Abstract
In persistent viral infections, the host's immune system is challenged by the constant exposure to antigen, potentially causing continuous activation of CD8+ T cells with subsequent immunopathology. Here we demonstrate, for experimental chronic lymphocytic choriomeningitis virus and human HIV infection, that upon prolonged in vivo exposure to antigen, TCR-triggered Ca2+ flux, degranulation, and cytotoxicity are maintained on a cellular level, whereas cytokine production is severely impaired because of a selective defect in activation-induced NFAT nuclear translocation. During chronic infection, this differential regulation of pathways leading to diverse effector functions may allow CD8+ T cells to sustain some degree of local viral control by direct cytotoxicity while limiting systemic immune pathology by silencing cytokine production.
Keywords: dysfunction, cytotoxic T lymphocyte, persistent infection, cytokine production
Control of infections with intracellular pathogens largely depends on CD8+ T lymphocytes. Activation of naïve CD8+ T cells occurs in secondary lymphoid organs, leading to proliferation and differentiation to effector cells (1–3) and is followed by contraction and development of memory CD8+ T cells, which are maintained at elevated frequencies independently of antigen (4, 5). Certain viruses induce protracted or chronic infection, including HIV (6), CMV (7), EBV (8), hepatitis B (9), hepatitis C (10, 11) and human T-lymphotrophic virus type 1 (12) in humans or lymphocytic choriomeningitis virus (LCMV) (13–15), murine cytomegalovirus (16), and gamma herpes virus 68 (17) in the mouse. Viruses can establish persistent infection by different mechanisms, depending on their specific biology. DNA viruses generally do not cause prolonged high-level viremic infections but mainly use the strategy of “camouflage and/or sabotage” to establish persistence. These strategies include viral latency (e.g., EBV, CMV, and herpes simplex virus), infection of cells in immuno-privileged sites (e.g., Varicella–Zoster virus and papilloma virus), interference with immunological cytokine and chemokine networks (e.g., EBV), and subversion of antigen processing and presentation in infected host cells (e.g., CMV) (18). In contrast, RNA viruses mainly rely on speed of replication and shape change as a strategy to persist, leading to high-level viremic infections. In such situations, modulation of specific immune responses is achieved by viral escape from immune-mediated pressure [e.g., HIV and hepatitis C virus (HCV)] or exhaustion (deletion and/or functional inactivation) of specific immune cells (e.g., LCMV, HIV, and HCV) (14, 18–22). On a cellular level, dysfunctional virus-specific CD8+ T cells often exhibit reduced or absent IL-2, TNF-α, and IFN-γ production capacity after long-term in vivo antigen exposure (9, 20, 22–28).
In this study, we investigated the modulation of virus-specific T cell function in situations of chronic high-level virus infections. We show, for murine LCMV infection and human HIV-1 infection, that molding of effector functions during chronic viral infection broadly affects the cytokine production potential by impairment of activation-induced NFAT nuclear translocation. In contrast, Ca2+ influx-dependent degranulation, nuclear translocation of NF-κB, ERK phosphorylation, and cytotoxic effector function are fully maintained, which may significantly contribute to local viral control.
Results
Functional Competence of LCMV-Specific CD8+ T Cells After Acute or Chronic Infection.
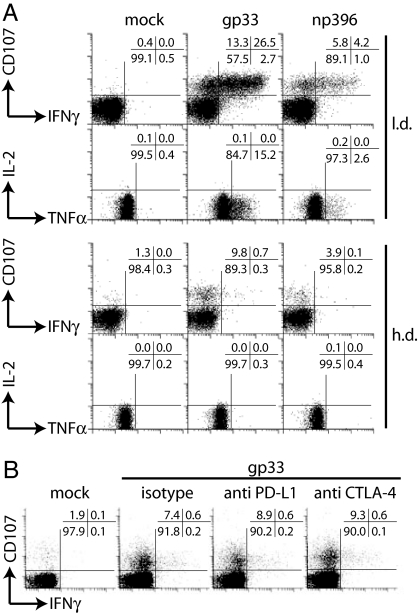
To compare the functional capacities of LCMV-specific CD8+ T cells after acute/resolved (low-dose, l.d.) or chronic (high-dose, h.d.) infection, we analyzed their ability to degranulate and to produce IFN-γ, TNF-α, or IL-2 (Fig. 1A). LCMV-specific CD8+ T cell numbers and viral titers are shown in supporting information (SI) Fig. 6. The majority of gp33- and np396-specific CD8+ T cells from l.d.-infected mice were able to degranulate and to produce IFN-γ and TNF-α. In contrast, gp33- and np396-specific CD8+ T cells from h.d.-infected mice were incapable of producing significant amounts of IFN-γ, TNF-α, or IL-2. To our surprise, LCMV-specific CD8+ T cells retained their degranulation capacity. Comparable results were obtained for lymphocytes isolated from lymph node and peripheral tissues (liver and lung, not shown). Programmed death 1 (PD-1) is highly expressed on virus-specific CD8+ T cells during chronic infections, and signaling through PD-1 might be implicated in CD8+ T cell dysfunction (29–32). We therefore tested whether blocking of PD-1/PD-L1 or cytotoxic T lymphocyte antigen (CTLA)-4/B7 interactions during restimulation would lead to increased cytokine production. Both PD-1 and CTLA-4 blocking did not restore cytokine production capacities of LCMV-specific CD8+ T cells (Fig. 1B).
Fig. 1.
Functional assessment of CD8+ T cells. (A) C57BL/6 mice were infected i.v. with 200 pfu (l.d.) or 106 pfu (h.d.) LCMV Docile, and 20 days later, gp33- or np396-induced degranulation (CD107) or IFN-γ, TNF-α, and IL-2 production were analyzed for splenocytes. (B) Monoclonal anti-PD-L1 or -CTLA-4 antibodies were added during stimulation. Plots are gated on CD8+ T cells. One of three similar experiments is shown.
Functional Properties of gp33-Specific TCR Transgenic (tg) CD8+ T Cells.
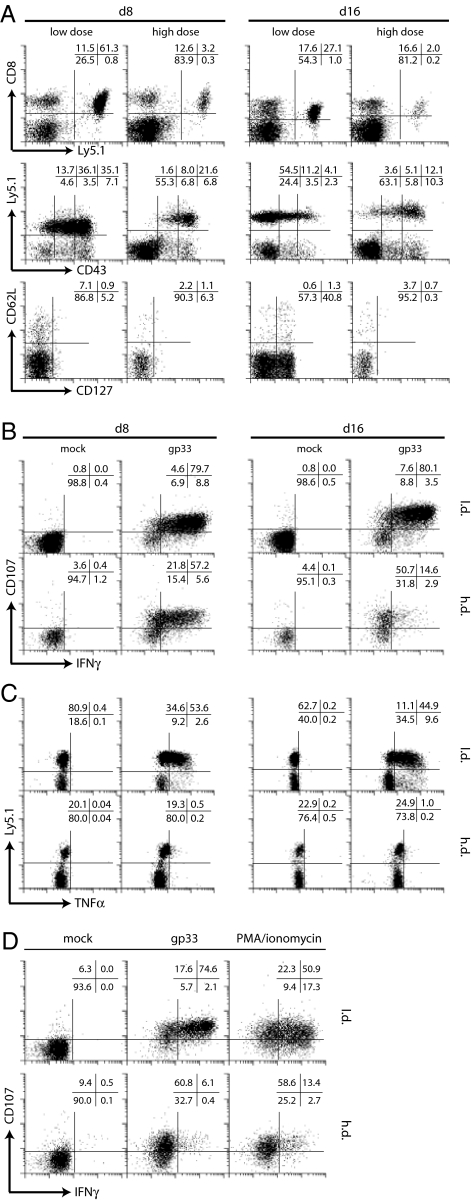
To investigate whether selective dysfunction of CD8+ T cells was also apparent in a monoclonal CD8+ T cell population, we adoptively transferred a low number (104) of naïve Ly5.1+ TCR tg CD8+ T cells, specific for the LCMV gp33–41 epitope, into naïve Ly5.2+ C57BL/6 recipient mice followed by l.d. or h.d. LCMV infection. Transfer of 104 naïve Ly5.1+ TCR tg CD8+ T cells did not influence viral kinetics (SI Fig. 7) or induce selection of CTL escape variants in vivo (SI Figs. 8 and 9 and SI Materials and Methods). Eight days after l.d. infection, TCR tg T cells expressed intermediate to high levels of CD43 and were CD62L− and CD127−, virtually all cells were able to degranulate and produce IFN-γ, and a majority produced TNF-α (Fig. 2). In h.d. infection, TCR tg cells expressed high levels of CD43, were CD62L− and CD127−, and produced IFN-γ but not TNF-α at day 8 after infection. Importantly, degranulation was not affected in h.d. infection. At day 16 after h.d. infection, TCR tg CD8+ T cells still expressed high levels of CD43, were CD62L− and CD127−, and degranulation remained functional, whereas cytokine production was further compromised, because IFN-γ and TNF-α synthesis were virtually absent. Similar results were obtained for day 50 after h.d. infection. Impaired cytokine production of TCR tg CD8+ T cells from chronically infected mice was also evident on mRNA level (data not shown).
Fig. 2.
Phenotypical and functional assessment of TCR tg CD8+ T cells. (A) Spleen cells were stained for Ly5.1 and CD43 (gated on CD8+ T cells) or for CD62L and CD127 (gated on CD8+Ly5.1+ T cells). Spleen cells were stimulated with gp33 peptide and degranulation, and IFN-γ production (B) or TNF-α production (C) was assessed. Plots are gated on TCR tg CD8+ T cells (B) or CD8+ T cells (C). (D) Phorbol 12-myristate 13-acetate/ionomycin or gp33 stimulation 16 days after infection. Plots are gated on TCR tg CD8+ T cells.
It is conceivable that the impaired cytokine production of CD8+ T cells from h.d.-infected mice is because of dysfunctional proximal TCR signaling. We therefore compared MHC/peptide stimulation with phorbol 12-myristate 13-acetate/ionomycin stimulation, which bypasses proximal TCR signaling (Fig. 2D). Both stimulation protocols induced degranulation but not IFN-γ production, indicating that impaired cytokine production is not a consequence of dysfunctional proximal TCR signaling.
Degranulation and Cytotoxicity Are Maintained During Chronic Infection.
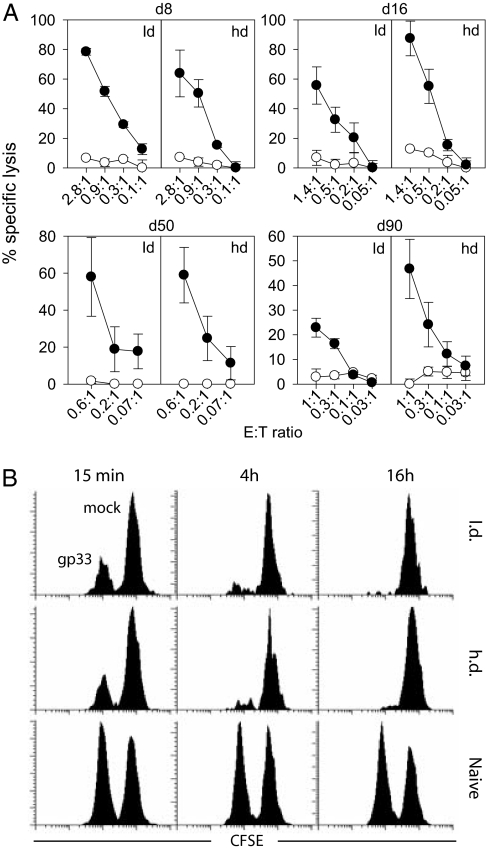
We next assessed whether the sustained degranulation potential of CD8+ T cells from h.d.-infected mice was associated with cytotoxic activity, because we have previously shown that degranulation is necessary but not sufficient for immediate cytotoxicity (33). We used the adoptive transfer system and isolated CD8+ T cells 8, 16, 50, and 90 days after l.d. or h.d. infection. Cells were adjusted to contain equal numbers of Ly5.1+ TCR tg CD8+ T cells and were tested in 51Cr release assays (Fig. 3A). TCR tg CD8+ T cells from h.d.-infected mice were at least as cytolytic as cells from l.d.-infected mice. We confirmed that cytotoxicity was perforin-mediated, because killing of fas-negative mbl-2 targets was comparable to fas-transfected mbl-2/fas targets (not shown).
Fig. 3.
Cytotoxic function of LCMV-specific CD8+ T cells. (A) In vitro cytotoxicity. At day 8, 16, 50, or 90 after infection, CD8+ T cells were purified from the spleens of adoptively transfused l.d.- or h.d.-infected mice and adjusted for equal numbers of TCR tg CD8+ T cells for 51Cr release assays (gp33-pulsed: closed circles, mock-pulsed: open circles). Specific lysis was determined after 16 h. Effector-to-target cell ratios indicate the ratio between Ly5.1+ TCR tg CD8+ T cells and target cells. (B) In vivo cytotoxicity. Gp33 peptide-loaded (CFSElo) and unloaded (CFSEhi) target cells were adoptively transferred into infected (day 16) or naïve recipient mice. Target cells were quantified in blood 15 min, 4 h, or 16 h after transfer. Representative plots of three mice per group and of three experiments are shown.
We next compared the cytotoxic activity of gp33-specific CD8+ T cells directly in vivo in l.d.- or h.d.-infected mice. Sixteen days after infection, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled target cells were transfused, and elimination of gp33-loaded target cells was assessed after 15 min, 4 h, and 16 h (Fig. 3B). Specific target cell elimination occurred with similar kinetics in l.d.- and h.d.-infected mice, corroborating that the cytotoxic activity of CD8+ T cells was maintained in vivo during persistent LCMV infection.
In Vivo Elimination of gp33-Expressing Antigen-Presenting Cells (APCs) Is Perforin-Dependent in Chronic Infection.
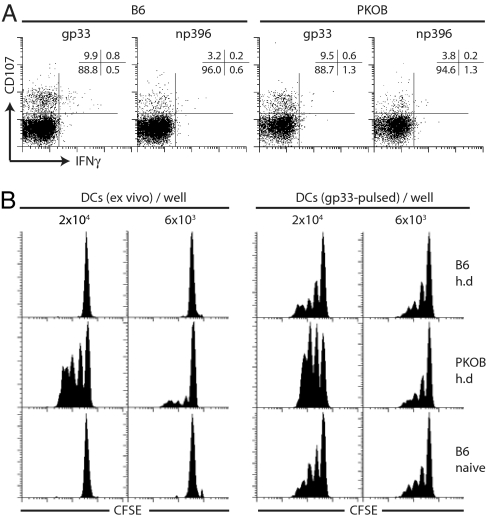
To assess whether maintained cytotoxicity of gp33-specific CD8+ T cells during h.d. infection was associated with enhanced virus control in vivo, we compared T cell functionality, LCMV titers, and frequencies of gp33-expressing APCs in C57BL/6 and perforin-deficient mice (PKOB) 19 days after h.d. infection. Comparable frequencies of gp33- and np396-specific CD8+ T cells were able to degranulate upon specific stimulation but failed to produce IFN-γ (Fig. 4A). LCMV titers in spleens, lymph nodes, and liver were 90% reduced in C57BL/6 mice compared with PKOB mice (data not shown). A more direct evaluation of antigen load, as encountered by gp33-specific CD8+ T cells, is the direct ex vivo analysis of gp33-expressing cells. We therefore isolated splenic dendritic cells (DCs), which are preferential in vivo targets for LCMV (34) from h.d.-infected C57BL/6 and PKOB mice and tested their ability to stimulate naïve TCR tg CD8+ T cells (Fig. 4B). Only DCs from h.d.-infected PKOB mice were able to induce proliferation of TCR tg CD8+ T cells, corroborating that perforin-mediated cytotoxicity is indeed functional in vivo during chronic LCMV infection.
Fig. 4.
Chronic LCMV infection in C57BL/6 and PKOB mice. (A) CD8+ T cell function in C57BL/6 and PKOB mice. C57BL/6 and PKOB mice were infected with 106 pfu LCMV Docile, and gp33- and np396-specific CD8+ T cells were analyzed at day 19 in spleen for degranulation and IFN-γ production (gated on CD8+ T cells). (B) Ex vivo quantification of gp33+APCs. CD11c+ DCs were purified to high purity from spleens, were either mock treated (Left) or pulsed with gp33 peptide (Right), serially diluted, and incubated with 2 × 105 purified naïve CFSE-labeled Ly5.1+ TCR tg CD8+ T cells. CFSE dilution profiles were analyzed after 3 days (plots are gated on CD8+ Ly5.1+ cells). One representative plot from three mice and three independent experiments is shown.
Degranulation Is Ca2+-Dependent but Calcineurin-Independent.
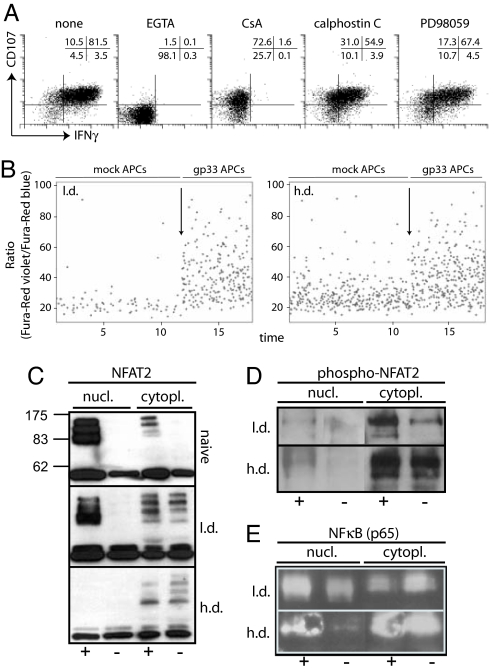
Our results indicate a functional dichotomy of CD8+ T cells during chronic infection. To address the signaling requirements for degranulation and IFN-γ production in LCMV-specific CD8+ T cells directly ex vivo, we tested signaling inhibitors, including the mitogen-activated protein kinase kinase (MEK)1/2 Ca2+ chelator EGTA, the calcineurin inhibitor cyclosporine A (CsA), the protein kinase C inhibitor calphostin C (35), or the MEK1/2 inhibitor PD98059 (36) (Fig. 5A). EGTA completely abrogated degranulation and IFN-γ production, whereas CsA selectively affected IFN-γ production. Both calphostin C and PD98059 only marginally affected degranulation and IFN-γ production. Thus, Ca2+ influx without involvement of the downstream calcineurin pathway is required for degranulation, implying that degranulation-competent CD8+ T cells from h.d.-infected mice are capable of TCR-triggered Ca2+ influx. To directly test this, we measured Ca2+ flux in response to engagement with gp33-presenting APCs. Significant Ca2+ influx was observed in gp33-specific CD8+ T cells from mice with l.d. or h.d. infection, indicating that Ca2+ influx, triggered by TCR MHC/peptide interactions on APCs, is functional in CD8+ T cells from h.d.-infected mice (Fig. 5B).
Fig. 5.
TCR signaling in LCMV-specific CD8+ T cells from h.d. or l.d. infected mice. (A) Signaling requirements for degranulation and IFN-γ production. Spleen cells from adoptively transfused and infected mice (day 20) were stimulated with gp33 peptide in the presence of EGTA, cyclosporine A, calphostin C, or PD98059 and degranulation and IFN-γ production were analyzed. Plots are gated on Ly5.1+ TCR tg CD8+ T cells. One representative staining of two mice and three independent experiments is shown. (B) Ca2+ flux analysis. Spleen cells were isolated, labeled with Fura-red, and stained for Ly5.1 followed by incubation with mock- or gp33-pulsed C57BL/6 peritoneal macrophages; arrows indicate the start of analysis of Ca2+ flux in the presence of gp33-pulsed macrophages. Plots are gated on Ly5.1+ T cells. Representative plots of two mice and three independent experiments are shown. (C–E) Ly5.1 TCR tg CD8+ T cells were sorted from adoptively transfused and h.d.- or l.d.-infected mice or from naïve TCR tg mice. Sorted cells were incubated in presence (+) or absence (−) of gp33 peptide for 16 h. Nuclear and cytoplasmic extracts were prepared and assayed in Western blots for presence of NFAT2 (C; molecular mass, 90, 110, or 140 kDa), p-NFAT2 (D), or NF-κB (E). One of two or three similar experiments is shown.
Impaired Cytokine Production Due to Defective NFAT Nuclear Translocation.
Next, we investigated the mechanisms that lead to impaired cytokine production despite maintained TCR-triggered Ca2+ flux. We used the adoptive transfer system and isolated TCR tg Ly5.1+ CD8+ T cells 18 days after l.d. or h.d. infection or from naïve TCR tg mice. Sorted cells were incubated for 16 h in vitro in the presence or absence of gp33 peptide, and thereafter nuclear and cytoplasmic extracts were prepared for Western blot analysis. Stimulation of naïve TCR tg CD8+ T cells or TCR tg CD8+ T cells from l.d.-infected mice resulted in pronounced NFAT2 nuclear translocation (Fig. 5C). In sharp contrast, no NFAT2 nuclear translocation was observed in CD8+ T cells from h.d.-infected mice, despite comparable levels of cytoplasmic p-NFAT2 in CD8+ T cells from h.d.- or l.d.-infected mice (Fig. 5 C and D). We further analyzed NF-κB nuclear translocation upon peptide stimulation (Fig. 5E). TCR tg CD8+ T cells from both l.d.- and h.d.-infected mice showed nuclear import of NF-κB upon stimulation. Furthermore, TCR tg CD8+ T cells from l.d.- or h.d.-infected mice showed significantly increased levels of p-ERK upon activation (SI Fig. 10 and SI Materials and Methods). Thus, there is a selective defect in NFAT nuclear translocation, whereas Ca2+ influx, NF-κB nuclear transport, or ERK phosphorylation are functional in CD8+ T cells from chronically infected mice.
Maintained Degranulation and Impaired Cytokine Production of CD8+ T Cells During HIV Infection.
Finally, we investigated whether this split functionality of CD8+ T cells is a general feature in situations of persistent high antigen load. We recruited 12 HLA-A2+ patients with advanced untreated HIV-1 infection (SI Table 1) and compared the ability of their HIV- and CMV-specific CD8+ T cells to degranulate and to produce IFN-γ upon specific stimulation (SI Fig. 11 and SI Materials and Methods). A significant proportion of CD8+ T cells were able to degranulate and to produce IFN-γ upon staphylococcal enterotoxin B or CMV stimulation, whereas a majority of HIV-specific CD8+ T cells tended to degranulate but not produce IFN-γ, which is reminiscent of LCMV-specific CD8+ T cells during chronic infection.
Discussion
Modulation of T cell effector function and frequency is of fundamental importance for the host to avoid lethal immunopathology in the setting of a chronic high-level viral infection. We have previously shown that high frequencies of fully functional T cells cause cachexia and death during persistent infection by type 1 cytokine-mediated immunopathology (19). Conversely, impaired T cell functionality might contribute to poor control of persistent infections, and recent studies have highlighted a role for PD-1 (29–32, 37) and IL-10 (38, 39) in induction of T cell dysfunction during chronic infections.
Here we report a differential susceptibility of CD8+ T cell functions toward exhaustion during chronic viral infections, and we have investigated in detail the involved mechanisms. Although functional inactivation was apparent for cytokine synthesis potential, degranulation and cytotoxicity were not compromised in virus-specific CD8+ T cells from chronically LCMV-infected mice.
The degree to which CD8+ T cell are rendered dysfunctional is likely to depend on the amount of viral antigen in the infected host, i.e., the abundance of MHC/peptide complexes. In latent-reactivating infections such as CMV or EBV, where antigen load is relatively low, functionality or CD8+ T cells, such as proliferation and IFN-γ production, is largely maintained, and virus-specific CD8+ T cell frequencies are generally high (8, 40–42). Persistent infections associated with high levels of viral replication, and thus high antigen loads are often associated with CD8+ T cell dysfunction. In untreated HIV infection and viremic hepatitis B and C infection, considerable fractions of virus-specific CD8+ T cells were shown to be dysfunctional with respect to IFN-γ and IL-2 production (9, 25–28, 43–45), and our results confirm these findings. It should be noted, however, that HIV-specific CD8+ T cell dysfunction with respect to cytokine secretion is not consistently observed in HIV-infected individuals, and HIV-specific CD8+ T cells were described as being incompletely differentiated with respect to effector phenotype and perforin content (46, 47). A more recent study showed that the functional competence of HIV-specific CD8+ T cells depends on the clinical status of the patients; progressors exhibited a more limited functional profile than nonprogressors (48), suggesting that the level of antigen exposure is related to the degree of dysfunction.
Chronic LCMV infection leads to exhaustion of LCMV-specific CD8+ T lymphocytes (14, 20–23, 49). Initially, exhaustion was defined by absence of primary or secondary CTL responses measured in 51Cr release assays (14). However, these assays at the time were not standardized for equivalent numbers of LCMV-specific effector cells. Therefore, effector functions were likely to be underestimated because of reduced overall numbers of virus-specific CD8+ T cells in chronically infected mice. More recently, it was recognized that CD8+ T cells with different epitope specificities were either deleted during persistent infection or maintained on a physical level with functional impairment in cytokine production (20–23, 49) and in some reports also in cytolytic activity (22, 24, 50).
In this study, we assessed different CD8+ T cell effector functions in chronic LCMV Docile infection on a cellular level and found no evidence for impaired cytotoxic potential. This is in contrast to some previous reports describing an apparent loss of cytolytic activity, mostly shown for chronic LCMV clone 13 infection (22, 24). It is conceivable that variations in cell tropism and replication capacity of different LCMV strains might lead to different antigen distribution regarding amount and APC type, and that such differences might translate into variable degrees of CD8+ T cell dysfunction.
In contrast to the maintained cytolytic activity, secretion of soluble effector molecules such as IFN-γ and TNF-α was severely compromised. Such differential regulation of effector functions in face of chronic antigen exposure might be crucial for the survival of the host by limiting systemic immunopathology such as toxic shock and cachexia, which is mediated by soluble effector molecules such as TNF-α (19, 51, 52). The maintenance of cytolytic activity in chronically infected mice was associated with reduced viral titers and a significant reduction in virus antigen-expressing target cells in immunocompetent vs. perforin-deficient animals, thus contributing to partial viral control, which might eventually enhance virus clearance.
We further demonstrate a selective silencing of signaling pathways during chronic LCMV infection. Degranulation depended on Ca2+ influx but was independent of calcineurin activity and both TCR-triggered Ca2+ influx and degranulation were intact in CD8+ T cells from chronically infected mice. In contrast, cytokine production completely depended on calcineurin activity, suggesting this signaling pathway is predominantly impaired. Indeed, we show that activation-induced nuclear translocation of NFAT2 was specifically abolished despite normal levels of cytoplasmic p-NFAT2, intact TCR-triggered Ca2+ influx, intact ERK phosphorylation, and NF-κB nuclear translocation.
Taken together, we propose that modulation of CD8+ T cell function during persistent viral infection aims at maintaining local effector cell functions while limiting systemic immune pathology. This is achieved by reducing absolute numbers of virus-specific CD8+ T cells and impairment of effector functions with systemic consequences, such as secretion of soluble mediators (IFN-γ and TNF-α) by selective silencing of the relevant signal transduction pathway. In contrast, cell-directed effector functions such as degranulation and cytotoxicity are largely maintained, thus maximizing specific target cell-directed control of viral replication.
Materials and Methods
Mice.
Tg mice expressing a TCR specific for LCMV peptide gp33–41 and PKOB mice were described previously (51, 53). C57BL/6 mice were purchased from Harlan (Indianapolis, IN) and kept under specific pathogen-free conditions. Animal experiments were performed according to the regulations of the Cantonal Veterinary Office.
Virus, Virus Load Determination, and Peptides.
LCMV Docile was propagated on MDCK cells at low multiplicity of infection. Quantification of infectious virus titers was performed as described (54).
LCMV peptides gp33–41 (gp33, KAVYNFATM) and np396–404 (np396, FQPQNGQFI) were purchased from NeoMPS (Strasbourg, France).
Antibodies and Peptide MHC Class I Tetramers.
APC- or PE-conjugated peptide/MHC class I tetrameric complexes were generated as described (55). Anti-CD107a, -IFN-γ, -TNF-α, -IL-2, -CD8, and -CD45.1 were purchased from BD (Basel, Switzerland).
Lymphocyte Isolation and Stimulation.
Lymphocytes were harvested from spleen or lymph node or isolated from lung and liver of perfused mice and stimulated with 1 μg/ml gp33 or np396 peptide in the presence of anti-mouse CD107a and Monensin A (Sigma, St. Louis, MO). Where indicated, lymphocytes were pretreated for 1 h at 37°C with 10 μg/ml anti-PD-L1 or -CTLA-4 or isotype control antibody (eBioscience, San Diego, CA) before stimulation in continued presence of antibodies. Where indicated, cells were pretreated with 2 mM EGTA (Sigma), 1 μM cyclosporine A (Novartis, Basel, Switzerland), 10 μM calphostin C (Chemie Brunschwig, Basel, Switzerland), or 100 μM PD98059 (Sigma) for 2 h before addition of gp33 and Monensin A in the continued presence of inhibitors (for effectiveness of drugs, refer to SI Fig. 10).
Immunofluorescent Staining and Analysis.
Cells were stained as described (33). Four-color flow cytometric analysis was performed by using a FACSCalibur flow cytometer (BD) with CellQuest software (BD). List-mode data were analyzed by using WinList software (Verity Software House, Topsham, ME).
Ca2+ Flux Analysis.
Cells were adjusted to 107 cells/ml and labeled with Fura-red according to the instructions of the supplier (Invitrogen, Carlsbad, CA). Cells were stained for Ly5.1 at 4°C followed by washing and resuspension in medium at 4°C. Peritoneal macrophages (107) were labeled with 1 μM gp33 peptide or mock-treated for 1 h at 37°C, washed twice in PBS, and resuspended in medium at 4°C. Fura-red-labeled cells (5 × 106) were mixed either with 3 × 106 mock- or 3 × 106 gp33-pulsed macrophages on ice, centrifuged at 250× g for 1 min at 4°C, and kept on ice. Cells were warmed to 37°C for 3 min just before and during analysis. Ly5.1+ cells were gated within a large forward-scatter/sideward-scatter gate containing T cell–macrophage conjugates. Data acquisition and analysis were done on a LSRII flow cytometer (BD) by using FACSDiva software (BD).
Cell Purification.
CD11c+ DCs were purified from spleens by magnetic activated cell sorting (MACS) according to the instructions of the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany). TCR tg Ly5.1+ CD8+ T cells were purified from l.d.- or h.d.-infected mice by MACS sorting of CD8+ T cells followed by FACS sorting of Ly5.1+ cells on a FACS Aria cell sorter (BD).
Ex Vivo Quantification of gp33+ DCs.
Purified DCs were either used directly ex vivo or pulsed with 1 μM gp33 peptide for 1 h at 37°C followed by two washing steps. Serial dilutions of DCs were incubated with 2 × 105 CFSE-labeled CD8+ T cells purified from naïve Ly5.1+ TCR tg mice. After 3 days, cells were harvested and stained for CD8 and Ly5.1, and CFSE dilution profiles of TCR tg CD8+ T cells were analyzed by flow cytometry.
Ex Vivo Cytotoxicity Assay.
Ex vivo 51Cr release assays were performed on EL4 target cells as described (33). Spontaneous release was <45% in 16-h assays.
In Vivo Cytotoxicity Assay.
Splenocytes from naïve C57BL/6 mice were labeled with either 25 or 2.5 μM CFSE (Invitrogen) and pulsed with 10−6 M gp33 peptide for 1 h at 37°C or incubated in medium, washed three times with PBS, mixed at equal amounts, and a total of 1 × 107 cells were adoptively transferred.
Western Blots.
Ly5.1+ CD8+ T cells were purified from adoptively transfused and l.d. or h.d. LCMV-infected mice or from naïve TCR tg mice, and 2–3 × 106 sorted cells were stimulated for 16 h in vitro ± 1 μg/ml gp33 peptide. Cytoplasmic and nuclear extracts were prepared (SI Fig. 12) according to the instructions of the supplier (NE-PER, Perbio, Bonn, Germany). Nuclear and cytoplasmic fractions corresponding to 6 × 105 cells were separated by SDS/PAGE (8.5%) followed by blotting onto a PVDF membrane (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (100 mM NaCl/20 mM Tris·HCl, pH 7.6) with 0.1% Tween20, incubated with anti-NFAT2 (clone 7A6), anti-p-NFAT2 (polyclonal), or anti-NF-κB (clone F-6) antibodies (LabForce, Basel, Switzerland), followed by a secondary HRP-labeled antibody, and developed by chemiluminescence (Chemie Brunschwig).
Supplementary Material
Acknowledgments
We thank Martin Bachmann, Andy Sewell, and Katrin Schwarz for helpful discussions; Eva Niederer for excellent technical help in cell sorting; and Ulrike Kutay (Institute of Biochemistry, Eidgenössische Technische Hochschule) for providing tubulin- and RCC1-specific antibodies. This work was supported by the Roche Research Fund for Biology, the Swiss National Science Foundation, and the Vontobel Foundation.
Abbreviations
- LCMV
lymphocytic choriomeningitis virus
- l.d.
low dose
- h.d.
high dose
- tg
transgenic
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- APC
antigen-presenting cell
- DC
dendritic cell
- CTL
cytotoxic T lymphocyte
- PD-1
programmed death 1.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610335104/DC1.
References
- 1.Kaech SM, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Hemby S, Kersh E, Ahmed R. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 4.Doherty PC, Hou S, Tripp RA. Curr Opin Immunol. 1994;6:545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 6.Sewell AK, Price DA, Oxenius A, Kelleher AD, Phillips RE. Stem Cells. 2000;18:230–244. doi: 10.1634/stemcells.18-4-230. [DOI] [PubMed] [Google Scholar]
- 7.Barouch DH, Letvin NL. Curr Opin Immunol. 2001;13:479–482. doi: 10.1016/s0952-7915(00)00244-2. [DOI] [PubMed] [Google Scholar]
- 8.Callan MF. Viral Immunol. 2003;16:3–16. doi: 10.1089/088282403763635401. [DOI] [PubMed] [Google Scholar]
- 9.Chisari FV, Ferrari C. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 10.Rehermann B, Chisari FV. Curr Top Microbiol Immunol. 2000;242:299–325. doi: 10.1007/978-3-642-59605-6_14. [DOI] [PubMed] [Google Scholar]
- 11.Barnes E, Lauer G, Walker B, Klenerman P. Viral Immunol. 2002;15:285–293. doi: 10.1089/08828240260066233. [DOI] [PubMed] [Google Scholar]
- 12.Bangham CR. J Clin Pathol. 2000;53:581–586. doi: 10.1136/jcp.53.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone M. J Exp Med. 1984;60:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moskophidis D, Lechner F, Pircher HP, Zinkernagel RM. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 15.Zinkernagel RM. Curr Top Microbiol Immunol. 2002;263:1–5. doi: 10.1007/978-3-642-56055-2_1. [DOI] [PubMed] [Google Scholar]
- 16.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Microbes Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Philos Trans R Soc London B. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay BB, McFadden G. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Oxenius A, Zinkernagel RM, Hengartner H. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 20.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou R, Zhou S, Huang L, Moskophidis D. J Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller MJ, Zajac AJ. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 24.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. J Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 25.Oxenius A, Sewell AK, Dawson SJ, Gunthard HF, Fischer M, Gillespie GM, Rowland-Jones SL, Fagard C, Hirschel B, Phillips RE, et al. J Clin Immunol. 2002;22:363–374. doi: 10.1023/a:1020656300027. [DOI] [PubMed] [Google Scholar]
- 26.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, et al. Nat Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 27.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Jr, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. J Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 32.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolint P, Betts MR, Koup RA, Oxenius A. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 36.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 42.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trimble LA, Lieberman J. Blood. 1998;91:585–594. [PubMed] [Google Scholar]
- 44.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 45.Vogel TU, Allen TM, Altman JD, Watkins DI. J Virol. 2001;75:2458–2461. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. J Exp Med. 2000;192:63–76. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 48.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel RM. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. J Virol. 2004;78:3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack E, Zinkernagel RM, Hengartner H. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 52.Badovinac VP, Hamilton SE, Harty JT. Immunity. 2003;18:463–474. doi: 10.1016/s1074-7613(03)00079-7. [DOI] [PubMed] [Google Scholar]
- 53.Pircher HP, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel RM. Nature. 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 54.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel RM. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 55.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Science. 1996;274:94–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.