Abstract
We investigated the focal adhesion proteins paxillin and Fak, and the cell-cell adhesion protein cadherin in developing zebrafish (Danio rerio) embryos. Cadherins are expressed in presomitic mesoderm where they delineate cells. The initiation of somite formation coincides with an increase in the phosphorylation of Fak, and the accumulation of Fak, phosphorylated Fak, paxillin, and fibronectin at nascent somite boundaries. In the notochord, cadherins are expressed on cells during intercalation, and phosphorylated Fak accumulates in circumferential rings where the notochord cells contact laminin in the perichordal sheath. Subsequently, changes in the orientations of collagen fibers in the sheath suggest that Fak-mediated adhesion allows longitudinal expansion of the notochord, but not lateral expansion, resulting in notochord elongation. Novel observations showed that focal adhesion kinase and paxillin concentrate at sites of cell-cell adhesion in the epithelial enveloping layer and may associate with actin cytoskeleton at epithelial junctions containing cadherins. Fak is phosphorylated at these epithelial junctions but is not phosphorylated on Tyr397, implicating a noncanonical mechanism of regulation. These data suggest that Fak and paxillin may function in the integration of cadherin-based and integrin-based cell adhesion during the morphogenesis of the early zebrafish embryo.
INTRODUCTION
The morphogenesis of embryos depends both on interactions between cells and their surrounding extracellular matrix (ECM) via integrin complexes and on direct cell-cell interactions via cadherins. Although these interactions are perceived as discrete adhesion systems, they may interact with each other during morphogenesis. The integrin family of transmembrane receptors physically connects the actin cytoskeleton of the cells to the ECM at focal adhesions, and thus, mediates migration and adhesion in many cells (reviewed in Turner, 2000a; Schwartz, 2001). The importance of focal adhesions in early development is well illustrated by mouse embryos deficient in fibronectin or focal adhesion components, including focal adhesion kinase (FAK), paxillin, or integrins. These embryos die early in development (days 5–10) with mesodermal defects (Furuta et al., 1995; Georges-Labouesse et al., 1996; Yang et al., 1999; Hagel et al., 2002).
Cadherins are prominent in adherens junctions and desmosomes used in the formation of epithelial tissues. They are transmembrane proteins, which form homophilic interactions between neighboring cells and connect the plasma membrane with the actin cytoskeleton. They are involved in signaling, polarization of epithelia, cell sorting, and probably cell migration (reviewed in Gumbiner, 2000). Cadherins are also thought to regulate many aspects of morphogenesis: mouse embryos deficient in N-cadherin (Cdh2) develop mesodermal defects such as irregularly shaped somites and an undulating notochord and die at day 10 (Radice et al., 1997). Mosaic expression of a dominant-negative cadherin in Xenopus embryos results in failure of the expressing cells to intercalate during notochord morphogenesis (Delarue et al., 1998). In N-cadherin mutants (parachute) of zebrafish (Danio rerio), convergent cell movements in the neural tube are severely compromised (Lele et al., 2002).
The integrin-mediated and cadherin-mediated adhesion-modulating systems may interact. Somitogenesis in vertebrate embryos involves presumptive somite border cells with cadherin-based adhesion apically (Linask et al., 1998) while forming integrin-based focal adhesions to the ECM basolaterally (Hens and DeSimone, 1995; Henry et al., 2001). In addition, mesoderm migrates as a multilayered coherent mass held together by cadherins in Xenopus embryos (Winklbauer et al., 1996). It seems plausible that the mechanism that coordinates these activities may involve proteins that participate in both processes.
Paxillin functions as an adaptor protein coordinating the activities of many focal adhesion proteins (Turner, 2000b). Thus, paxillin is in a position to play a role in the integration and regulation of adhesion and signaling, yet little is known regarding its function during embryogenesis (Turner, 1991; Hagel et al., 2002). FAK (abbreviated “Fak” in zebrafish) is a nonreceptor tyrosine kinase that binds paxillin and is a well-characterized component of focal adhesions. Integrin-mediated FAK-autophosphorylation of Tyr397 generates a binding site for Src, which in turn phosphorylates a number of other tyrosine residues on FAK, notably Tyr576/577 in the kinase domain and Tyr861 in the proline-rich domain (Schlaepfer and Hunter, 1998; Cary et al., 2002). Consequently, the phosphorylation state of Tyr397 is considered indicative of FAK's activity. FAK has been localized at sites of strong cell-ECM adhesion in embryos. Hens and DeSimone (1995) and Polte et al. (1994) showed that in Xenopus and mouse embryos FAK is prominent at somite borders and in the brain. We have shown it to be prominent in somite borders and in the notochord cells in zebrafish embryos (Henry et al., 2001).
To elucidate the roles of cell-cell and cell-ECM adhesion in morphogenesis and to characterize these important regulators of adhesion, we have cloned cDNAs representing paxillin (this study) and two isoforms of fak (Henry et al., 2001; this study). We examined the differential phosphorylation of tyrosine residues in Fak in the context of possible interactions with paxillin and cadherins in the developing fish embryo. We found that Fak is dynamically phosphorylated after gastrulation when the somites form and the notochord extends the body-axis. We found that a novel pattern of Fak phosphorylation occurs in the epithelial enveloping layer (EVL) at epithelial junctions where Fak colocalizes with paxillin and cadherins. Our analysis of the expression and phosphorylation dynamics of these proteins, combined with ultrastructural observations, provides insight into the mechanisms of morphogenesis in the EVL, somites, and notochord.
MATERIALS AND METHODS
Cloning of Paxillin cDNA
Polymerase chain reaction (PCR) was used to isolate a probe for zebrafish paxillin. The probe was a 603-base pair DNA fragment amplified from a zebrafish gastrula stage cDNA library (kindly provided by Dr. Randall T. Moon, University of Washington, Seattle, WA) by using 5′-TTC CCT GCT GAA GAA CCT-3′ and 5′-CTC GCA GTC GTC ACA GCG-3′ as primers. A 2566-base pair cDNA of paxillin was isolated from a zebrafish gastrula stage library provided by Drs. Thiery Lepage and David Kimelman (University of Washington, Seattle, WA). The GenBank accession number for the full-length zebrafish paxillin sequence is AY144487.
Cloning of fak1b cDNA
Sequences of fak1b were initially identified by a comparison of the PCR products from a zebrafish PAC genomic library generously supplied by Dr. Bruce W. Draper (Frederick Hutchinson Cancer Research Center, Seattle, WA), by using specific and degenerate primers for the amino acid sequences “PPTANLDR” and “EYVPMVKEVG” in the FAT domain. Degenerate sequences were design by CODEHOP (Fred Hutch Cancer Research Center and http://bioinformatics.weizmann.ac.il/blocks/codehop.html). The fak1b PCR product was used to isolate clones of fak1b from the Lepage/Kimelman gastrula zebrafish library, which was cloned into a Uni-ZAP XR unidirectional vector (Stratagene, San Diego, CA). To determine the specificity of the FAK C-20 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), which we have previously demonstrated recognizes Fak1a in zebrafish (Henry et al., 2001), we expressed Fak1b and assayed cross-reactivity by Western blot. The fak1b clone was subcloned into a pGEX-5X-3 vector (Amersham Biosciences, Piscataway, NJ) and transcribed and translated using PROTEINscript-PRO (Ambion, Austin, TX). The 5′ end of fak1b was sequenced from a TA-clone derived from a 5′ rapid amplification of cDNA ends (RACE) cDNA library generously supplied by Chris J. Thorpe (University of Washington, Seattle, WA), by using SMART RACE (BD Biosciences Clontech, Palo Alto, CA) and 5′-CGTCACATTCTCATACACTT-3′ as the reverse fak1b primer. The GenBank accession number for the full-length zebrafish fak1b sequence is AY196213.
Mapping of paxillin and fak1b
The meiotic mapping of paxillin was performed as in Henry et al. (2001). Genomic DNAs from the mapping panels were amplified using primers from the 3′-untranslated region of paxillin to give a 342-base pair fragment (forward paxillin +53 CCTTGTGGCGGTAGTGAGCA, reverse paxillin –394 AGACATGATACGGCCGAGGAAGAA). Genomic DNAs from the mapping panels were similarly amplified using primers from the 3′-untranslated region of fak1b.
Whole-Mount In Situ Hybridization
Single in situ hybridizations were performed as in Henry et al. (2001). The antisense in situ probes were synthesized to hybridize with the 3′-untranslated region of the paxillin, fak1a, and fak1b transcripts. Sense controls showed no specific staining (our unpublished data). Images of stained embryos were prepared using Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Western Blot Analysis
Methods were similar to those in Henry et al. (2001). For each stage to be analyzed, embryos were partially deyolked by poking the yolk ball with sharp forceps and gently squeezing the embryo to force out the majority of yolk. Deflated embryos were suspended in ice-cold embryo medium (Westerfield, 1995) with 10 mM orthovanadate and one tablet of protease inhibitors/5 ml medium (double the recommended dose of Mini Complete tablets; Roche Diagnostics, Indianapolis, IN), settled on ice, and boiled in 2× reducing SDS-PAGE sample buffer at a concentration of 1 embryo/μl. Ten microliters of embryo extracts was run per lane in a 10% gel (Invitrogen, Carlsbad, CA), blotted, and processed for enhanced chemiluminescence detection as in Henry et al. (2001). Primary antibodies were used diluted at 1:1000, except for anti-paxillin, which was used at 1:5000, and secondary antibodies were used at 1:5000. To detect weak signals during epiboly, films were exposed to the blots for 7 to 10 times longer than for postbud stages. Films were scanned using an Umax 6500 scanner and prepared using Photoshop 7.0 (Adobe Systems). For blots that were probed with multiple antibodies, membranes were stripped using Restore Western blot stripping buffer (Pierce Chemical, Rockford, IL) and redetected with secondary antibodies between probes to ensure complete removal of primary antibodies. Antibodies used were polyclonal antibodies to the C-terminal end of FAK (C-20; Santa Cruz Biotechnology) and affinity-purified polyclonal antibodies to the SH2 binding sites pY397FAK, pY576FAK, pY577FAK, pY861FAK, and pY925FAK (BioSource International, Camarillo, CA), which are conserved in human, mouse, and chick. The antibody against paxillin was a monoclonal to human paxillin (Transduction Laboratories, Lexington, KY).
The Tyr397 antigenic SH2 binding sites of zebrafish Fak1a and Fak1b are identical with that of human FAK (see Figure 2; Dre Fak1a: Henry et al., 2001 and GenBank AAK31154; Dre Fak1b: GenBank AY196213). In comparison, the sequence at this site in human cell adhesion kinase-β (CAK-β) is dissimilar at four amino acids and becomes very dissimilar as the length of the peptide is extended. BioSource International shows that their human pY397-FAK peptide, but not their human pY397-CAK-β competes with their pY397 affinity-purified polyclonal antibody (http://www.biosource.com/; catalog no. 44-624). Thus, their antibody to pY397-FAK is specific for FAK. The CAK-β gene in zebrafish has not yet been identified.
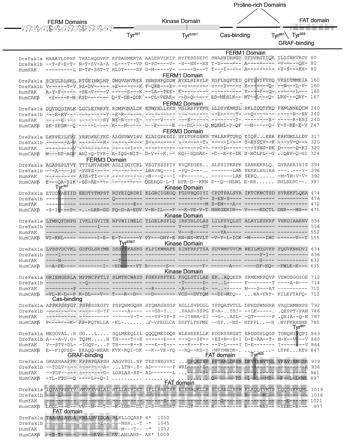
Figure 2.
Zebrafish Fak1b is conserved with respect to zebrafish Fak1a and human FAK1 and differs significantly from human CAKβ. The FERM, kinase, and the FAT domains are shaded and labeled. The SH2 binding domains have their tyrosine residues darkening. The proline-rich Cas- and GRAF-binding domains are labeled. Dre designates the zebrafish D. rerio sequences and Hum the human sequences. Dre Fak1a is in Henry et al. (2001) and GenBank AAK31154; Dre Fak1b in GenBank AY196213; Hum FAK1 from GenBank Q05397; and Hum CAKβ from GenBank Q14289.
The Tyr925 SH2 binding site is identical for human FAK and zebrafish Fak1a and Fak1b (see Figure 2). The anti-pY925 antibody, however, is weak and only lightly stains zebrafish proteins on Western blots and does not stain whole mounts. The SH2 binding sites for Tyr576/577 and Tyr861 have one or two conservative amino acid differences between humans and zebrafish. In contrast, the site for human CAK-β Tyr576/577 is very different in charge, and CAK-β has no Tyr861 SH2 binding site (see Figure 2). The anti-pY576/577 and anti-pY861 antibodies gave strong signals in both Western blots and whole mounts of fish embryos.
The polyclonal antibody to the C-terminal end human FAK (C-20; Santa Cruz Biotechnology) was shown by us to recognize the expressed Dre Fak1a protein (Henry et al., 2001). By Western Blot analysis of the in vitro-expressed proteins, we have shown that the FAK (C-20) antibody does not recognize with Dre Fak1b and confirmed that it recognizes Dre Fak1a (our unpublished data). The C termini of human CAK-β do not significantly match that of human FAK (see Figure 2). Thus, all of our antibodies except that to C-20 FAK recognize both Fak1a and Fak1b and not CAK-β. The antibody to C-20 Fak recognizes only Fak1a.
Whole-Mount Antibody Immunofluorescence
Whole-mount immunostaining was used to determine the subcellular localization of various antigens. In addition to the antisera used in Western blot analysis, we used rabbit anti-laminin and anti-fibronectin (NeoMarkers, Fremont, CA), mouse anti-pTyr (pY99; Santa Cruz Biotechnology), and rabbit anti-PanCadherin (Sigma-Aldrich, St. Louis, MO), which was used previously by Bitzur et al. (1994) on zebrafish. With the exception of experiments with the anti-FAK polyclonal antibody, the embryos were dechorionated and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) with 10 mM orthovanadate for 24 h at 4°C. All embryos were rinsed with PBDT (1% bovine serum albumin, 1% dimethyl sulfoxide, and 0.1% Triton X-100 in PBS pH 7.3) and then blocked in PBDT with 5% bovine serum albumin at room temperature for 2 h. Embryos were incubated with primary antibodies diluted at 1:200 overnight at 4°C. Embryos were washed three times in PBDT, incubated 2 h with secondary antibodies (goat anti-mouse Alexa-488, goat anti-rabbit Alexa-568; Molecular Probes) at 1:250 dilution at room temperature, and washed three times in PBDT, mounted, and viewed using a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA) fitted with 20× 0.8 numerical aperture dry and 63× 1.3 numerical aperture water immersion objectives. Microscopic data were processed and analyzed using ImageJ 1.27z (http://rsb.info.nih.gov/ij/) and prepared for publication using Photoshop 7.0 (Adobe Systems). For actin-staining experiments, Alexa-568-conjugated phalloidin (Molecular Probes) was added to the secondary incubation at 1:100. For negative control experiments, diluted primary antibody was heat inactivated for 5 min at 70°C before use. No specific staining was observed in any negative control. Embryos stained with anti-FAK were fixed in 2% trichloroacetic acid as in Henry et al. (2001).
Detection of Apoptosis by Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL)
Apoptotic cell death in the EVL of normal embryos was detected using the TUNEL technique. Embryos were fixed in 4% paraformaldehyde overnight at 4°C, made permeable by washing 3 × 15 min and 1 × 5 min in PBS + 1% Triton X-100, biotinylated with dUTP according to the Roche protocol, and labeled nuclei detected using streptavidin-Alexa488 (Molecular Probes).
Transmission Electron Microscopy (TEM)
Embryos were dechorionated and fixed in Karnofsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde, 5% sucrose, 0.1% CaCl2, in 0.2 M cacodylate buffer pH 7.2) overnight at 4°C. For TEM embryos were postfixed in 4% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA) in 0.2 M cacodylate buffer for 1 h and then dehydrated through graded ethanols, embedded in LX-112 resin overnight, and polymerized at 70°C. Blocks were sectioned, stained with uranyl acetate and lead citrate, and viewed on a Phillips CM100 transmission electron microscope. Images were prepared using Photoshop 7.0 (Adobe Systems).
RESULTS
Zebrafish Paxillin Is Conserved
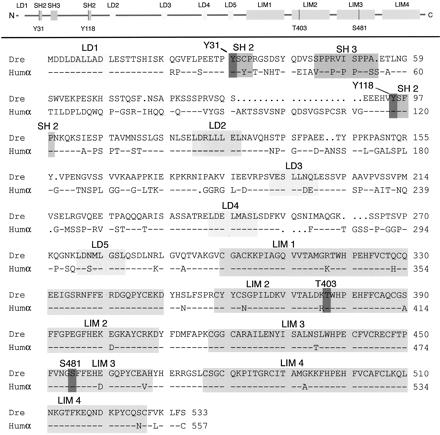
The functional domains of paxillin are highly conserved. The amino acid sequence of zebrafish paxillin is 75% identical compared with human α-paxillin (Figure 1). In the amino half of human paxillin, there are five conserved leucine-rich motifs (LDxLLxxL), termed LD domains, which bind to a number of other proteins, including FAK, vinculin, and p95PKL, and indirectly to the Rho family of GTPases (Tong et al., 1997; West et al. 2001; Brown et al., 2002; Roy et al., 2002). These LD domains are conserved in zebrafish paxillin. Paxillin's carboxyl terminus consists of four tandemly repeated LIM domains essential for localization to focal adhesions, which show 94% identity between the fish and human sequences. Important sites of phosphorylation (Tyr31/118, Thr403, and Ser481) and the surrounding SH2 binding sites are also conserved (Figure 1, dark highlights). Less well conserved is a proline-rich SH3 binding site near the N terminus. The nuclear export signal overlapping the LD2 domain in Xenopus, human, and chicken paxillin (LxxLxxLxLxL; Ogawa et al. 2001) is conserved in the zebrafish sequence as well. However, we do not detect paxillin in the nuclei of cells under our conditions. Notable differences between the zebrafish and human sequences include the 21 amino acid insertion in the human sequence between LD1 and LD2, and considerable sequence variability in the N-terminal region of the protein, which is also less conserved in other vertebrates.
Figure 1.
Zebrafish paxillin is conserved. The LD and LIM domains, and the SH2 and SH3 binding sites are shaded and labeled. Typically, phosphorylated amino acid residues are shaded and marked as Y31, Y118, T403, and S481. Dre is the zebrafish D. rerio sequence and Humα the α-paxillin isoform of human. D. rerio protein and cDNA sequences are in GenBank AY144487.
Zebrafish paxillin maps to linkage group 5 (LG5) with a high statistical significance (courtesy of Yi-Lin Yan and John Postlethwait, University of Oregon, Eugene, OR). It placed 3.05 cR from Z6323 with a Z6323 LOD of 14.1. Analyses of apparent orthologs show no synteny with human pavilion, which maps to human chromosome 14.
Zebrafish Has Two fak Genes, fak1a and fak1b
We have cloned the duplicated Fake gene, fak1b, which is very similar to the previously reported fak1a (Henry et al., 2001) and, like fak1a, is more similar in derived amino acid sequence to mammalian FAK than to CAK-β. The amino acid sequence of zebra fish Fak1b is 69% identical compared with zebra fish Fak1a or with human FAK1 (Figure 2). The kinase domain is very highly conserved being 94% identical to zebra fish Fak1a and 93% identical to human FAK1 (Figure 2, shaded area). The focal adhesion-targeting domain (FAT) of zebra fish Fak1b is also highly conserved, 93% with zebra fish Fak1a and 92% with human FAK1 (Figure 2, cross-hatched). The proline-rich Cas binding domain (amino acids 714–724; Figure 2) is identical between all three FAK sequences. The FERM2 phosphotyrosine binding domain, NYFY at amino acids 144–147, is identical for Fak1a and Fak1b and similar to Hum FAK (NFFY) (Garcia-Alvarez et al., 2003). Of importance, for our observations, is that the SH2 binding sites surrounding Tyr397 and Try925 are identical for zebrafish Fak1a, Fak 1b, and human FAK1 and that the SH2 binding sites for Tyr576/577 and Tyr861 are identical except for a few conservative changes (Figure 2, highlighted). Although the SH2 binding sites are similar for the zebrafish and human FAK proteins, there are significant difference between them and the SH2 binding sites of human CAK-β (Figure 2). Those areas that differ most significantly between zebrafish Fak1a, Fak1b, and human FAK are the N-terminal ends, two areas of the FERM3 domain, and the areas between the proline-rich Cas binding domain and the FAT domain. These areas are not usually conserved between species.
Zebrafish fak1b maps to linkage group 19 (LG19), which is a duplicate of LG 16 containing fak1a (see AAK31154 and AY196213). Both LG19 and LG 16 share syntenies with the distal tip of human chromosome 8, the site of the human FAK locus.
paxillin, fak1a, and fak1b mRNAs Are Expressed throughout Embryogenesis
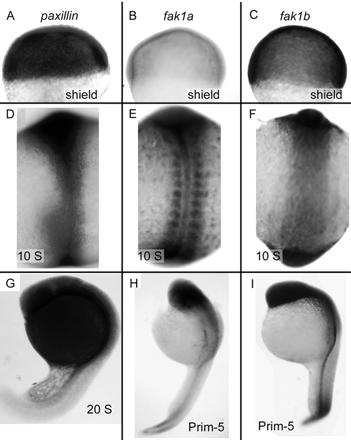
We previously found that fak1a mRNA is segmentally expressed in the paraxial mesoderm and is regulated at least in part by the Notch signaling pathway (Figure 3E; Henry et al., 2001). We compared the expression of paxillin and fak1b with that of fak1a. In situ hybridization reveals that paxillin mRNA is expressed ubiquitously throughout the embryo at the shield stage (Figure 3A), when very little fak1a mRNA is detected (Figure 3B; Henry et al., 2001). The expression of fak1b is similar to paxillin at shield stage (Figure 3C). At the 10-somite stages, paxillin, fak1a, and fak1b mRNAs accumulate in the axial region, the head, and the tail. However, in the paraxial region paxillin and fak1b are expressed homogeneously (Figure 3, D and F, respectively), whereas fak1a is expressed segmentally (Figure 3E). At the 20-somite stage, paxillin is detected strongly in the head and EVL surrounding the yolk, and less strongly in the tail (Figure 3G). In 20-somite embryos, fak1a and fak1b are both expressed strongly in the head region and in the dorsal axis, but not strongly in the EVL (Figure 3, H and I). fak1a is also segmentally expressed in the newly formed somites in the tail (Figure 3H), The mRNA expression is reflected in the protein expression (see below; Figures 5 and 9).
Figure 3.
Expression of paxillin, fak1a, and fak1b mRNAs in the zebrafish embryo. (A–C) Shield stage embryos (6 hpf) hybridized with paxillin (A), fak1a (B), and fak1b (C) probes. Animal pole is up. (D–F) Ten-somite embryos (14 hpf) hybridized with paxillin (D), fak1a (E), and fak1b (F) probes. Presumptive head is up. (G) A 20-somite embryo (19 hpf) hybridized with a paxillin probe (G). (H and I) Prim-5 embryos (24 hpf) hybridized with fak1a (H) and fak1b (I) probes. Head is up and pointing left.
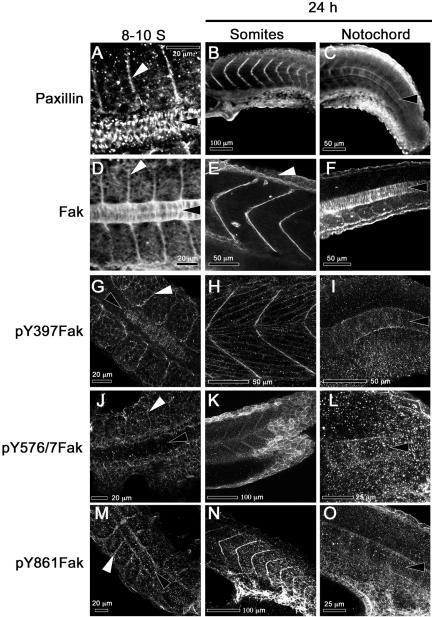
Figure 5.
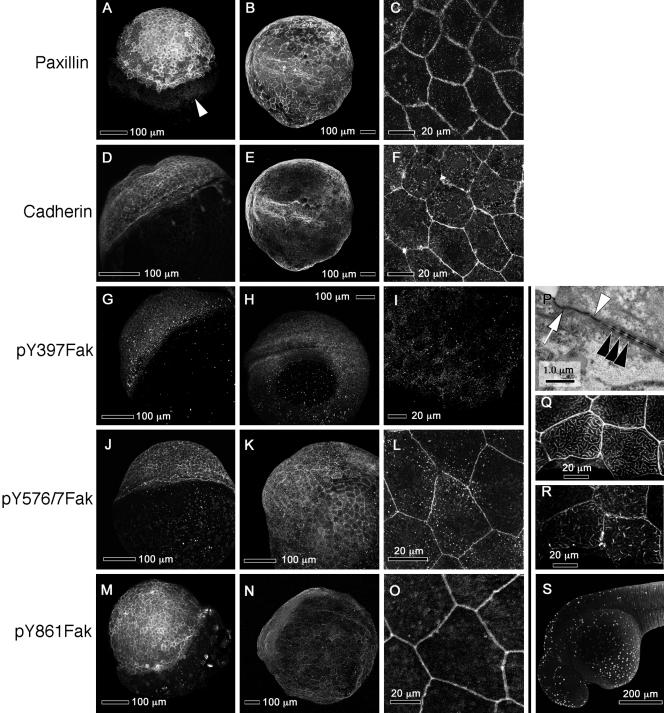
Paxillin and Fak are concentrated at somite boundaries and in the notochord, where Fak is phosphorylated at sites of cell-matrix adhesion. The left column of panels shows confocal images of dorsal views of 8- to 10-somite embryos (13.5 hpf, anterior to the left). The middle column shows lateral views of prim-5 embryos (24 hpf, dorsal up) that are focused on the somites. The right column shows lateral views of prim-5 embryos (dorsal up) that are focused on the notochord. The specificities of the antibodies used are indicated at the left. White arrowheads denote somite boundaries and black arrowheads the notochord, except in 5E, where the white arrowhead indicates faint Fak immunoreactivity in the EVL.
Figure 9.
Paxillin, cadherin, and noncanonically phosphorylated Fak concentrate at the margins of the EVL cells. (A–O) Each row shows confocal images of immunostaining with an antibody against the indicated target. The first column (A, D, G, J, and M) shows embryos at 50% epiboly (5 hpf). The second column (B, E, H, K, and N) shows embryos at the six-somite stage (13 hpf), and the third column (C, F, I, L, and O) shows high-magnification confocal images of the EVL in prim-5 embryos (24 hpf). (A) White arrowhead shows paxillin on the surface of the equatorial region of the yolk. (B and E) Single embryo double-labeled for paxillin and cadherin. (C and F) High-magnification of a single embryo double-labeled for paxillin and cadherin. (P) TEM of a section through the EVL of a prim-5 embryo showing apical tight junctions (white arrow) and adherens junctions (white arrowhead) and desmosomes (black arrowheads) between lateral membranes of EVL cells. (Q and R) High magnifications of confocal images the EVL of a single prim-5 stage embryo showing phalloidin labeling of actin in Q and paxillin labeling in R. The images are near the apical surface of the cell. (S) TUNEL labeling of EVL nuclei in a prim-15 embryo (30 hpf), showing apoptosis. (A and M) Animal poles are up and tilted toward the viewer. (D, G, and J) Animal poles are toward the upper left and slightly away from the viewer. (B, E, and H) Anterior is to the left and dorsal toward the viewer. (K and N) Dorsal is up. (S) Anterior is to the left and dorsal up. (C, F, I, L, and O) Single confocal sections of the EVL. All other panels are confocal projections of Z-stacks.
We also examined the expression of paxillin mRNA in the fused somite class of mutations, which are believed to disrupt Ephrin and Notch signaling and in which expression patterns of fak1a mRNA are disrupted (Henry et al. 2001, 2002; Holley et al., 2002). Expression of paxillin is ubiquitous throughout the paraxial mesoderm in fused-somite (fss/Tbx24), beamter (bea), deadly seven (des/notch 1), and after-eight (aei/DeltaD) embryos and is indistinguishable from wild-type embryos (our unpublished data). Thus, the transcriptional regulation of paxillin is markedly different from fak1a, in that only fak1a is segmentally expressed and regulated by the Notch pathway.
Expression and Phosphorylation of Focal Adhesion Proteins Change Dramatically after Gastrulation
FAK is phosphorylated at several SH2 binding sites, both because of autophosphorylation in response to integrin signaling, and because of the activity of other kinases such as Src (Hanks and Polte, 1997). We used several affinity-purified rabbit antibodies that recognize conserved phosphorylated SH2 binding sites from human FAK to analyze the phosphorylation of the zebrafish Fak epitopes in whole-embryo extracts. The SH2 binding sites of both Fak1a and Fak1b are identical for the zebrafish and human sequences flanking Tyr397, and those sequences flanking Tyr925. The sequences flanking Tyr576/577 and Tyr861 have just one or two conserved changes between humans and zebrafish (see MATERIALS AND METHODS). Several lines of evidence indicate that zebrafish CAK-β is not recognized by these antibodies. The comparable Tyr397 and Tyr576/577 sequences in human CAK-β are very dissimilar, the Tyr861 SH2 binding site does not exist, and binding of the antiserum to the FAK Tyr397 peptide is not competitively inhibited by CAK-β peptides (see MATERIALS AND METHODS).
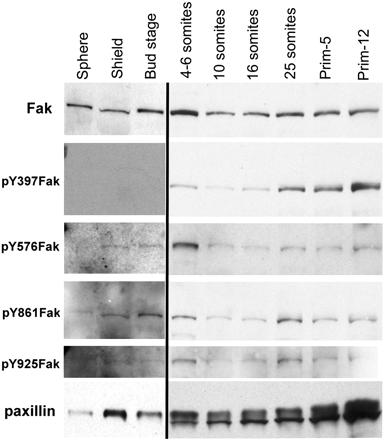
Dramatic increases in the abundance and phosphorylation of Fak and the abundance of paxillin occur during somitogenesis (Figure 4). In Figure 4, this increase is underrepresented because of the long exposures used to detect signals during epiboly and gastrulation (first three lanes). During epiboly and gastrulation, no Fak phosphorylation is detectable on Tyr397 even after long exposures (Figure 4, lanes 1–3). However, the phosphorylation of other tyrosine residues of Fak, including Tyr576/577, Tyr861, and Tyr925, are just detectable in long exposures (Figure 4, lanes 1–3). By the beginning of somitogenesis, the Tyr397 residue of Fak is increasingly phosphorylated. The phosphorylation of all four tyrosine residues of Fak increases during somitogenesis. This pattern is consistent with our previous observations (Henry et al., 2001) and will be revisited later in relationship to spatial patterns of Fak phosphorylation in the EVL and at somite boundaries.
Figure 4.
Phosphorylation of Fak and abundance of both paxillin and Fak increase at the end of gastrulation. Each lane represents Western blots from extracts of ten embryos probed with either a polyclonal antibody to the C termini of Fak, or affinity-purified polyclonal antibodies to the phosphorylated SH2 binding sites of Fak, or a monoclonal antibody to paxillin as described in MATERIALS AND METHODS. To maximize detection of antigens before the onset of somitogenesis, the exposures were 7 to 10 times that used for the later stages.
A qualitative change in the detection of paxillin also occurs at the beginning of somitogenesis, in the appearance of a doublet separated by an apparent molecular mass of ∼5 kDa. This doublet does not seem to be due to nonspecific proteolysis, because other proteins detected on the same blot show no sign of degradation. We have not been able to assign the change to a phosphorylation difference: a general phosphatase does not consolidate the bands and both bands are phosphorylated. The faster moving band is probably not the related Hic-5 protein, because Hic-5 is significantly smaller than paxillin (50 compared with 68 kDa), and we have evidence by immunocytochemistry that Hic-5 is not expressed during early somitogenesis, whereas it is during late somitogenesis. In addition, we observed that the slow-migrating band is cytosolic and the faster migrating paxillin band occurs in a low-speed membrane pellet (our unpublished data). Possible explanations for this doublet include expression of a second paxillin gene, alternate splicing, posttranslational modifications, and paxillin-specific proteolysis that is not blocked by the protease inhibitors in our extraction buffers. Although we have found no evidence for the existence of a second paxillin gene or splice variants in any of our libraries or in database searches, future completion of the zebrafish genome sequence may resolve this question. Nevertheless, it is clear that a qualitative change is taking place after the onset of somitogenesis. We will designate this period of increased protein synthesis, changes in the patterns of Fak and paxillin synthesis, and increases in the phosphorylation of Fak as the postgastrula mesodermal transition (PGMT).
Paxillin, Fak, Fibronectin, and Laminin Localize at Boundaries of Somites as They Form
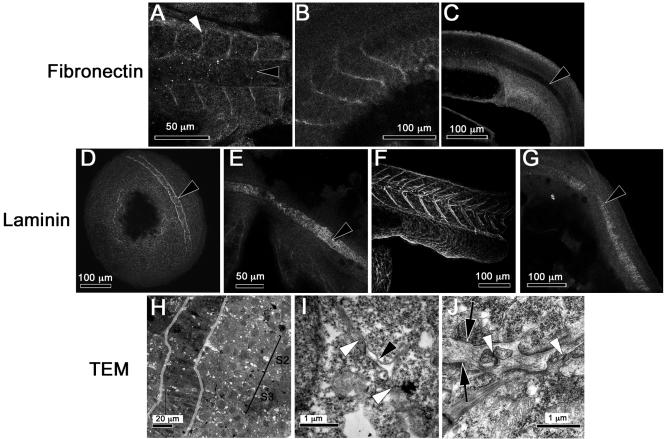
In zebrafish embryos, somites form by the alignment and polarization of their border cells (Henry et al., 2000). Thus, we hypothesize that matrix deposition and adhesion at newly formed somite boundaries may be important in the stabilization of somite boundaries. We asked whether paxillin colocalizes with Fak in somites and whether these proteins are found adjacent to the extracellular matrix glycoproteins fibronectin and laminin. Figure 5, A and D, illustrate the abundance of paxillin and Fak in the axial and somitic mesoderm during somitogenesis. Paxillin concentrates at the boundaries of forming somites (Figure 5A, white arrowhead) and in later stages at the boundaries of the chevron-shaped myotomes (Figure 5B). That paxillin protein remains abundant after paxillin mRNA has declined suggests that the protein is relatively stable. The pattern of paxillin distribution in somites is similar to that described for Fak (Figure 5, D and E; Henry et al., 2001), despite apparent differences in the regulation of these genes at the transcriptional level. All tyrosine-phosphorylated forms of Fak that we are able to detect by immunostaining (pY397, pY576/577, and pY861Fak) concentrate at the somite boundaries during somitogenesis and later stages (Figure 5, G, H, J, K, M, and N). In addition, anti-pY397Fak labels the fused myotome cells that extend between somite boundaries (Figure 5H). Fibronectin concentrates at the somite boundaries during somitogenesis (Figure 6A, white arrowhead) and in 24-h embryos (Figure 6B). We did not detect laminin in forming somite boundaries at the eight-somite stage (Figure 6E), but laminin is abundant at the somite boundaries by the 15-somite stage (Parsons et al., 2002) and at 24 hours postfertilization (hpf) (Figure 6F). In TEMs, we saw that by prim-5 (24 hpf) the somites are separated by rich, fibrous ECM (Figure 6J, between the black arrows), consistent with the presence of both fibronectin and laminin. We also occasionally observe cell processes that cross the somite boundary making adherens and/or gap junctions with the cells of neighboring somites (Figure 6J, white arrowheads). The distributions of fibronectin and laminin are similar to those observed in chick and frog embryos at the somite boundaries (Duband et al., 1987; Krotoski and Bronner-Fraser, 1990) and are consistent with our previous suggestion (Henry et al., 2001) that focal adhesion contacts mediate the stabilization of somite boundaries.
Figure 6.
Fibronectin is concentrated at somite borders, whereas laminin is concentrated in the forming notochord sheath and at somite borders only after the eight-somite stage. The antigens are given to the left of each row of confocal images. (A) Eight-somite embryo (13 hpf). (B) Prim-5 embryo (24 hpf). (C) Tail of a prim-5 embryo focused on the notochord. (D) Bud-stage embryo (10 hpf), showing staining surrounding the notochord. (E) Eight-somite embryo showing the notochord. (F) Tail of a prim-5 embryo focused on the somites. (G) Prim-5 embryo focused on the notochord. (A, D, and E) Dorsal view, anterior is to the left or left and up. (B, C, F, and G) Lateral view, anterior is to the left. (A–G) Black arrowheads denote the notochord and white arrowheads somite boundaries. (H) Low-magnification TEM of a horizontal section through a three-somite embryo (11 hpf). Two somites are marked (S2 and S3). The notochord is outlined in gray. (I) High-magnification TEM of the section in H focused on a membrane gap with small “island” of matrix forming at the presumptive S2/S3 somite boundary (black arrowhead). Membranes of abutting somite border cells in close contact are marked with white arrowheads. (J) High-magnification TEM of a horizontal section through the somite boundary of a prim-5 embryo (24 hpf) showing rich ECM between somites or black arrows and cellular projections that cross this ECM and form junctions with cells in adjacent somites (white arrowheads).
Ontogeny of Somite Boundaries
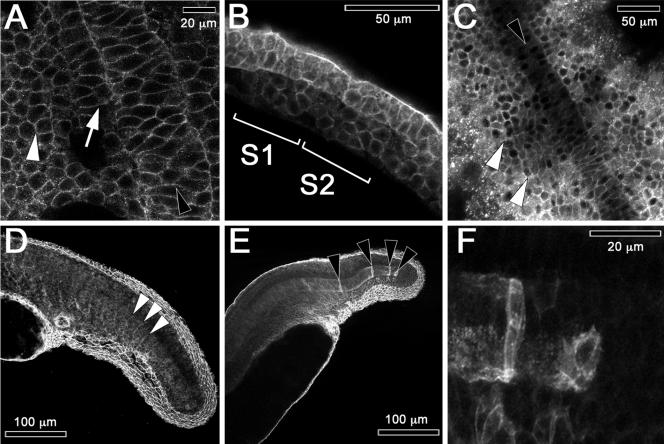
Cadherins have been implicated in the morphogenesis of axial mesoderm in mice and frogs (Radice et al., 1997; Delarue et al., 1998) and in the somites of chick embryos (Duband et al., 1987; Linask et al., 1998). In bud-stage fish embryos (Figure 7A) cadherins are concentrated in membranes of the axial mesoderm of the notochord (black arrowhead), and in membranes of the adaxial and paraxial cells of the presomitic mesoderm (white arrow and white arrowhead, respectively). In lateral views of early somitogenesis, we see that cadherins are concentrated in the internal cells of forming somites between the somite boundaries (Figure 7B, see middle of S1 and S2). In dorsal views later in development, cadherins are still present around all cells, but are more concentrated at the somite boundaries (Figure 7, C and D, white arrowheads).
Figure 7.
Cadherin concentrates at plasma membranes in somite and notochord cells. Embryos are labeled with anti-cadherin antibody in these confocal images. (A) Bud-stage embryo (10 hpf, dorsal view, anterior up). Intercalating notochord (black arrowhead), adaxial cells (white arrow), and presomitic mesoderm (white arrowhead). (B) Lateral view of a two-somite embryo (10.6 hpf, anterior to the left, dorsal up). Somites 1 and 2 are labeled as S1 and S2, respectively. (C) Eight-somite embryo (13 hpf, dorsal view, anterior up). White arrowheads denote somite boundaries and a black arrowhead the notochord. (D) Lateral view of the tail of a prim-5 embryo (24 hpf) that is focused on formed somites to the left. White arrowheads mark outlines of recently formed somites. (E) A lateral view of the tail of a prim-5 embryo focused on the notochord and the fin-fold. Black arrowheads mark isolated caudal notochord cells expressing high levels of cadherin. (F) High-magnification view of the embryo in E showing the caudal tip of the notochord.
Somite boundaries emerge between mesodermal cells that are in direct cell-cell contact. Wood and Thorogood (1994) suggested the formation of the boundary starts in the lateral somitic mesoderm and then spreads into adaxial somitic mesoderm. In contrast, Henry et al. (2000) suggested a random initiation of boundary formation at multiple sites. To test these hypotheses, we examined the ultrastructure of forming somite boundaries of a three-somite embryo where the location of the forming somite boundaries could be established (Figure 6H, black brackets). At high magnification, we observed, several small “islands” of ECM (Figure 6I, black arrowhead) between stretches of direct cell-cell contact that extend both medially and laterally along nascent somite boundaries (white arrowheads). These small patches of matrix are in stark contrast to a mature somite boundary (Figure 6J), which has rich ECM separating cells of adjacent somites. Our observations suggest that somite boundaries form at multiple sites between adjacent somites.
Notochord Cells Initially Express Cadherins and Form Circumferential Focal Adhesions after Intercalation
As previously noted, the earliest adhesion proteins found in the notochord are cadherins that concentrate in the membranes of notochord cells in bud-stage embryos (Figure 7A, black arrowhead). In eight-somite embryos the level of cadherin detected in the anterior notochord cells (Figure 7C, black arrowhead) becomes reduced compared with posterior regions that are still undergoing intercalation. These observations suggest that cadherins are required primarily during intercalation. By prim-5, high levels of cadherins are detected only in isolated cells in the caudal region of the notochord (Figure 7E, black arrowheads; and F). This residual staining suggests that these cells are still undergoing intercalation.
Paxillin and Fak are expressed abundantly in the cytoplasm of intercalating notochord cells (Figure 5, A, C, D, and F, black arrowheads). Their localization on plasma membranes cannot be verified as distinct from nuclear exclusion of the proteins, which gives the impression of their location in the cortex of the cell. Most obvious, however, is that fibronectin and laminin are absent from the regions where cells are intercalating (Figure 6, A, C, and D, black arrowheads). Instead, laminin is present at the periphery of the notochord (Figure 6, D, E, and G). To assess the interaction of notochord cells with the perichordal sheath, we investigated the pattern of Fak phosphorylation. Figure 5G shows a single confocal section of a 10-somite embryo labeled with an antibody to pY397Fak. We can resolve fine circumferential striations where notochord cells are in contact with the perichordal ECM (black arrowhead). Labeling is restricted to the periphery of the notochord. These circumferential striations persist and are shown in a projection of confocal sections comprising the notochord of a prim-5 embryo (24 hpf) stained with anti-pY397Fak in Figure 5I (black arrowhead) and with anti-pY861Fak in Figure 5O (black arrowhead). These observations imply a fine structure in the pattern of adhesion to the extracellular matrix that has not been previously described.
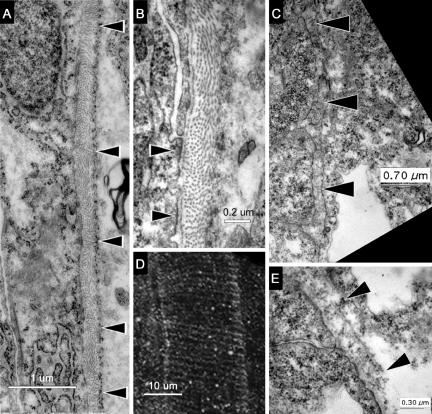
To determine what, if any, relationship these circumferential patterns of Fak-immunolabeling have to the ultrastructure of the notochord sheath surrounding the notochord, we used electron microscopy. In the TEMs of prim-5 embryos (Figure 8A), a fibrous sheath denoted by black arrowheads can be seen beside the notochord cells, which are to the left of the sheath. At high magnification, we see what may be focal adhesions where the notochord cell membrane contacts the perichordal sheath (Figure 8B, black arrowheads). The layer of matrix closest to the notochord and these sites of adhesion is laminin-like, based on ultrastructure. The dominant component of the sheath, however, is probably a fibrillar collagen. The orientations of collagen-like fibers occur in alternating groups, which are either perpendicular to the plane of section, and thus, circumferential to the notochord, or else parallel to the plane of section and thus, longitudinal to the notochord. In prim-5 embryos, the distance between groups of circumferential fibers is 1.56 ± 0.35 μm (n = 14) (Figure 8A, black arrowheads), and the distance between circumferential striations of immunostaining for pY861Fak is 1.45 ± 0.27 μm (n = 26) (Figure 8D). These are statistically indistinguishable patterns. We hypothesize that the pattern of phosphorylation of Fak represents a spatially periodic pattern of adhesion by notochord cells to the sheath. During notochord elongation, this pattern of adhesion may generate an uneven mechanical load on the sheath, resulting in the observed alternating pattern of orientation of the collagen-like fibers.
Figure 8.
Ultrastructure of the notochord sheath reveals patterns of stress that correlate with rings of phosphorylated Fak. (A) TEM of a horizontal section through the notochord of a prim-5 embryo (24 hpf). Black arrowheads indicate changes in the orientation of fibers in the inner layer. The notochord is to the left of the sheath and somites to the right. (B) High-magnification TEM of this notochord sheath showing sites where the membrane of a notochord cell adheres to the fibrous sheath (black arrowheads). (D) Projection of confocal sections of the notochord of a prim-5 embryo stained with anti-pY861Fak shows circumferential striations (anterior up, dorsal to the right). (C) TEM of a horizontal section through the notochord of a three-somite embryo (11 hpf) showing the immature notochord sheath marked with black arrowheads. The notochord cells are to the left of the sheath. (E) High-magnification TEMs of the notochord sheath (black arrowheads) in a three-somite embryo showing only circumferential fiber orientations.
To test this hypothesis, we looked at the sheath of the notochord in three-somite embryos before notochord extension has occurred. The fibers of the matrix surrounding the notochord in the three-somite embryos occur only in circumferential orientation (Figure 8, C and E). We also measured the distance between circumferential striations of pY397Fak around the notochord periphery in an embryo that did not yet have a straight body-axis. The distance between striations in the 10-somite embryos in Figure 5G is 0.63 ± 0.18 μm (n = 24), which is significantly smaller that that of the older prim-5 embryo (P ≪ 0.01) (Figure 8E; see above). Consistent with our hypothesis, the longitudinal collagen fibers indicative of stretching are absent from the notochord sheath in early embryos. Thus, as the notochord elongates, the spacing between striations in Fak phosphorylation expand and the longitudinal collagen fibers occur.
Paxillin, Cadherin, and Noncanonically Phosphorylated Fak Codistribute at the Lateral Membranes of the Enveloping Layer Cells
The first tissue to develop in the zebrafish embryos is the EVL. The EVL is an epithelial sheet that migrates over the yolk and gastrulating embryo, forming an extraembryonic membrane (Kimmel et al., 1995). The cells of the EVL remain a coherent epithelium that extends and flattens, enveloping the yolk and the rest of the embryo. The mechanism of its migration is not clear (Betchaku and Trinkaus, 1978; Cooper and Kimmel, 1998; Zalik et al., 1999). This epithelium undergoes apoptosis at around prim-15 (30 hpf) shown by TUNEL labeling of apoptotic EVL cell nuclei (Figure 9S).
To better understand the migration of this epithelial EVL, we investigated the distribution and activity of cell adhesion proteins during epiboly of the EVL by using confocal microscopy. Anti-paxillin and anti-cadherin immunolabeling shows concentrations of both proteins at the lateral margins of EVL cells at 50% epiboly (Figure 9, A and D). This codistribution of paxillin and cadherin continues as long as the EVL is present and is shown herein in a six-somite embryo (Figure 9, B and E) and in a prim-5 embryo (Figure 9, C and F). At higher magnification, the paxillin staining (Figure 9C) is often more diffuse than cadherin staining (Figure 9F) and may extend into the cytosol with actin.
The existence of tight and adherens junctions and desmosomes (macula adherens) between cells of the EVL were confirmed by TEM. Tight junctions hold these cells together at their apical-lateral points of contact (Figure 9P, white arrow). Basal to the tight junctions are an adherens junction followed by a row of desmosomes surrounded by dense material that is probably intermediate filaments (Figure 9P, white and black arrowheads). This structure is consistent with tension being applied to these cells as they spread over the embryo.
The surprising observations that paxillin is concentrated at the lateral margins of EVL cells and that some tyrosine residues of Fak, but not Tyr397, are phosphorylated during epiboly (Figure 4) led us to investigate the localization of phosphorylated Fak during epiboly and gastrulation. fak1b, the dominant isoform of Fak expressed early in development (Figure 3, B and C), is not recognized by anti-Fak-C20 and is therefore not shown. However, faint immunoreactivity can be seen in the EVL of prim-5 embryos stained with anti-Fak-C20 (Figure 5E, arrowhead), suggesting that both isoforms of Fak may be expressed in the EVL. The phosphorylated residues of Fak detectable at EVL cell boundaries are pY576/7Fak (Figure 9, J–L) and pY861Fak (Figure 5, M–O). Phosphorylation on Tyr397 is not detected at the borders of EVL cells in any stages examined (Figure 9, G–I). Punctate background staining was seen in both cellular and acellular regions of the embryo when using anti-pY397Fak antibodies (Figure 9, G–I), but no labeling was ever seen at the lateral membranes of EVL cells. Similar findings are shown in Figure 5 where anti-pY576Fak and anti-pY861Fak stain the epithelium of EVL cell margins (Figure 5, K, L, and O), but anti-pY397Fak does not (Figure 5I). These observations are consistent with the phosphorylation patterns of Fak seen in Western blots before the bud stage (Figure 4). How phosphorylated-Fak functions in the EVL is not yet clear, but it does not seem to depend on stable phosphorylation of Tyr397.
Zalik et al. (1999) and Betchaku and Trinkaus (1978)) have shown that EVL cells contain conspicuous cortical actin belts and that the apical surfaces of the EVL cells are covered with small aggregates of actin coincident with microvilli or ridges. We investigated the relationship between paxillin, cortical belts, and these microvilli. In the EVL at the prim-5 stage (24 hpf), paxillin is abundant (Figure 9R) in areas of actin concentration (Figure 9Q) that is in the cortical belt and microvilli-like extension. One possible interpretation is that paxillin is associated with actin support filaments spreading from adherens or tight junctions.
In summary, the EVL migrates as an epithelial layer connected at their lateral margins by tight and cadherin-based adherens junctions. The focal adhesion proteins paxillin and Fak associate near these junctions. The distribution of paxillin, pY576/577Fak and pY861Fak, cadherin, and actin at the lateral margins of the EVL cells are strikingly similar from 50% epiboly through prim-15, suggesting a cooperation in their functions. The stably phosphorylated tyrosine residues of Fak in the EVL are in the kinase domain, near the Cas-binding site, and in the Map kinase-signaling domain, but not at the autophosphorylation site.
DISCUSSION
Focal adhesion complexes and cadherin-based cell-cell interactions play distinct roles in cell migration and cell adhesion, and therefore in morphogenesis. The regulation of these two modes of cell interaction in embryos is not understood. We have described several tissues where distribution of adherens junctions and/or cadherin proteins overlap with activated focal adhesion proteins and have made some unexpected observations.
Regulation of Focal Adhesion Components
The transcriptional regulation of the segmentally expressed fak1a mRNA was shown to be strongly affected by mutants in Notch signaling (Henry et al., 2001). The expression pattern of the nonsegmentally expressed paxillin mRNA is independent of Notch or other signaling that confers segmentation in the paraxial mesoderm (Conlon et al., 1995; Barrantes et al., 1999). Despite these differences in transcriptional regulation, paxillin and Fak1a proteins show many similarities in expression pattern at the protein level especially in somitogenesis and notogenesis. Both proteins accumulate at the boundaries of the forming somites where fibronectin is secreted, persist after the formation of the intersomitic borders, and remain through myotome formation. Both proteins are abundant in the notochord and both proteins seem to be participating in the cell-cell adhesion between EVL cells.
Cell Adhesion in Somite Boundary Formation
Focal adhesions do not act alone in forming somites. Cadherin proteins occur early on the membranes of every cell of the presomitic mesoderm. In early zebrafish embryos, we see a reduction in the abundance of cadherins at the interface with neighboring somite border cells, similar to that in chick embryos where there is strong expression of cadherin proteins in the inner mesenchymal cells of forming chick somites and down-regulation near the somite borders (Duband et al., 1987; Radice et al., 1997). The observations in chicks suggested that the formation of cell-cell adhesions drives the formation of groups of somite cells. In other words, the adhesions of the central mesenchymal cells pull together groups of cells to form discrete somites, segmenting the presomitic mesoderm. We have shown previously that, at least in zebrafish, the central mesenchyme cells are not needed for the formation of somite boundaries (Henry et al., 2000). Herein, we also show with TEM images that a matrix is secreted between somite boundaries in patches as the borders form.
Our observations that phosphorylated Fak and paxillin concentrate at somite boundaries where fibronectin is secreted is consistent with integrin-based adhesion to fibronectin at one edge of these polarized cells (Henry et al., 2001; this study). Laminin is not required because mutations in laminin β1 and γ1 do not disrupt somite boundary formation. Rather, laminin mutations disrupt the formation of the notochord, which is surrounded by laminin, and the differentiation of somites at a stage when we see laminin in somites (grumpy and sleepy; Stemple et al., 1996; Parsons et al., 2002). We see also in the TEM sections that cell-cell contacts extend across somite boundaries in late-stage embryos, which may contain the cadherin proteins we observe in mature somites and may stabilize these boundaries. Thus, the formation of somites is mediated by both cell-cell (cadherin) and cell-matrix (fibronectin/integrin/Fak) junctions.
Cell Adhesion in Notogenesis
The nature of the cell-cell or cell-matrix interactions involved in the intercalation of notochord cells is obscure (Keller et al., 1989; Winklbauer and Keller, 1996; Henry et al., 2001). We found cadherin proteins and little, if any, matrix material between the intercalating cells of the early notochord. Furthermore, we detected phosphorylated Fak only at the interface between notochord cells and the perichordal sheath, not in the middle of the notochord where the cells are extending processes that intercalate between one another. Thus, integrin-based adhesion to the ECM may not provide traction for the intercalating cells. In zebrafish, the N-cadherin (Cdh2) gene parachute clearly mediates convergence of dorsal cells in the midbrain, a process similar to notochord cell intercalation (Lele et al., 2002). Zebrafish E-cadherin (Cdh1) mRNA is present in the midline during early development and could be involved in the ventral notochord cell movements (Babb et al., 2001). We and others (Duband et al., 1987; Krotoski and Bronner-Fraser, 1990; Delarue et al., 1998) have shown that cadherins are present surrounding notochord cells, and we suggest that cadherin-based cell-cell adhesion could provide the major traction force required for notochord intercalation. This idea is consistent with results of mosaic expression of a dominant-negative cadherin construct in Xenopus embryos, in which axial mesoderm cells expressing the construct fail to intercalate (Delarue et al., 1998).
Notogenesis involves the deposition of an ECM sheath around the presumptive notochord cells and the intercalation of the notochord cells into a single column of disk-shaped cells that contact the ECM at their peripheries, like a stack of pennies in a tube. The notochord cells then develop large internal vacuoles, the inflation of which results in elongation and stiffening of the notochord. One possible mechanism for this elongation is that the mechanical properties of the sheath and the interactions of the cells with the sheath result in a system that resists lateral expansion, while permitting longitudinal expansion (Adams et al., 1990). Our observations with respect to the ultrastructure of the zebrafish notochord sheath and the pattern of Fak phosphorylation within the notochord are consistent with such a model.
Viewed by TEM, the zebrafish notochord sheath has an extensive fibrillar layer, which seems structurally similar to collagens. Zebrafish notochord cells express type II collagen mRNA (Yan et al., 1995; Lele and Krone, 1997), and types I and II collagen are well-characterized components of the perichordal sheath in other vertebrates (Ghanem, 1996; Zhu et al., 2001). The collagen-like fibrillar matrix is bound on the notochord side by laminin, which is required for notogenesis: mutations in laminin β1 and γ1 block differentiation of the notochord, which is rescued by exogenous sources of laminin (Parsons et al., 2002).
We see a change in the orientation of the collagen-like fibrillar matrix that correlates with notochord elongation and with a change in distance between the circular patterns of Fak phosphorylation on the periphery of the notochord. In the early embryo, the collagen-like fibers within the sheath are consistently circumferential, and circumferential striations in phospho-Fak immunolabeling are close together. Later in development, we see alternating patterns of circumferential and longitudinal collagen-like fibers and the circumferential striations in phospho-Fak immunolabeling are further apart. If the notochord cells were adhering to the fibrillar sheath by periodic junctions such as focal adhesion complexes while the notochord was undergoing expansion by vacuolization, these periodic junctions would move apart and the fibers between the junctions would be stretched longitudinally, like a coil-spring being stretched. We observe this pattern in the perichordal sheath.
Focal Adhesion Proteins in the Enveloping Epithelial Layer
Previous investigations have shown that cadherins are abundant at the lateral margins of EVL cells (Zalik et al., 1999) and are important in maintaining the integrity of the ectoderm during epiboly in Xenopus embryos (Levine et al., 1994, Lee and Gumbiner, 1995). Although Betchaku and Trinkaus (1978) have studied the morphology and ultrastructure of the EVL in the teleost, Fundulus heteroclitis, apart from the studies of Zalik et al. (1999), little is known about the nature of the EVL in zebrafish.
The vegetal spreading of the EVL is an interesting example of a migrating epithelial sheet. Typically, cells of an epithelium share tight junctions and cadherin-based adherens junctions and desmosomes (reviewed in Jamora and Fuchs, 2002). ECM and integrins are ostensibly absent between neighboring cells in an epithelium. This view of the EVL is supported by the close apposition of the membranes and the absence of detectable ECM in TEMs and high magnification confocal micrographs (Figure 9P). However, we find both paxillin and phosphorylated Fak, hallmarks of integrin-mediated cell-ECM adhesion, concentrated along the lateral membranes of these squamous epithelial cells where cadherin is also present. It is provocative that Fak in the EVL is not detectably phosphorylated on Tyr397, but is phosphorylated on Try576/577 and Tyr861. McLean et al. (2000) showed that v-Src phosphorylates residues in FAK in fibroblasts without phosphorylating Tyr397 and results in a decrease in the fibronectin-stimulated phosphorylation of FAK. Src phosphorylation of FAK may also drive the deregulation of E-cadherin-associated adhesion in colon cancer cells (Avizienyte et al., 2002). However, the noncanonical pattern of FAK phosphorylation has not been documented in the context of a normal tissue nor its biological significance understood. Tsai and Kinsey (2002)) showed that the tyrosine kinase Yes is active in zebrafish embryos during epiboly and thus Yes could potentially contribute to the phosphorylation of Fak1b. Another Src-like kinase, Fyn, is activated by fertilization of zebrafish eggs (Rongish and Kinsey, 2000); however, there is no evidence yet that Fyn is active during epiboly. We propose that, in the epithelium of zebrafish EVL, modulation of the actin cytoskeleton at cadherin-based cell-cell adhesions may involve Tyr397-independent phosphorylation of Fak by Src-like kinases such as Yes, although we cannot rule out rapid dephosphorylation of Tyr397.
The integration of Fak and paxillin into the protein complex on the cytoplasmic face of EVL cells may be mediated through 1) a direct cadherin-paxillin interaction, because E-cadherin has a potential paxillin binding sequence (Brown et al., 1998), or 2) through an indirect interaction with vinculin, which binds paxillin and thus FAK (Tong et al., 1997). Cell-cell junctions in the corneal epithelium and neuroepithelium also bind paxillin (Sugrue and Zieske, 1997; Chenn et al., 1998).
Several potential roles exist for paxillin and Fak in this migrating epithelial sheet. It is unlikely that Fak in these cells is a signal for cell division because EVL cells divide only once after the 10th cleavage cycle of the embryo (Kane et al., 1992). One possibility is that Fak signaling blocks premature apoptosis (Almeida et al., 2000). Fak also has a FERM domain, which would allow it to interact with molecules such as phosphatidylinositol phosphate kinase type1γ that are involved in membrane recycling (Liddington et al., 2003). Thus, Fak1b may be involved in membrane recycling during the expansion of the EVL epithelial layer to 50% epiboly as it envelops the yolk cell, or in remodeling of the EVL membrane as it moves over the lower equator of the yolk cells. Paxillin may function to stabilize the microvilli on the apical surface of EVL cells, which in turn might provide storage for membrane and cytoskeletal material needed as the EVL expands over the yolk. Our data support roles for paxillin and Fak in the organization of actin around tight junctions or adherens junctions: functions that would help stabilize or remodel cell-cell borders during the migration of this epithelial sheet.
The PGMT
The shift in activity patterns of focal adhesion proteins observed during development is of great interest. Dramatic changes in the expression as well as qualitative and quantitative changes in phosphorylation of focal adhesion proteins are indicative of a fundamental change in the adhesive milieu of mesoderm cells at the onset of somitogenesis. Hagel et al. (2002) note in the mouse embryo, similar to our observations in the zebrafish embryo, that there is expression of paxillin in extraembryonic membranes early in development, and a dramatic increase in paxillin abundance in the mesoderm after gastrulation. Bitzur et al. (1994) also noted in the zebrafish a de novo synthesis of one subtype of cadherin at the end of gastrulation, which persists through somitogenesis. We also observed an increased expression of fak1a mRNA during somitogenesis. The molecular triggers and consequences of this PGMT as well as the interactions between cadherin-based and focal adhesion-based cell adhesion probably play central roles in the morphogenesis of vertebrate embryos. We have only begun to scratch the surface of our understanding of these processes.
Acknowledgments
We thank Dr. Mark S. Cooper for many exciting discussions and advice. We thank the following for generous gifts and advise: Drs. Thierry Lepage and David Kimelman for the cDNA library, Dr. Bruce W. Draper for the PAC library, and Dr. Chris J. Thorpe for the RACE library. We thank Paul Kong, Rainy Reagan, Cara Poage, and Salina Park for technical support, and Drs. Ingrith Deyrup-Olsen, Jessica Smith, and Steven Kulak for reviewing this manuscript. M.B.H. thanks the faculty at the Institute of Neuroscience (University of Oregon) for generous support and interactions during sabbatical leave, especially Dr. Charles B. Kimmel for discussions, Yi-Lin Yan and Dr. John Postlethwait for mapping data, and The Zebrafish International Resource Center (National Institutes of Health-National Center for Research Resources RR12546) for fish support. B.D.C thanks Dr. Dave Pilgrim for generous support during the preparation of this manuscript. C.A.H. was supported in part by Public Health Service National Research Service Award T32 GM07270 from National Institute of General Medical Sciences. The UW Royalty Research Fund and a March of Dimes grant to M.B.H. are acknowledged with gratitude.
Abbreviations used: CAK-β, cell adhesion kinase-β, which is also known as protein tyrosine kinase 2β (PTK2β), and focal adhesion kinase 2 (FADK 2); ECM, extracellular matrix; EVL, enveloping layer; FAK, mouse, Xenopus, or human focal adhesion kinase protein; Fak, zebrafish focal adhesion kinase protein; all numbering of Fak residues is according to the known phosphorylated human sites; fak, mRNA or DNA focal adhesions kinase gene; hpf, hours postfertilization; PGMT, postgastrula mesoderm transition; TEM, transmission electron microscopy.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-08-0537. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0537.
References
- Adams, D.S., Keller, R., and Ma, K. (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115–130. [DOI] [PubMed] [Google Scholar]
- Almeida, E.A.C., Ilic, D., Han, Q., Hauck, C.R., Jin, F., Kawakatsu, H., Schlaepfer, D.D., and Damsky, C.H. (2000). Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH2-terminal kinase. J. Cell Biol. 149, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avizienyte, E., Wyke, A.W., Jones, R.J., McLean, G.W., Westhoff, M.A., Brunton, V.G., and Frame, M.C. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat. Cell Biol. 4, 632–638. [DOI] [PubMed] [Google Scholar]
- Babb, S.G., Barnett, J., Doedens, A.L., Cobb, N., Liu, Q., Sorkin, B.C., Yelick, P.C., Raymond, P.A., and Marrs, J.A. (2001). Zebrafish E-cadherin: expression during early embryogenesis and regulation during brain development. Dev. Dyn. 22, 231–237. [DOI] [PubMed] [Google Scholar]
- Barrantes, I.B., Elia, A.J., Wunsch, K., De Angelis, M.H., Mak, T.W., Rossant, J., Conlon, R.A., Gossler, A., and de la Pompa, J.L. (1999). Interaction between notch signaling and lunatic fringe during somite boundary formation in the mouse. Curr. Biol. 9, 470–480. [DOI] [PubMed] [Google Scholar]
- Betchaku, T., and Trinkaus, J.P. (1978). Contact relations, surface activity, and cortical microfilaments in marginal cells of the enveloping layer of the yolk syncytial and yolk cytoplasmic layers of Fundulus before and during epiboly. J. Exp. Zool. 206, 381–426. [DOI] [PubMed] [Google Scholar]
- Bitzur, S., Kam, Z., and Geiger, B. (1994). Structure and distribution of N-cadherin in developing zebrafish embryos: morphogenetic effects of ectopic over-expression. Dev. Dyn. 201, 121–136. [DOI] [PubMed] [Google Scholar]
- Brown, M.C., Curtis, M.S., and Turner, C.E. (1998). Paxillin LD motifs may define a new family of protein recognition domains. Nat. Struct. Biol. 5, 677–678. [DOI] [PubMed] [Google Scholar]
- Brown, M.C., West, K.A., and Turner, C.E. (2002). Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesion involves a multistep activation pathway. Mol. Biol. Cell 13, 1550–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary, L.A., Klinghoffer, R.A., Sachsenmaier, C., and Cooper, J.A. (2002). SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22, 2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn, A., Zhang, Y.A., Chang, B.T., and McConnell, S.K. (1998). Intrinsic polarity of mammalian neuroepithelial cells. Mol. Cell Neurosci. 11, 183–193. [DOI] [PubMed] [Google Scholar]
- Conlon, R.A., Reaume, A.G., and Rossant, J. (1995). Notch1 is required for the coordinate segmentation of somites. Development 121, 1533–1545. [DOI] [PubMed] [Google Scholar]
- Cooper, M.S., and Kimmel, C.B. (1998). Morphogenetic cell behaviors and specification of cell fate during early teleost development, in Motion Analysis of Living Cells, ed. D.R. Soll and D. Wessels, New York: Wiley-Liss, 177–220.
- Delarue, M., Saez, F.J., Boucaut, J.-C., Thiery, J.-P., and Broders, F. (1998). Medial cell mixing during axial morphogenesis of the amphibian embryo requires cadherin function. Dev. Dyn. 213, 248–260. [DOI] [PubMed] [Google Scholar]
- Duband, J.L., Dufour, S., Hatta, K., Takeichi, M., Edelman, G.M., and Thiery, J.P. (1987). Adhesion molecules during somitogenesis in the avian embryo. J. Cell Biol. 104, 1361–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta, Y., Ilic, D., Kanazawa, S., Takeda, N., Yamamoto, T., and Aizawa, S. (1995). Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene 11, 1989–1995. [PubMed] [Google Scholar]
- Garcia-Alvarez, B., de Pereda, J.M., Calderwood, D.A., Ulmer, T.S., Critchley, D., Campbell, I.D., Ginsberg, M.H., and Liddington, R.C. (2003). Structural determinants of integrin recognition by talin. Mol. Cell. 11, 49–58. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse, E.N., George, E.L., Rayburn, H., and Hynes, R.O. (1996). Mesodermal development in mouse embryos mutant for fibronectin. Dev. Dyn. 207, 145–156. [DOI] [PubMed] [Google Scholar]
- Ghanem, E. (1996). Immunohistochemical localization of type I and II collagens in the involuting chick notochords in vivo and in vitro. Cell Biol. Int. 20, 681–685. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (2000). Regulation of cadherin adhesive activity. J. Cell Biol. 148, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel, M., George, E.L., Kim, A., Tamimi, R., Opitz, S.L., Turner, C.E., Imamoto, A., and Thomas, S.M. (2002). The adapter protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22, 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks, S.K., and Polte, T.R. (1997). Signaling through focal adhesion kinase. Bioessays 19, 137–145. [DOI] [PubMed] [Google Scholar]
- Henry, C.A., Hall, L.A., Hille, M.B., Solnica-Krezel, L., and Cooper, M.S. (2000). Somites in zebrafish doubly mutant for knypek and trilobite from without internal mesenchymal cells or compaction. Curr. Biol. 10, 1063–1066. [DOI] [PubMed] [Google Scholar]
- Henry, C.A., Crawford, B.D., Yan, Y.L., Postlethwait, J., Cooper, M.S., and Hille, M.B. (2001). Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis. Dev. Biol. 240, 474–487. [DOI] [PubMed] [Google Scholar]
- Henry, C.A., et al. (2002). Two linked hairy/Enhancer of split-related Zebrafish genes, her1 and her7, function together to refine alternating somite boundaries. Development 129, 3693–3704. [DOI] [PubMed] [Google Scholar]
- Hens, M.D., and DeSimone, D.W. (1995). Molecular analysis and developmental expression of the focal adhesion kinase pp125FAK in Xenopus laevis. Dev. Biol. 170, 274–288. [DOI] [PubMed] [Google Scholar]
- Holley, S.A., Jülich, D., Rauch, G.-J., Geisler, R., and Nüsslein-Volhard, C. (2002). her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development 129, 1175–1183. [DOI] [PubMed] [Google Scholar]
- Jamora, C., and Fuchs, E. (2002). Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 4, 101–108. [DOI] [PubMed] [Google Scholar]
- Kane, D.A., Warga, R.M., and Kimmel, C.B. (1992). Mitotic domains in the early embryo of the zebrafish. Nature 360, 735–737. [DOI] [PubMed] [Google Scholar]
- Keller, R., Cooper, M.S., Danilchik, M., Tibbetts, P., and Wilson, P.A. (1989). Cell intercalation during notochord development in Xenopus laevis. J. Exp. Zool. 251, 134–154. [DOI] [PubMed] [Google Scholar]
- Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B., and Schilling, T.F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Krotoski, D., and Bronner-Fraser, M. (1990). Distribution of integrins and their ligands in the trunk of Xenopus laevis during neural crest cell migration. J. Exp. Zool. 253, 139–150. [DOI] [PubMed] [Google Scholar]
- Lee, C.-H., and Gumbiner, B.M. (1995). Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev. Biol. 171, 363–373. [DOI] [PubMed] [Google Scholar]
- Lele, Z., Folchert, A., Concha, M., Rauch, G.J., Geisler, R., Rosa, F., Wilson, S.W., Hammerschmidt, M., and Bally-Cuif, L. (2002). Parachute/N-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development 129, 3281–3294. [DOI] [PubMed] [Google Scholar]
- Lele, Z., and Krone, P.H. (1997). Expression of genes encoding the collagen-binding heat shock protein (Hsp47) and type II collage in developing zebrafish embryos. Mech. Dev. 61, 89–98. [DOI] [PubMed] [Google Scholar]
- Levine, E., Lee, C.H., Kintner, C., and Gumbiner, B.M. (1994). Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development 120, 901–909. [DOI] [PubMed] [Google Scholar]
- Liddington, R.C., Bankston, L.A., and de Pereda, J.M. (2003). Cell adhesion: a FERM grasp of membrane dynamics. Curr. Biol. 13, R94–R95. [DOI] [PubMed] [Google Scholar]
- Linask, K.K., Ludwig, C., Han, M.D., Liu, X., Radice, G.L., and Knudsen, K.A. (1998). N-Cadherin/catenin-mediated morphoregulation of somite formation. Dev. Biol. 202, 85–102. [DOI] [PubMed] [Google Scholar]
- McLean, G.W., Fincham, V.J., and Frame, M.C. (2000). v-Src induces tyrosine phosphorylation of focal adhesion kinase independently of tyrosine 397 and formation of a complex with Src. J. Biol. Chem. 275, 23333–23339. [DOI] [PubMed] [Google Scholar]
- Ogawa, M., Hiraoka, Y., Taniguchi, K., Sakai, Y., and Aiso, S. (2001). mRNA sequence of the Xenopus laevis paxillin gene and its expression. Biochim. Biophys. Acta 1519, 235–240. [DOI] [PubMed] [Google Scholar]
- Parsons, M.J., Pollard, S.M., Saúde, L., Feldman, B., Coutinho, P., Hirst, E.M.A., and Stemple, D.L. (2002). Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137–3146. [DOI] [PubMed] [Google Scholar]
- Polte, T.R., Naftilan, A.J., and Hanks, S.K. (1994). Focal adhesion kinase is abundant in developing blood vessels and elevation of its phosphotyrosine content in vascular smooth muscle cells is a rapid response to angiotensin II. J. Cell. Biochem. 55, 106–119. [DOI] [PubMed] [Google Scholar]
- Radice, G.L., Rayburn, H., Matsunami, H., Knudsen, K.A., Takeichi, M., and Hynes, R.O. (1997). Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181, 64–78. [DOI] [PubMed] [Google Scholar]
- Rongish, B.J., and Kinsey, W.H. (2000). Transient nuclear localization of Fyn kinase during development in zebrafish. Anat. Rec. 260, 115–123. [DOI] [PubMed] [Google Scholar]
- Roy, S., Ruest, P.J., and Hanks, S.K. (2002). Fak regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J. Cell. Biochem. 84, 377–388. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., and Hunter, T. (1998). Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8, 151–157. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A. (2001). Integrin signaling revisited. Trends Cell Biol. 11, 466–470. [DOI] [PubMed] [Google Scholar]
- Sugrue, S.P., and Zieske, J.D. (1997). ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp. Eye Res. 64, 11–20. [DOI] [PubMed] [Google Scholar]
- Stemple, D.L. (1996). Mutations affecting development of the notochord in zebrafish. Development 123, 117–128. [DOI] [PubMed] [Google Scholar]
- Tsai, W., and Kinsey, W.H. (2002). Role of c-Yes kinase during gastrulation of the zebrafish embryos. Mol. Biol. Cell 13, 117a. [Google Scholar]
- Tong, X., Salgia, R., Li, J.-L., Griffin, J.D., and Howley, P.M. (1997). The bovine papillomavirus E6 protein binds to the LD motif repeats of paxillin and blocks its interaction with vinculin and the focal adhesion kinase. J. Biol. Chem. 272, 33373–33376. [DOI] [PubMed] [Google Scholar]
- Turner, C.E. (1991). Paxillin is a major phosphotyrosine-containing protein during embryonic development. J. Cell Biol. 115, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C.E. (2000a). Paxillin and focal adhesion signalling. Nat. Cell Biol. 2, E231–E236. [DOI] [PubMed] [Google Scholar]
- Turner, C.E. (2000b). Paxillin interactions. J. Cell Sci. 113, 4139–4140. [DOI] [PubMed] [Google Scholar]
- West, K.A., Zhang, H., Brown, M.C., Nikolopoulos, S.N., Riedy, M.C., Horwitz, A.F., and Turner, C.E. (2001). The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL). J. Cell Biol. 154, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield, M. (1995). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press.
- Winklbauer, R., and Keller, R.E. (1996). Fibronectin, mesodermal migration and gastrulation. Dev. Biol. 177, 413–426. [DOI] [PubMed] [Google Scholar]
- Winklbauer, R., Nagel, M., Selchow, A., and Wacker, S. (1996). Mesoderm migration in the Xenopus gastrula. Int. J. Dev. Biol. 40, 305–311. [PubMed] [Google Scholar]
- Wood, A., and Thorogood, P. (1994). Patterns of cell behaviour underlying somitogenesis and notochord formation in intact vertebrate embryos. Dev. Dyn. 201, 151–167. [DOI] [PubMed] [Google Scholar]
- Yan, Y.L., Hatta, K., Riggleman, B., and Postlethwait, J.H. (1995). Expression of a type II collagen gene in the zebrafish embryonic axis. Dev. Dyn. 203, 363–376. [DOI] [PubMed] [Google Scholar]
- Yang, J.T., Bader, B.L., Kreidberg, J.A., Ullman-Cullere, M., Trevithick, J.E., and Hynes, R.O. (1999). Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev. Biol. 215, 264–277. [DOI] [PubMed] [Google Scholar]
- Zalik, S.E., Lewandowski, E., Kam, Z., and Geiger, B. (1999). Cell adhesion and the actin cytoskeleton of the enveloping layer in the zebrafish embryo during epiboly. Biochem. Cell Biol. 77, 527–542. [PubMed] [Google Scholar]
- Zhu, Y., McAlinden, A., and Sandell, L.J. (2001). Type IIA procollagen in development of the human intervertebral disc: regulated expression of the NH(2)-propeptide by enzymic processing reveals a unique developmental pathway. Dev. Dyn. 220, 350–362. [DOI] [PubMed] [Google Scholar]