-sensitive genes. In this assay, both the hop and red clover extracts were significantly active. Genistein and 8-PN had significant activity, but the weaker estrogens biochanin A, genistein, and IX did not have activity in the Ishikawa cell line. When ERE-luciferase activity was measured in MCF-7 cells, the hop and red clover extracts had significant induction compared with DMSO. There was a marked difference in the compounds that responded. Genistein and 8-PN were active, as were compounds that had less affinity for ERα, biochanin A and IX. Joung et al. used a stably transfected ERE-luciferase MCF-7 cell line treated with genistein, daidzein, or biochanin A for a similar time period, and found that genistein and biochanin A gave a dose dependent response (37). Our results for 8-PN are in agreement with Zierau et al. who used the MVLN bioluminescent MCF-7-derived cell line to verify the modulation of estrogenic activity (20).
The induction of the estrogen sensitive gene PR in the ERα positive Ishikawa endometrial cell line was designed to evaluate the upregulation of mRNA using quantitative real time RT-PCR. Following the formation of the ER-ERE complex, transcription of the target gene begins, in this case PR. As seen in the ERE-luciferase assay using the Ishikawa cell line, hop and red clover extracts significantly upregulated PR, as did 8-PN. Results for biochanin A, daidzein were consistent with the results from the ERE-luciferase assay performed in the Ishikawa cell line. It was surprising that genistein did not have a statistically significant response; however, there was a trend toward estrogenic activity. Interestingly, 8-PN had greater than ten-fold induction compared with genistein and had a similar fold induction for PR with that of estradiol when all compounds, including estradiol, were tested at the same concentration (100 nM). This may be a key point since 8-PN and genistein had essentially the same affinity for ERα, but they had considerable differences in ERE-dependent transcriptional activity, although not to the extent that was seen in the target gene induction. It appears, at least in the Ishikawa cell line, that 8-PN has a 10-fold higher activity compared with genistein. This is consistent with 8-PN being considered one of the most potent phytoestrogens (16), and it acting as a pure estrogen with activity similar to estrone (45).
Both the hops and red clover extracts were evaluated for PR gene induction in the ERα positive MCF-7 breast cancer cell line, and showed significant activity. Furthermore, genistein, 8-PN, and the weaker estrogens, biochanin A and IX, also demonstrated significant induction compared with DMSO. Biochanin A was also found to be estrogenic even though poor binding affinity to both receptors was observed in the competitive binding assay. This was attributed to the conversion of biochanin A to genistein, and would also explain the estrogenic activity of biochanin A that was also was seen with the ERE-luciferase assay. We confirmed (unpublished results) that the estrogenic activity of biochanin A in the MCF-7 cell line is due to the metabolism of biochanin A to genistein as previously reported (46). The conversion of biochanin A to genistein was not observed in the Ishikawa cell line (unpublished results) explaining why no significant induction of the PR gene was observed.
The AP induction assay was designed to evaluate the estrogen-induced production of the AP enzyme. As expected based on the ERα competitive binding assay, both the hop and red clover extracts compared similarly in this assay with EC50 values of 1.9 and 1.1 μg/mL, respectively. Genistein and 8-PN were the most active compounds; however, 8-PN was 10-times more active than genistein. As was seen with the ERE-luciferase assay, there appears to be some ERE affinity and transcriptional regulation that effectively caused 8-PN, which had similar ERα binding, to have a ten-fold increase in PR gene upregulation, which was also seen in the production of alkaline phosphatase.
In the AP assay, formononetin and XN gave results that were slightly different from previous reports. Formononetin was previously reported to have an EC50 value around 12 μM (23). We demonstrated 50% activity at approximately the same concentration as previously reported; however, concentrations at 20 and 40 μM consistently gave between 50% and 60% activity. We therefore conclude that formononetin has a maximum induction of 50% in the AP enzyme at concentrations of 12 μM and above. It should also be noted that Gerhäuser et al. (47) reported antiestrogenic activity for XN of 6.6 ± 0.2 μM. We did not find any antiestrogenic activity for XN, or any of the other pure compounds reported in this paper. One caveat of the antiestrogenic assay is that false positives can occur due to cell death, a cytotoxicity assay is run concurrent with the AP induction assay. During this study, we found that XN had a cytotoxic effect as determined using the SRB assay described in ref. (36) with an LC50 of 7.1 ± 2.3 μM. This is essentially the same concentration that was reported for the antiestrogenic IC50 of XN. While Gerhäuser et al. did perform a cytotoxicity assay, there were methodological differences between their investigation (47) and the current study, which might explain the differences in antiestrogenic activity for XN.
Overall, the hop and red clover extracts evaluated in the present study (a chloroform partition of a methanol extract from a previously CO2-extracted Nugget hops cultivar and a red clover ethanol extract containing 30% isoflavones prepared for a phase II clinical trial) gave results that are similar to previously reported data from our laboratory which were assessed using a methanol extract of a Galena hops cultivar and a 15% isoflavone methanol extract of red clover (23). Both extracts demonstrated significant activities in the ER competitive binding, activation of transiently transfected ERE-luciferase, quantitative real time RT-PCR of an estrogen-inducible gene, and AP enzyme induction assays. The equivalent estrogenic potency of the hop extract compared with that of red clover suggests future in vivo studies are warranted to further validate the estrogenic nature of a standardized hop extract in comparison with a red clover extract already in clinical trials.
Acknowledgments
The authors thank Dr. R. B. Hochberg of Yale University for the Ishikawa cell line. We are grateful to Jim Boyd of Yakima Chief for the generous supply of the hops plant material.
Footnotes
FINANCIAL SUPPORT
This research was supported by Ruth L. Kirschstein NIH predoctoral fellowship F31 AT24232 to C.R.O. and by NIH Grant P50 AT00155 from the Office of Dietary Supplements (ODS), the National Institute of General Medical Sciences (NIGMS), the Office for Research on Women’s Health (ORWH), and the National Center for Complementary and Alternative Medicine (NCCAM).
References
- 1.Shaver JL, Giblin E, Paulsen V. Sleep quality subtypes in midlife women. Sleep. 1991;14:18–23. doi: 10.1093/sleep/14.1.18. [DOI] [PubMed] [Google Scholar]
- 2.Barlow DH. Who understands the menopause? Br J Obstet Gynaecol. 1997;104:879–880. doi: 10.1111/j.1471-0528.1997.tb14344.x. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29:319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 4.Genazzani AR, Gambacciani M. HRT in the third millennium. Maturitas. 2001;38 Suppl 1:S49–55. doi: 10.1016/s0378-5122(01)00204-3. [DOI] [PubMed] [Google Scholar]
- 5.Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 8.Million Women Study Collaborators . Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 9.Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448–454. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 10.Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. J Am Med Assoc. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 12.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Piersen CE. Phytoestrogens in botanical dietary supplements: implications for cancer. Integr Cancer Ther. 2003;2:120–138. doi: 10.1177/1534735403002002004. [DOI] [PubMed] [Google Scholar]
- 14.Stevens JF, Miranda CL, Buhler DR, Deinzer ML. Chemistry and biology of hop flavonoids. J Am Soc Brew Chem. 1998;56:136–145. [Google Scholar]
- 15.Koch W, Heim G. Estrogens in hops and beer. Munch Med Wochenschr. 1953;95:845. [PubMed] [Google Scholar]
- 16.Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, De Keukeleire D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab. 1999;84:2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- 17.Milligan SR, Kalita JC, Pocock V, Van De Kauter V, Stevens JF, Deinzer ML, Rong H, De Keukeleire D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab. 2000;85:4912–4915. doi: 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- 18.Hänsel RV, Schulz J. Desmethylxanthohumol: Isolierung aus Hopfen und Cyclisierung zu Flavanonen. Arch Pharm Weinheim. 1988;321:37–40. [Google Scholar]
- 19.Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Froehlich R, Fong HH, Farnsworth NR, Pauli GF. Estrogens and Congeners from Spent Hops (Humulus lupulus L.) J Nat Prod. 2004;67:2024–2032. doi: 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zierau O, Hauswald S, Schwab P, Metz P, Vollmer G. Two major metabolites of 8-prenylnaringenin are estrogenic in vitro. J Steroid Biochem Mol Biol. 2004;92:107–110. doi: 10.1016/j.jsbmb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Zierau O, Gester S, Schwab P, Metz P, Kolba S, Wulf M, Vollmer G. Estrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl)naringenin and 8-prenylnaringenin. Planta Med. 2002;68(5):449–451. doi: 10.1055/s-2002-32089. [DOI] [PubMed] [Google Scholar]
- 22.Diel P, Thomae RB, Caldarelli A, Zierau O, Kolba S, Schmidt S, Schwab P, Metz P, Vollmer G. Regulation of gene expression by 8-prenylnaringenin in uterus and liver of Wistar rats. Planta Med. 2004;70:39–44. doi: 10.1055/s-2004-815453. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 24.Liggins J, Bluck LJ, Coward WA, Bingham SA. Extraction and quantification of daidzein and genistein in food. Anal Biochem. 1998;264:1–7. doi: 10.1006/abio.1998.2825. [DOI] [PubMed] [Google Scholar]
- 25.Piersen CE, Booth NL, Sun Y, Liang W, Burdette JE, van Breemen RB, Geller SE, Gu C, Banuvar S, Shulman LP, Bolton JL, Farnsworth NR. Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Curr Med Chem. 2004;11:1361–1374. doi: 10.2174/0929867043365134. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Krausz K, Chen J, Ge X, Li J, Gelboin HL, Gonzalez FJ. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31:924–931. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Gu C, Liu X, Liang W, Yao P, Bolton JL, van Breemen RB. Ultrafiltration tandem mass spectrometry of estrogens for characterization of structure and affinity for human estrogen receptors. J Am Soc Mass Spectrom. 2005;16:271–279. doi: 10.1016/j.jasms.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- 30.Zava DT, Dollbaum CM, Blen M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med. 1998;217:369–378. doi: 10.3181/00379727-217-44247. [DOI] [PubMed] [Google Scholar]
- 31.Zava DT, Blen M, Duwe G. Estrogenic activity of natural and synthetic estrogens in human breast cancer cells in culture. Environ Health Perspect. 1997;105(Suppl 3):637–645. doi: 10.1289/ehp.97105s3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth NL, Nikolic D, van Breemen RB, Geller SE, Banuvar S, Shulman LP, Farnsworth NR. Confusion regarding anticoagulant coumarins in dietary supplements. Clin Pharmacol Ther. 2004;76:511–516. doi: 10.1016/j.clpt.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Obourn JD, Koszewski NJ, Notides AC. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993;32:6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- 34.Catherino WH, Jordan VC. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Lett. 1995;92:39–47. doi: 10.1016/0304-3835(95)03755-l. [DOI] [PubMed] [Google Scholar]
- 35.Mata-Greenwood E, Cuendet M, Sher D, Gustin D, Stock W, Pezzuto JM. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc. Leukemia. 2002;16:2275–2284. doi: 10.1038/sj.leu.2402696. [DOI] [PubMed] [Google Scholar]
- 36.Pisha E, Pezzuto JM. Cell-based assay for the determination of estrogenic and anti-estrogenic activities. Methods Cell Sci. 1997;19:37–43. [Google Scholar]
- 37.Joung KE, Kim YW, Sheen YY. Assessment of the estrogenicity of isoflavonoids, using MCF-7-ERE-Luc cells. Arch Pharm Res. 2003;26:756–762. doi: 10.1007/BF02976687. [DOI] [PubMed] [Google Scholar]
- 38.Holinka CF, Hata H, Kuramoto H, Gurpide E. Effects of steroid hormones and antisteroids on alkaline phosphatase activity in human endometrial cancer cells (Ishikawa line) Cancer Res. 1986;46:2771–2774. [PubMed] [Google Scholar]
- 39.Bramlett KS, Burris TP. Target specificity of selective estrogen receptor modulators within human endometrial cancer cells. J Steroid Biochem Mol Biol. 2003;86:27–34. doi: 10.1016/s0960-0760(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 40.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 41.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, De Keukeleire D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–242. [PubMed] [Google Scholar]
- 43.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 44.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer O, Humpel M, Fritzemeier KH, Bohlmann R, Schleuning WD. 8-Prenyl naringenin is a potent ERalpha selective phytoestrogen present in hops and beer. J Steroid Biochem Mol Biol. 2003;84:359–360. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 46.Peterson TG, Ji GP, Kirk M, Coward L, Falany CN, Barnes S. Department of, P.; Toxicology; Comprehensive Cancer Center Mass Spectrometry Shared Facility, U. o. A. a. B. U. S. A. Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. The American journal of clinical nutrition. 1998;68(6 Suppl):1505S–1511S. doi: 10.1093/ajcn/68.6.1505S. [DOI] [PubMed] [Google Scholar]
- 47.Gerhauser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, Becker H. Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop. Mol Cancer Ther. 2002;1:959–969. [PubMed] [Google Scholar]


