Abstract
We have shown that protein kinase A phosphorylation of t-SNAREs inhibits SNARE assembly and suppresses endo- and exocytosis in yeast. Herein, we show that protein kinase A phosphorylation of the Sso exocytic t-SNAREs promotes the binding of Vsm1, a potential SNARE regulator identified previously in our laboratory. Phosphorylation of Sso increases its affinity for Vsm1 by more than fivefold in vitro and both phosphorylated Sso1, as well as Sso1 bearing an aspartate substitution at position 79, interact tightly with Vsm1. Vsm1 binding is dependent upon the NH2-terminal autoinhibitory domain of Sso, and constitutively “open” forms of the t-SNARE show a reduction in Vsm1 binding in vivo. The substitution of serine-79 in Sso1 with an alanine residue or the treatment of yeast with C2-ceramide, which results in the dephosphorylation of serine-79, both inhibit Vsm1 binding in vivo. Importantly, Vsm1 binding to Sso seems to preclude Sso binding to its partner t-SNARE, Sec9, and vice versa. This is consistent with the idea that Vsm1 is an inhibitor of SNARE assembly in yeast. Thus, one way by which phosphorylation inhibits SNARE assembly could be by regulating the association of inhibitory factors that control the ability of t-SNAREs to form complexes in vivo.
INTRODUCTION
Intracellular membrane fusion is mediated by three major families of membrane-associated proteins (e.g., vesicle-associated membrane protein [VAMP], syntaxin, and soluble N-ethylmaleimide-sensitive factor attachment protein-25 [SNAP-25]) known as soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (Rothman and Warren, 1994). Cognate SNAREs present on both donor and acceptor membranes (designated as v-SNAREs and t-SNAREs, respectively), interact in trans to form a tight four-helix bundle (Sutton et al., 1998), which brings the bilayers into proximity and is thought to lead to membrane fusion (Lin and Scheller, 2000; Waters and Hughson; 2000). After fusion, SNAREs reside in the same membrane in an inactive cis conformation. Soluble N-ethylmaleimide-sensitive fusion protein (NSF) acts to disassemble cis complexes and primes the SNAREs for the next round of fusion (Lin and Scheller, 2000; Wickner and Haas, 2000). Although other factors may be involved in vivo (Peters et al., 2001), SNAREs constitute the minimal machinery required for membrane fusion in vitro (Weber et al., 1998).
The events leading to SNARE assembly in vivo involve a number of accessory proteins, though their functions are not entirely known. For example, Sec1/Munc18 (SM) interacts with members of the Sso/syntaxin family of t-SNAREs (Hata et al., 1993; Garcia et al., 1994; Pevsner et al., 1994). Yeast Sec1 binds to exocytic SNARE complexes containing Sso, Sec9 (a SNAP-25 ortholog), and Snc (a VAMP ortholog), but apparently not to free Sso (Carr et al., 1999). Sly1, a Sec1 homolog that acts upon endoplasmic reticulum (ER)-Golgi transport, binds to the Sed5 t-SNARE, and confers specificity to SNARE assembly (Peng and Gallwitz, 2002). Likewise, Vps45 (an endosomal Sec1 homolog) associates with the Tlg2 endocytic t-SNARE and facilitates assembly with Tlg1 and Vti1 (Bryant and James, 2001). Thus, yeast SM proteins act in a positive manner to confer complex formation. Although mammalian Sec1 binds to free and uncomplexed syntaxin in vitro (Pevsner et al., 1994; Yang et al., 2000) and maintains the t-SNARE in a “closed” inactive conformation (Nicholson et al., 1998), recent studies in animal cells also suggest that SM proteins facilitate assembly (Verhage et al., 2000). Another SNARE regulator, LMA1, binds to the primed vacuolar t-SNARE, Vam3, to prevent the reformation of cis SNARE complexes (Xu et al., 1998; Wickner and Haas, 2000). The release of LMA1 from Vam3 is, therefore, necessary to fusion to proceed. Our laboratory identified Vsm1/Ddi1 as a negative regulator of the exocytic SNAREs in yeast (Lustgarten and Gerst, 1999). Vsm1 binds directly to the Snc1,2 v-SNAREs and its overproduction results in the accumulation of low-density secretory vesicles, coupled with an inhibition of growth and secretion in cells bearing a mutant Sec9 t-SNARE.
Both SNAREs and SNARE regulatory proteins are phosphorylated in vitro (for reviews, see Gerst, 1999; Lin and Scheller, 2000). For instance, the phosphorylation of syntaxins by protein kinase C, protein kinase A (PKA), and other kinases reduces their affinity for SNARE partners, while increasing their affinity for the synaptotagmin SNARE regulator (Gerst, 1999; Lin and Scheller, 2000). Studies on vacuolar fusion in yeast have shown that the release of LMA1 from Vam3 is dependent upon the function of protein phosphatase 1 (PP1) (Peters et al., 1999). Thus, the dephosphorylation of Vam3 may be necessary for vacuolar fusion (Wickner and Haas, 2000). Recently, we demonstrated that phosphorylation of the Sso exocytic t-SNAREs by PKA inhibits SNARE assembly and vesicle fusion (Marash and Gerst, 2001). Mutation of a PKA site (serine-79) to alanine in the autoinhibitory domain of Sso1, or its dephosphorylation by a ceramide-activated protein phosphatase (CAPP), increased the ability of Sso to assemble into complexes with the Sec9 t-SNARE. This restored exocytosis and normal growth in certain secretion mutants. Similarly, phosphorylation of the endosomal Tlg t-SNAREs was found to inhibit Tlg SNARE assembly and endocytosis (Gurunathan et al., 2002). Thus, phosphorylation plays a critical role in regulating the availability of t-SNAREs to assemble into v-t SNARE complexes and to confer membrane fusion in vivo.
We now report that the mechanism of phosphorylation-dependent inhibition of the exocytic t-SNAREs in yeast may involve the recruitment of a SNARE regulator. Phosphorylation of the Sso t-SNAREs by PKA increases their affinity for Vsm1 and modulates its binding both in vivo and in vitro. Moreover, Vsm1 binding to this t-SNARE may reduce the availability of Sso to bind the Sec9 t-SNARE and, thus, inhibit SNARE assembly. In contrast, unphosphorylated Sso shows a reduced ability to bind Vsm1 and an increased ability to interact with Sec9, both in vivo and in vitro. Thus, Vsm1 binding may be mutually exclusive with Sec9 binding. Vsm1 requires the NH2-terminal autoinhibitory domain of the t-SNARE for this interaction and constitutively “open” conformations of Sso1 show a reduced ability to bind Vsm1. Finally, removal of the ubiquitin-association domain (UBA) of Vsm1 did not affect Sso binding, and neither VSM1 overexpression nor deletion altered t-SNARE stability. Thus, Vsm1 does not regulate Sso degradation, but it may modulate the ability of the t-SNARE to enter into functional SNARE complexes.
MATERIALS AND METHODS
Yeast Strains
Yeast strains are listed in Table 1.
Table 1.
Yeast strains used in this study
| Name | Genotype | Source |
|---|---|---|
| SP1 | MATa ura3 leu2 trp1 ade8 can1 his3 | M. Wigler |
| NY782 | MATa ura3-52 leu2-3,112 sec9-4 | P. Novick |
| VL3 | MATa his3 leu2 trp1 ade2 vsm1::URA3 | J. Gerst |
| NY1217 | MATa ura3-52 leu2-3,112 sec18-1 | P. Novick |
| FHY102 | MATa his3 leu2 ura3 sso1::kanMX4 sso2::kanMX4 pMM250 (SSO1-CEN-URA3) | F. Hughson |
Plasmids
Plasmids used in this work are listed in Table 2.
Table 2.
Plasmids used in this study
| Name | Gene Expressed | Vector | Insertion sites | Source |
|---|---|---|---|---|
| pADH-mycSSO1a | SSO1 | pAD6 | SalI and SacI | J. Gerst |
| pADH-mycNTSSO1 | SSO12-146 | pAD6 | SalI and SacI | This study |
| pADH-mycSSO1Δ1-103a | SSO1Δ1-103 | pAD6 | SalI and SacI | This study |
| pADH-mycSSO1Δ1-146a | SSO1Δ1-146 | pAD6 | SalI and SacI | This study |
| pADH-mycSSO1A66ac | SSO1A66 | pAD6 | SalI and SacI | J. Gerst |
| pADH-mycSSO1A79ac | SSO1A79 | pAD6 | SalI and SacI | J. Gerst |
| pADH-mycSSO1D79c | SSO1D79 | pAD6 | SalI and SacI | This study |
| pGexSSO11-265d | SSO11-265 | pGex-4T-3 | P. Brennwald | |
| pGexSSO11-265,A66ac | SSO11-265,A66 | pGex-4T-3 | EcoRI and XhoI | This study |
| pGexSSO11-265,A79ac | SSO11-265,A79 | pGex-4T-3 | EcoRI and XhoI | This study |
| pGexSSO11-265,D79c | SSO11-265,D79 | pGex-4T-3 | EcoRI and XhoI | This study |
| pMM251 | SSO1 | M. Munson | ||
| pMM255 | SSO1E84,E95,A148 | M. Munson | ||
| pMM256 | SSO1A95,A99,A119,A123,A148 | M. Munson | ||
| pMM255A66 | SSO1E84,A66,E95,A148 | This study | ||
| pMM255A79 | SSO1E84,A79,E95,A148 | This study | ||
| pHIS6-Vsm1b | VSM1 | pTrc-HisB | XhoI and KpnI | J. Gerst |
| pHIS6-HAVsm1 | HAVSM1 | pTrc-HisB | XhoI and KpnI | This study |
| pADHU-HAVsm1 | HAVSM1 | pUV2 | BamHI | J. Gerst |
| pADH-myc-Vsm12-402 | VSM12-402 | pAD6 | SalI and SacI | This study |
| pGex-TPK1a | TPK1 | pGex-4T-3 | EcoRI and XhoI | J. Gerst |
| pGex-Sec9402-651d | SEC9402-651 | pGex-4T-3 | P. Brennwald | |
| pHIS6-Snc12-94 | SNC12-94 | pTrc-HisA | SacI and HindIII | J. Gerst |
| pHIS6-Snc22-93 | SNC22-93 | pTrc-HisA | SacI and HindIII | J. Gerst |
Protein Phosphorylation
Glutathione S-transferase (GST)-Sso11-265, GST-Sso11-265,A66, GST-Sso11-265,A79, and His6-Vsm1 (3.4E-11 moles each) were phosphorylated with (up to 4 μg) Tpk1 in the presence of 50 μCi of [γ-32P]ATP (5 Ci/μmol) and visualized as described previously (Marash and Gerst, 2001).
Pulse-Chase Analysis
Intracellular processing of Sso was monitored by pulse-chase analysis by using [35S]methionine (Amersham Biosciences, Piscataway, NJ), as described previously (Couve et al., 1995).
Measurement of Protein Complexes
Immunoprecipitation (IP) from Lysates. IP of both SNARE and Sso-Vsm1 complexes from total cell lysates (TCLs) was performed by coimmunoprecipitation, by using the modifications described in Marash and Gerst, 2001. Anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Vsm1 (Lustgarten and Gerst, 1999) antibodies (abs) were used for IP (4 and 1 μl/reaction, respectively). Samples of TCLs and IPs were resolved by SDS-PAGE and detected by Western analysis to determine the amount of Sec9, Snc, Sso, and Vsm1 that either immunoprecipitated or coimmunoprecipitated with a given antiserum. Antibodies used for detection included anti-phosphoserine (1:1000) (Zymed Laboratories, South San Francisco, CA); anti-Sso (1:3000) (gift of S. Keranen, VTT Biotechnology, Espoo, Finland), anti-Sec9 (1:1000) (C terminus) (gift of P. Brennwald, University of North Carolina, Chapel Hill, NC), anti-Snc (1:500) (Protopopov et al., 1993), anti-Sed5 (1:3000) and anti-Tlg1 (1:2000) (gifts of D. Banfield, Hong Kong University of Science and Technology, Hong Kong, China), anti-Tlg2 (1:2000) (gift of H. Abeliovich, Hebrew University of Jerusalem, Rehovot, Israel), anti-Vsm1 (1:3000) (Lustgarten and Gerst, 1999), and anti-Vti1 antibodies (1:3000) (gift of G. Fischer von Mollard, Georg-August Universitat Gottingen, Germany). Detection was performed by chemiluminescence. To improve detection and accuracy, TCL samples were normalized to account for variations in protein expression and recovery.
Sso-Vsm1 Assembly In Vitro. Recombinant affinity-purified GST-Sso11-265, GST-Sso11-265,A66, and GST-Sso11-265,A79 (phosphorylated or nonphosphorylated) and His6-Vsm1 proteins were mixed together at a ratio 1:1 (3.4E-11 moles) in buffer containing 0.5% NP-40 in phosphate-buffered saline, and allowed to incubate overnight at 4°C. Thereafter, proteins were immunoprecipitated with anti-Vsm1 abs (1 μl/reaction), resolved by SDS-PAGE, and detected in blots with anti-Vsm1 and anti-Sso abs (1:3000).
Sso-Sec9 Assembly In Vitro. Purified GST-Sso11-265 (2E-11 moles) and GST-Sec9402-651 (1E-11 moles) were incubated in the absence or presence of increasing amounts of His6-Vsm1 (0.2–10E-11 moles) at 4°C, and resolved by IP and SDS-PAGE (Figure 1C). For competition binding studies (Figure 5), GST-Sso11-265 and GST-Sso11-265,A79 were mixed together at different ratios (0:1, 0.25:0.75, 0.5:0.5, 0.75: 0.25, and 1:0) to yield a final concentration of 3E-11 moles and incubated with 3E-11 moles each of His6-Vsm1 and GST-Sec9402-651.
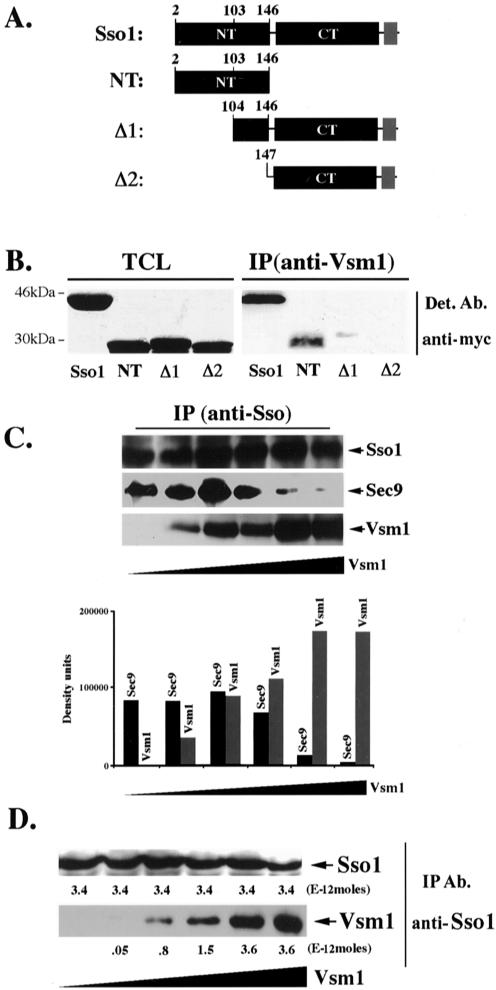
Figure 1.
Vsm1 binds directly to the Sso1 t-SNARE. (A) Scheme of Sso1 deletion mutants. Native Sso1 and deletion mutants (e.g., Sso12-146 [NT], Sso1Δ1-103 [Δ1], and Sso1Δ1-146 [Δ2]) are depicted schematically. NT indicates the NH2-terminal autoinhibitory domain, and CT indicates the COOH-terminal SNARE binding domain and flanking region. The transmembrane domain of Sso1 is shown in gray. Proteins were tagged at the NH2 terminus by using the myc epitope. (B) The NH2 terminus of Sso1 is required for Vsm1 binding. WT cells producing myc-tagged Sso1, Sso12-146, Sso1Δ1-103 and Sso1Δ1-146 were lysed and subjected to IP with anti-Vsm1 abs. Immunoprecipitated complexes were resolved on the gels and detected (Det.) with anti-myc and anti-Vsm1 abs (latter not shown). TCL (100 μg of protein). (C) Vsm1 displaces Sec9 binding to Sso1. GST-Sso11-265 and GST-Sec9402-651 (2E-11 and 1E-11 moles, respectively) were mixed with increasing amounts of His6-Vsm1 (between 0.2 and 10E-11 moles) and incubated overnight (o.n.) at 4°C (see MATERIALS AND METHODS). Proteins were immunoprecipitated with anti-Sso abs and detected in blots. (D) The stoichiometry of Vsm1-Sso1 binding is 1:1. Moles (2.3E-11) of GST-Sso11-265 were mixed with increasing amounts of His6-Vsm1 (between 1 and 16E-11 moles) and incubated overnight at 4°C. Proteins were immunoprecipitated with anti-Sso abs and detected in blots. Molar quantification of the proteins was determined using known amounts of GST-Sso11-265 and His6-Vsm1 that were electrophoresed and detected in parallel to the immunoprecipitated proteins. The stoichiometry is defined as the ratio of the number of moles of GST-Sso11-265 (3.4E-12 moles) immunoprecipitated to the number moles of His6-Vsm1 (3.6E-12 moles) coimmunoprecipitated at saturation.
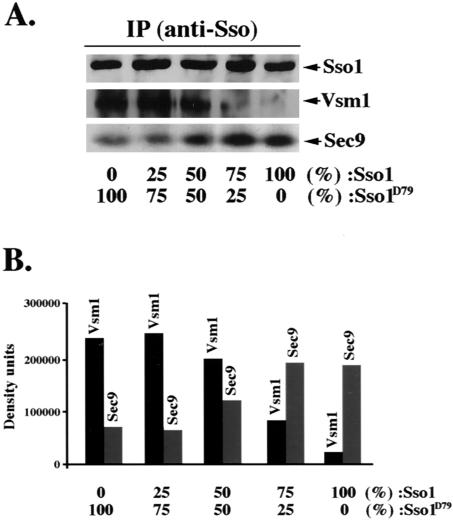
Figure 5.
Phosphorylation modulates the interaction of Sso with Vsm1, at the expense of Sec9. GST-Sso11–265 and the aspartate substitution mutant, GST-Sso11-265,D79, were mixed together to yield a final concentration of 3E-11 moles at the following ratios: 0:1, 0.25: 0.75, 0.5:0.5, 0.75:0.25, and 1:0, respectively. Samples were incubated at 4°C with 3E-11 moles of His6-Vsm1 and GST-Sec9402-651 for 24 h (see MATERIALS AND METHODS). Sso1-containing complexes were immunoprecipitated with anti-Sso abs and detected quantitatively in Westerns by using anti-Sec9, -Vsm1, or -Sso abs. The histogram represents the amounts of His6-Vsm1 and GST-Sec9 bound to GST-Sso11-265.
For both experiments, complexes were immunoprecipitated using anti-Sso abs (1 μl/reaction) and detected quantitatively in Westerns by using anti-Sec9 (1:1000), -Vsm1 (1:3000), or -Sso (1:3000) abs.
Measurement of Sso1-Vsm1 Stoichiometry In Vitro. Moles (2.6E-11) of either GST-Sso11-265 or GST-Sso11-265,D79 were mixed with increasing concentrations of His6-Vsm1 (between 1 and 16E-11 moles) and incubated overnight at 4°C in buffer containing 0.5% NP-40 in phosphate-buffered saline. Proteins were then subjected to IP with anti-Sso abs (1 μl/reaction), resolved by SDS-PAGE, and detected quantitatively in blots by using anti-Sso and anti-Vsm1 (1:3000) abs. Molar quantification of the proteins was determined using known quantities of GST-Sso11-265 and His6-Vsm1 that were purified over glutathione-Sepharose, or nickel beads (Pharmacia, Peapack, NJ), and electrophoresed and detected in parallel to the immunoprecipitated proteins.
Measurement of Sso1-Vsm1 Affinity In Vitro. To measure the affinity of the Sso1–Vsm1 interaction, 0.2E-11 moles of His6-Vsm1 and 0.8E-11 moles of either GST-Sso11-265 or GST-Sso11-265,D79 was incubated with increasing amounts of His6-HA-Vsm1 (e.g., 0, 0.3-, 1.3-, 3.1-, 8.7-, 15.8-, and 31.8E-11 moles). Complexes were precipitated using glutathione-Sepharose beads, separated by SDS-PAGE, and detected with either anti-Sso or anti-Vsm1 abs (1:3000) in blots. Molar quantification of the proteins was determined using known amounts of purified GST-Sso11-265, His6-Vsm1, and His6-HAVsm1 that were detected in parallel to the immunoprecipitated proteins. The constant for half-maximal binding of Vsm1 to Sso was calculated by measuring the displacement of His6-Vsm1 by His6-HAVsm1 in the Sso1-containing complexes. Inverse reciprocal plots of the molar amounts of bound Vsm1 (His6-HAVsm1 and His6-Vsm1) (y-axis) versus total added Vsm1 (x-axis) were made and the data subjected to linear regression. The absolute value of the crossing point of the regression line with the x-axis represents the inverse value of the binding constant.
Measurement of the Rate of Sso1-Vsm1 Binding In Vitro. Equal amounts of His6-Vsm1 (1.5E-11 moles) were added to either GST-Sso11-265 or GST-Sso11-265,D79 (3.4E-11 moles) and incubated for 0, 2, 4, 8, or 16 h. Protein complexes were precipitated with glutathione-Sepharose beads, resolved by SDS-PAGE, and detected with either anti-Sso or anti-Vsm1 abs (1:3000) in blots. Molar quantification of the proteins was determined using known amounts of GST-Sso11-265, His6-Vsm1, and GST-Sso11-265,D79 that were detected in parallel to the immunoprecipitated proteins.
RESULTS
Vsm1 Binds to the Sso t-SNAREs and Is a Competitive Inhibitor for Sec9
Vsm1/Ddi1 was identified as a negative regulator of secretion in yeast (Lustgarten and Gerst, 1999). Deletion of VSM1 in wild-type (WT) cells increases the secretion of proteins into medium, whereas its overexpression leads to the repression of cell growth and secretion, particularly in sec9-4 cells. In addition, direct physical interactions between Vsm1 and the Snc v-SNAREs were observed, suggesting that Vsm1 might exert its effect upon the secretory pathway via the regulation of v-SNARE function.
In studies designed to show the specificity of Vsm1 binding to the Snc v-SNAREs, we examined whether it also binds the Sso t-SNAREs. We precipitated Vsm1 from lysates prepared from WT cells expressing myc-tagged Sso1 and looked for the presence of the t-SNARE. Surprisingly, we found that Sso coimmunoprecipitates with Vsm1 (Figure 1B), suggesting that these t-SNAREs may be targets for Vsm1.
To help define the region of Sso required for Vsm1 binding, we constructed deletion mutants and expressed them in yeast (Figure 1, A and B). We tested myc-tagged mutants bearing either a partial (Δ1; Sso1Δ1-103) or full (Δ2; Sso1Δ1-146) deletion of the NH2-terminal autoinhibitory domain, as well as one lacking the COOH-terminal SNARE binding domain (Sso12-46). Next, we immunoprecipitated complexes formed between the Sso1 deletion mutants and Vsm1, by using anti-Vsm1 antibodies. We found that partial deletion of the NH2-terminal of Sso1 greatly reduced (by >20-fold) the binding of Sso1 to Vsm1, whereas deletion of the entire autoinhibitory domain resulted in complete abrogation of the interaction (Figure 1B). This suggests that the NH2-terminal domain of Sso1 is necessary for Vsm1 binding.
The autoinhibitory domain of Sso interacts with the SNARE-binding motif of the t-SNARE to form an intramolecular closed structure, thus, preventing SNARE complex assembly (Nicholson et al., 1998). Because this domain may be necessary for Vsm1 binding, we examined whether Vsm1 binds directly to it when expressed alone in yeast (Figure 1B). To ensure proper localization of the NH2-terminal fragment (Sso12-46) to the plasma membrane, we inserted a CAAX motif at its COOH terminus. Expression of this construct in WT cells had no deleterious effects upon growth (our unpublished data). Upon immunoprecipitation with anti-Vsm1 antibodies, we found that a portion of the autoinhibitory domain was bound to Vsm1. This suggests that the SNARE-binding domain of Sso is not required for its association with the Vsm1 SNARE regulator.
To determine whether Vsm1 binding affects the ability of Sso to bind to its t-SNARE partner, Sec9, we examined whether increasing amounts of Vsm1 could displace Sec9 binding in vitro. We used increasing amounts of recombinant His6-tagged Vsm1 (0.2–10E-10 moles) in binding reactions containing GST-tagged Sso11-265 and GST-Sec9402-651 (2E-11 and 1E-11 moles, respectively). As the concentration of Vsm1 in the assay increased, there was a corresponding decrease in the amount of Sec9 bound to Sso1 (Figure 1C). Thus, Vsm1 acts as a competitive inhibitor of SNARE assembly in vitro.
To determine stoichiometry of the Sso-Vsm1 interaction, we incubated fixed amounts of recombinant GST-Sso11-265 (2.3E-11 moles) with increasing amounts of His6-Vsm1 (0, 1, 2, 4, 8, and 16E-11 moles). To measure the molar equivalents of precipitated His6-Vsm1 and bound GST-Sso11-265, we used purified GST-Sso11-265 and His6-Vsm1 as protein standards that were detected in parallel in Westerns. This quantitative analysis revealed that His6-Vsm1 and GST-Sso11-265 interact in vitro and at a ratio of 1:1, at saturation (Figure 1D).
Phosphorylation of Sso1 at Serine-79 by Tpk1 Promotes Vsm1 Binding In Vitro
Given that PKA phosphorylation of the NH2 terminus domain of the Sso t-SNAREs inhibits SNARE assembly and that Vsm1 binds to this domain, we determined the effect of phosphorylation upon Vsm1 binding in vitro (Figure 2A). Purified GST-Sso11-265 (3.4E-11 moles) was phosphorylated in vitro with unlabeled ATP by using increasing amounts of recombinant Tpk1 (a catalytic subunit of PKA) and mixed with an equimolar amount of His6-Vsm1. Next, complexes were precipitated with anti-Sso antibodies and the amount of bound Vsm1 measured by quantitative Western analysis. In parallel, equal amounts of GST-Sso11-265 and His6-Vsm1 were phosphorylated under identical conditions by using radioactive [γ-32P]ATP. Interestingly, we found that an increase in Sso phosphorylation correlated with a large enhancement in Vsm1 binding (Figure 2A), whereas Vsm1 itself was not phosphorylated under these conditions (Figure 2A) or in vivo (Lustgarten and Gerst, 1999).
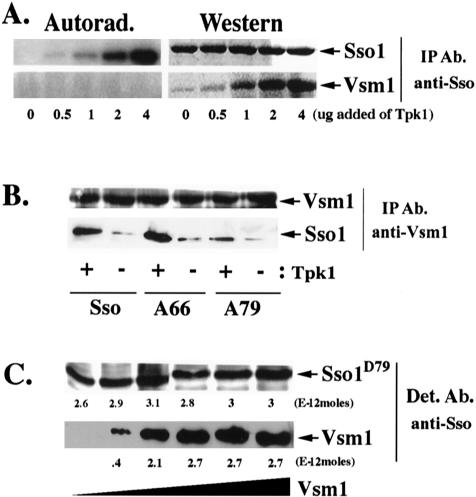
Figure 2.
Phosphorylation of Sso1 at serine-79 enhances Vsm1 binding in vitro. (A) PKA-dependent phosphorylation of Sso1 increases the Vsm1 binding in vitro. GST-Sso11-265 and His6-Vsm1 (3.4E-11 moles each) were mixed with increasing amounts of Tpk1 in the presence of either ATP or [γ-32P]ATP for 1 h at 30°C. Proteins were immunoprecipitated with anti-Sso abs and detected in blots by using anti-Sso and -Vsm1 abs or by autoradiography. (B) PKA-dependent phosphorylation of Sso1 at serine-79 is important for the Vsm1 binding in vitro. Phosphorylated and nonphosphorylated GST-tagged native Sso11-265 (Sso) and alanine substitution mutants Sso11-265,A66 (A66) and Sso11-265,D79 (A79) were incubated overnight at 4°C with His6-Vsm1 at a 1:1 molar ratio. Proteins were immunoprecipitated using anti-Vsm1 abs and were detected in blots with anti-Sso or -Vsm1 abs. (C) The stoichiometry of Vsm1 and Sso1D79 binding is 1:1. 2.3E-11 moles of GST-Sso11-265,D79 were mixed with increasing amounts of His6-Vsm1 (between 1 and 16E-11 moles) and incubated overnight at 4°C (see MATERIALS AND METHODS). Molar quantification of the proteins was determined using known amounts of GST-Sso11-265,D79 and His6-Vsm1 that were detected in parallel. The stoichiometry was defined as the ratio of the number of moles of GST-Sso11-265,D79 (3E-12 moles) immunoprecipitated to the number moles of His6-Vsm1 (2.7E-12 moles) coimmunoprecipitated at saturation.
Analysis of the Sso1 sequence reveals two putative PKA phosphorylation sites at threonine-66 and serine-79, although only serine-79 plays a role in SNARE assembly (Marash and Gerst, 2001). To define the extent of GST-Sso11-265 phosphorylation, we found that 1.9 moles of phosphate was incorporated per mole of GST-Sso11-265 (7.6E-11 moles of phosphate was incorporated into 3.96E-11 moles of Sso1) at saturation, suggesting that both PKA sites undergo phosphorylation by Tpk1 (our unpublished data; Marash and Gerst, 2001). To determine which PKA site plays a role in the binding of Vsm1, we phosphorylated mutants bearing alanine substitutions at the relevant PKA sites of Sso1 (e.g., GST-Sso11-265,A66 and GST-Sso11-265,A79) in vitro. The phosphorylated t-SNAREs were then mixed with His6-Vsm1 and immunoprecipitated with anti-Vsm1 antibodies. As shown above, Tpk1-dependent phosphorylation of GST-Sso11-265 increased the binding of Vsm1 (Figure 2, A and B). Presence of the alanine substitution at position 66 of Sso1 did not inhibit the interaction (Figure 2B), whereas, in contrast, an alanine substitution at position 79 decreased Vsm1 binding by threefold, even in the presence of Tpk1. Alanine substitutions at both positions did not inhibit the Vsm1–Sso1 interaction more than the single substitution at position 79 (our unpublished data). Thus, the phosphorylation of Sso1 at serine-79 is likely to be important for normal Vsm1 binding.
As shown above (Figure 1D), unphosphorylated Sso binds Vsm1 at a 1:1 ratio. To determine whether phosphorylation of serine-79 alters the stoichiometry of the Sso–Vsm1 interaction, we introduced an aspartate mutation that mimics phosphorylation into this site. We incubated fixed amounts of purified recombinant GST-Sso11-265,D79 (2.3E-11 moles) with increasing amounts of His6-Vsm1 (0, 1, 2, 4, 8, and 16E-11 moles) (Figure 2C). After precipitation and quantitative detection in blots, we found that His6-Vsm1 and GST-Sso11-265,D79 also interact in vitro in a ratio of 1:1.
Because the stoichiometries of Vsm1 binding to native Sso1 or Sso1D79 are equivalent, we examined whether the increased binding of Vsm1 to phosphorylated Sso1 results from a change in affinity. To measure the binding affinities of Sso1 and pseudophosphorylated Sso1 for Vsm1, we incubated a mixture of 0.2E-11 moles of His6-Vsm1 and 0.8E-11 moles of either GST-Sso11-265or GST-Sso11-265,D79 with increasing amounts of His6-HAVsm1 (e.g., 0–31.8E-11 moles) (Figure 3A). After quantitative detection, the constants for half-maximal binding were calculated from the molar concentrations of His6-HAVsm1 necessary to displace His6-Vsm1 in Sso1- and Sso1D79-containing complexes. We found that the affinity of Vsm1 for native Sso1 is fivefold lower (n = 2 experiments) than for Sso1D79 (1.9E-7M versus 4.2E-8M in the experiment shown in Figure 3A). This suggests that Sso phosphorylation greatly increases its affinity for Vsm1.
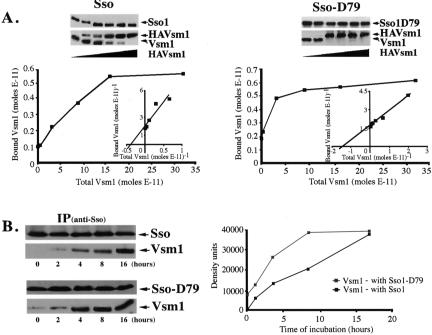
Figure 3.
Phosphorylation of Sso1 at serine-79 increases the affinity for Vsm1 and its rate of binding. (A) Phosphorylation of Sso1 at serine-79 increases its affinity for Vsm1. Moles (0.2E-11) of His6-Vsm1 and 0.8E-11 moles of either GST-Sso11-265or GST-Sso11-265,D79 were incubated with increasing amounts of His6-HAVsm1 (see MATERIALS AND METHODS). Molar quantification of the proteins was determined using known amounts of affinity-purified GST-Sso11-265,D79, GST-Sso11-265, and His6-Vsm1 that were detected in parallel. The affinity was determined according to the molar concentration of Vsm1 necessary for half-maximal binding to Sso (see MATERIALS AND METHODS). (B) Phosphorylation of Sso1 at serine-79 increases the rate of Vsm1 assembly. His6-Vsm1 (1.5E-11 moles) was mixed with either GST-Sso11-265 (3.4E-11 moles) or GST-Sso11-265,D79 (3.4E-11 moles) and incubated for 0–16 h (see MATERIALS AND METHODS). The data are also represented graphically (right), whereby His6-Vsm1 precipitated with GST-Sso11-265 is shown using black squares, whereas His6-Vsm1 precipitated with GST-Sso11-265,D79 is shown using gray squares.
To further characterize the effect of Sso phosphorylation on Vsm1 binding, we measured the rate of assembly between Vsm1 and either Sso11-265 or Sso11-265,D79. We added 1.5E-11 moles of His6-Vsm1 to equal amounts (3.4E-11 moles) of either GST-Sso11-265 or GST-Sso11-265,D79 and incubated them for 0–16 h (Figure 3B). We found that Vsm1 binding to Sso11-265,D79 reached saturation in half the time it took with native Sso1. Thus, Sso phosphorylation increases both the affinity for, and rate of assembly with, Vsm1.
Phosphorylation of Sso1 at Serine-79 Promotes Vsm1 Binding In Vivo
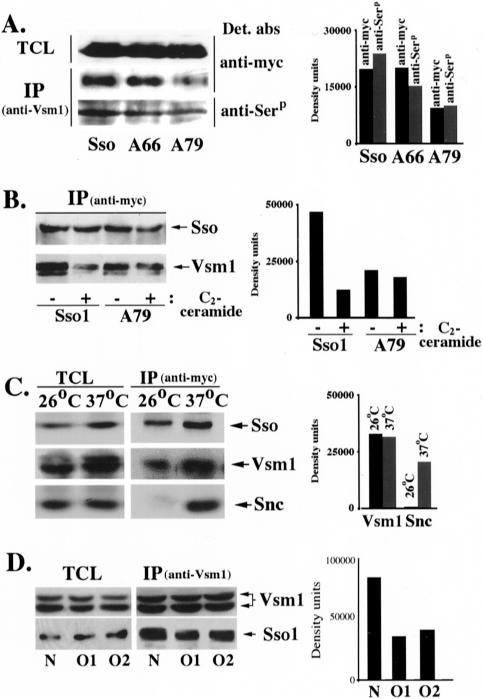
PKA-dependent phosphorylation of Sso inhibits SNARE complex formation by reducing the apparent affinity for its t-SNARE partner, Sec9 (Marash and Gerst, 2001). This work suggests that the phosphorylation of Sso1 on serine-79 has an additional effect, i.e., enhancing the binding of Vsm1 to Sso1 in vitro. To find out whether this effect is meaningful in living cells, we examined Vsm1 binding to Sso1 and the Sso1 alanine substitution mutants in vivo. We expressed myc-tagged forms of native Sso1, Sso1A66, and Sso1A79 in WT cells and examined their ability to bind Vsm1. We found that both native and mutant Sso1 bound Vsm1 (Figure 4A); however, Sso1A79 bound about half as much as either native Sso1 or Sso1A66. Because the alanine-79 mutant is significantly less phosphorylated in vivo (Marash and Gerst, 2001), it suggested that phosphorylation mediates the Vsm1-Sso1 interaction in vivo, as well as in vitro (Figures 2, A and B, and 3). To determine whether Sso bound to Vsm1 is phosphorylated, we used an anti-phosphoserine antibody that can be used to detect SNAREs that undergo phosphorylation in vivo (our unpublished data). When precipitated using anti-Vsm1 antibodies we found that native Sso1, as well as the Sso1A66 and Sso1A79 mutants, could be detected with the anti-phosphosphserine antibody though the signal corresponding to Sso1A79 was less. This was expected because the serine-to-alanine substitution at position 79 blocks the phosphorylation site used by PKA. That the signal is not completely blocked indicates that other serine residues in Sso1 may be phosphorylated by additional kinases in vivo. Nonetheless, the results imply that phosphorylated Sso associates with Vsm1 in vivo.
Figure 4.
Phosphorylation of Sso1 at serine-79 is important for Vsm1 binding in vivo. (A) PKA-dependent phosphorylation of Sso1 at serine-79 is important for Vsm1 binding in vivo. WT cells expressing myc-tagged Sso1, Sso1A66 (A66) and Sso1A79 (A79) were lysed and subjected to IP with anti-Vsm1 abs. Complexes were resolved on the gels and detected with anti-myc abs to detect tagged Sso or anti-phosphoserine (anti-Serp) antibodies to determine whether precipitated Sso was phosphorylated in vivo. The histogram (right) shows both the relative amounts of Sso that coimmunoprecipitated with anti-Vsm1 abs, as well as the level of serine phosphorylation present therein. (B) C2-ceramide inhibits the binding of Sso to Vsm1 in vivo. sec9-4 cells overexpressing myc-tagged Sso1 or Sso1A79 (A79) grown overnight in either presence (+) or absence (–) of added C2-ceramide. Cells were lysed and subjected to IP with anti-myc abs. Proteins were detected in blots by using either anti-myc or -Vsm1 abs. The histogram represents the amount of Vsm1 coimmunoprecipitated after normalization for expression. (C) Vsm1 binds to Sso that is not assembled into SNARE complexes. sec18-1 cells were maintained at 26°C or shifted to the restrictive temperature (37°C) for 15 min to accumulate SNARE complexes. Proteins were immunoprecipitated with anti-Sso abs and detected in Westerns by using anti-Sso, -Vsm1, or -Snc abs. The histogram (right) shows the IP data after normalization for increased Sso precipitation at 37°C. (D) Vsm1 binds better to native Sso1 than constitutively open mutants. Native Sso1 (N) and two open conformations (O1, O2) of Sso1 were expressed in the sso null yeast and complexes containing Vsm1, and the different forms of Sso1 were immunoprecipitated with anti-Vsm1 abs. Proteins were detected in blots by using either anti-Sso or -Vsm1 abs. Due to extended electrophoresis, the two molecular weight forms of Vsm1 (Lustgarten and Gerst, 1999) are visible. The histogram represents the amount of coimmunoprecipitated proteins after normalization for expression.
Because Sso phosphorylation enhances Vsm1 binding, we conjectured that t-SNARE dephosphorylation must reduce Sso-Vsm1 assembly. Previously, we showed that the activation of CAPP dephosphorylates Sso1 at the serine-79 position (Marash and Gerst, 2001). To determine whether CAPP activation affects the interaction of native Sso1 and Sso1A79 with Vsm1, we isolated Sso–Vsm1 complexes from C2-ceramide–treated and untreated sec9-4 cells overexpressing SSO1 or SSO1A79. We used sec9-4 cells because of their enhanced sensitivity to VSM1 overexpression (Lustgarten and Gerst, 1999). Addition of 10 μM C2-ceramide strongly reduced the binding of Vsm1 to Sso1 (by 3.7-fold), whereas little effect on the binding to Sso1A79 was seen (Figure 4B). This result suggests that CAPP-mediated dephosphorylation of Sso1 at position 79 regulates its interaction with Vsm1 in vivo.
Vsm1 Does Not Seem to Bind to Sso in SNARE Complexes Formed In Vivo
Because t-SNARE phosphorylation inhibits SNARE assembly (Marash and Gerst, 2001), but mediates the association of Vsm1 and Sso (Figures 2 and 3), it suggested that Vsm1 should not bind to assembled SNARE complexes. This idea is supported by in vitro binding data showing that Sec9 can be displaced by Vsm1 (Figure 1C). To test this idea in vivo, we examined Vsm1 binding to Sso in temperature-sensitive sec18-1 cells, which accumulate SNARE complexes at restrictive temperatures (Carr et al., 1999). We found that Vsm1, but not Snc, was bound to Sso at permissive temperatures (26°C) (Figure 4C). In contrast, a large increase in Snc v-SNARE binding was observed at 37°C (due to SNARE assembly), whereas no significant change in the amount of bound Vsm1 was detected, after normalization for the increase in Sso synthesis and precipitation at 37°C (Figure 4C). This suggests that Vsm1 is not likely to bind to Sso t-SNAREs that have assembled into SNARE complexes in vivo.
Constitutively Open Forms of Sso Bind Less Vsm1
Sso t-SNAREs exist in either an active (open) or inactive (closed) conformation, mediated by the NH2 terminus autoinhibitory domain (Nicholson et al., 1998). Switching from the closed to open conformation is necessary for Sso assembly into SNARE complexes with its cognate SNARE partners (i.e., the Sec9 t-SNAREs and Snc v-SNAREs). To determine which configuration binds Vsm1, we assessed the ability of Vsm1 to interact with either native Sso1 or constitutively open mutants (Munson and Hughson, 2002). The open mutants of Sso1 are fully functional and are able to replace the native form in yeast. We expected that if Vsm1 binds preferentially to the closed conformation of Sso1 then native Sso1 should recruit more Vsm1 than the open mutants. Native Sso1 and the two open (Sso1V84E,K95E,Y148A [O1] and Sso1K95A,K99A,R119A,L123A,Y148A [O2]) mutants were expressed in ssoΔ yeast and complexes containing Vsm1 and Sso1 precipitated using anti-Vsm1 antibodies. We found that Vsm1 binds twice as much native Sso1 than either of the two open forms (Figure 4D). Thus, the open conformation of Sso seems to inhibit Vsm1 binding.
Phosphorylation Alters the Equilibrium between Sso Binding to Its t-SNARE Partner, Sec9, or to Vsm1
Phosphorylation of Sso inhibits its interaction with Sec9 (Marash and Gerst, 2001) but increases that with Vsm1 (Figures 2 and 3). Moreover, Vsm1 preferentially binds to the native (Figure 4D) and uncomplexed form of Sso (Figure 4C) in vivo, and displaces Sec9 in vitro (Figure 1C). Thus, phosphorylation-dependent Vsm1 binding may prevent Sso from assembling into SNARE complexes. To verify this, we examined whether the binding of phosphorylated Sso to Vsm1 excludes Sec9. We mixed GST-Sso11-265 and GST-Sso11-265,D79 at different ratios (e.g., 0:1, 0.25:0.75, 0.5:0.5, 0.75:0.25, and 1:0), with equimolar amounts of His6-Vsm1 and GST-Sec9402-651. Next, we performed quantitative detection of the amounts of Vsm1 and Sec9402-651 that precipitated with Sso. We found that as the percentage of GST-Sso11-265,D79 increased, the amount of Vsm1 bound increased proportionally (Figure 5, A and B). Likewise, as the percentage of GST-Sso11-265,D79 decreased, there was a concomitant rise in Sec9 binding. Thus, Vsm1 binds to Sso at the expense of its SNARE partner.
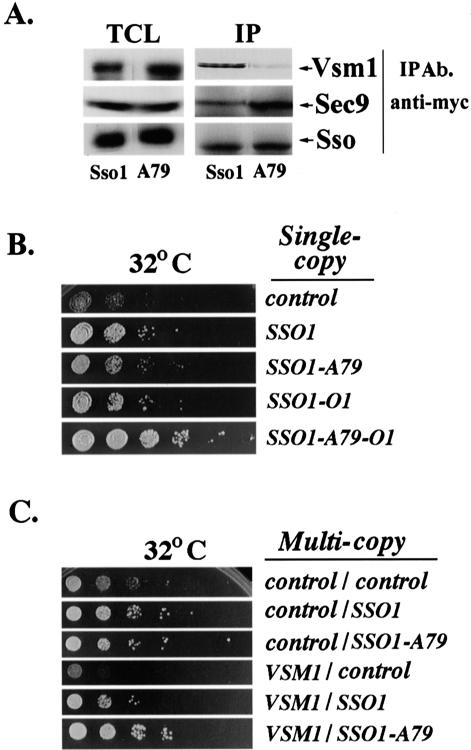
To determine whether this competition occurs in vivo, we monitored the amounts of Sso1 in complexes with either Vsm1 or Sec9 in sec9-4 cells overexpressing VSM1. Quantitative analysis revealed that 2.2-fold less Vsm1 was bound to Sso1A79 than to native Sso (Figure 6A). Correspondingly, there was a twofold increase in the amount of Sec9 bound to Sso1A79 versus native Sso1. Thus, the reduction in Vsm1 binding of Sso1A79 may lead to an increase in the amount of Sso available to form complexes with Sec9.
Figure 6.
An alanine-79 mutation in Sso1 enhances the open conformation of the t-SNARE. (A) Mutation of Sso1 at serine-79 abolishes the binding of Vsm1 and increases Sso-Sec9 SNARE complex assembly in sec9-4 cells at semirestrictive temperatures. sec9-4 cells overexpressing myc-tagged Sso1 or Sso1A79 (A79) were lysed and subjected to IP with anti-myc abs. Proteins were detected in blots by using anti-myc, -Vsm1, or -Sec9 abs. TCL (100 μg). (B) The open form of Sso1A79 abolishes the growth inhibition of sec9-4 cells overexpressing VSM1. sec9-4 cells were transformed with a multicopy plasmid expressing VSM1 alone or also with single-copy plasmids expressing either native SSO1, the open conformation of SSO1 (SSO1-O1), or their phosphorylation mutants that bear an alanine substitution at position 79 (SSO1-A79 and SSO1-O1-A79). Transformants were grown in liquid medium (24 h), diluted serially, and grown on plates at 32°C for 36 h. (C) Sso1A79 overproduction suppresses the growth inhibition of sec9-4 cells overexpressing VSM1 better than native Sso1. sec9-4 cells overproducing Sso1 or Sso1A79 (SSO1-A79) were transformed with a control vector or a second plasmid that overexpresses VSM1. Cells were grown to log phase at 26°C, diluted serially, and plated at 32°C (semirestrictive temperature).
An Alanine Substitution at Position 79 Enhances the Open Conformation of Sso
Because sec9-4 cells are sensitive to Vsm1 overproduction (Lustgarten and Gerst, 1999), we examined whether Sso1, open Sso1 (Sso1-O1), and their alanine-79 mutants (Sso1A79 and Sso1A79-O1, respectively) rescue sec9-4 cells overexpressing VSM1, when expressed from single-copy plasmids. As shown previously, VSM1 overexpression inhibited the growth of sec9-4 cells (Figure 6, B and C). However, Sso1 and Sso1-O1, as well as their alanine-79 mutants, can suppress Vsm1-mediated growth inhibition (Figure 6B). Notably, rescue by the Sso1A79-O1 mutant was much stronger than that of either Sso1-O1 or Sso1A79. This suggests that a lack of phosphorylation at serine-79 enhances functioning of the open (and active) form of the t-SNARE.
When overexpressed from multicopy plasmids, we found that Sso1A79 also restores the growth of sec9-4 cells overexpressing VSM1 (Figure 6C). This implies, but does not prove, that the alanine mutation at position 79 allows the t-SNARE to assume a more open conformation. Nevertheless, it would seem that the absence of phosphorylation at this site not only reduces the interaction between Sso and Vsm1 in vitro and in vivo, but also leads to the rescue of yeast sensitive to VSM1 overexpression.
Vsm1 Binds to Other Snc-interacting t-SNAREs
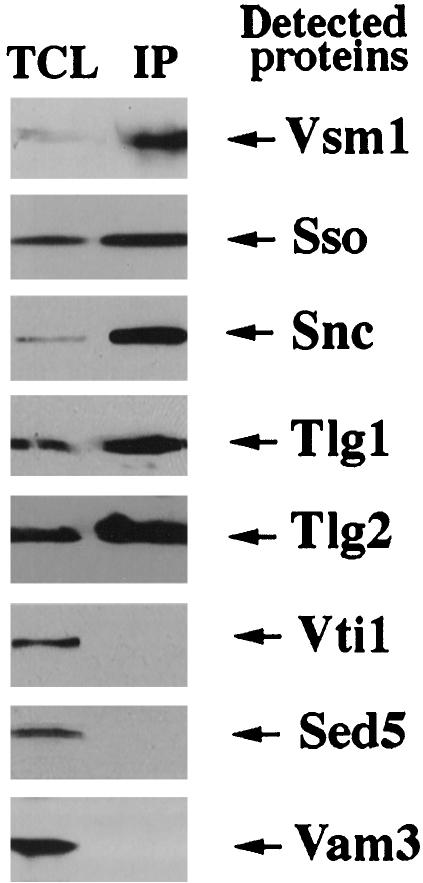
To investigate the specificity of Vsm1 interactions with late-acting SNAREs, we tested its ability to bind to SNAREs involved in other intracellular fusion events. We immunoprecipitated Vsm1 from WT cells and examined the precipitates for the presence of Sso, Sed5 (a cis-Golgi t-SNARE), Vam3 (a vacuolar t-SNARE), Tlg2 (an endosomal t-SNARE), Tlg1 (an endosomal and trans-Golgi t-SNARE), Snc, and Vti1 (an endosomal t-SNARE). We found that Vsm1 interacts with Tlg1 and Tlg2, as well as with the Snc and Sso proteins (Figure 7). Interestingly, Vsm1 was not found in the complexes with SNAREs that act upon either ER-Golgi or vacuolar fusion. This suggests that Vsm1 acts upon SNAREs involved in exo- and endocytosis, and specifically with known Snc v-SNARE partners, e.g., Tlg1, Tlg2, and Sso. That Vsm1 does not interact with Vti1, a putative component of the endocytic SNARE complex, suggests that its interactions are specific to certain endosomal t-SNAREs.
Figure 7.
Vsm1 binds to other Snc-interacting t-SNAREs. Vsm1-containing complexes were immunoprecipitated from lysates by using anti-Vsm1 abs. Complexes were resolved on gels and subjected to the Western analysis by using anti-Vsm1, -Sso, -Snc, -Tlg1, -Tlg2, -Vti1, -Sed5, and -Vam3 abs. TCL (100 μg/lane).
The UBA Domain of Vsm1 Is Not Required for the Binding to Sso
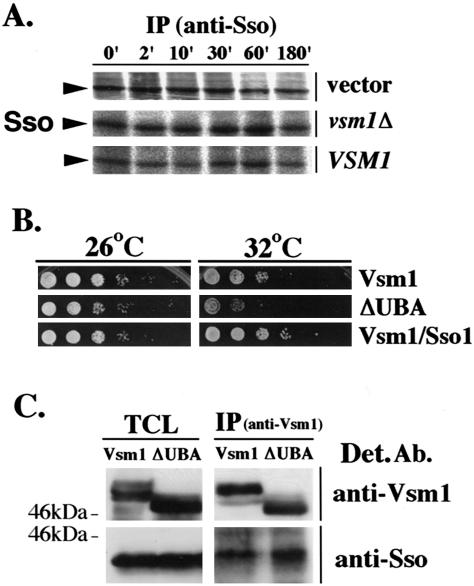
Vsm1/Ddi1 interacts directly with ubiquitin through a UBA domain at its COOH terminus (Bertolaet et al., 2001) and is involved in the degradation of the Pds1 checkpoint factor (Clarke et al., 2001). This suggests that Vsm1 regulates the stability of some proteins. To determine whether Vsm1 modulates t-SNARE stability, we measured the rate of Sso degradation in sec9-4 cells bearing either a deletion of VSM1 or overexpressing it from a multi-copy vector. Sso processing was identical in all cell types and the t-SNARE was stable for up to 3 h (Figure 8A). This suggests that Vsm1 does not exert its effect upon the secretory pathway via the modulation of Sso degradation.
Figure 8.
Vsm1 does not regulate t-SNARE stability. (A) Vsm1 does not affect the stability of Sso. Pulse-chase experiments were performed in sec9-4 cells bearing a control vector (vector), a deletion in VSM1 (vsm1), or overexpressing VSM1 (VSM1). Cells were labeled with [35S]methionine for 3 min and chased with unlabeled methionine/cysteine for up to 180 min. Sso proteins were immunoprecipitated from lysates by using anti-Sso abs and detected in gels by autoradiography. (B) The UBA domain of Vsm1 is not required for the growth suppression of sec9-4 cells. sec9-4 cells overproducing either native Vsm1 (Vsm1) or Vsm1 lacking its UBA domain (ΔUBA) were diluted serially and grown on plates at 26°C and 32°C for 36 h. sec9-4 cells overproducing both Vsm1 and Sso1 (Vsm1/Sso1) were used as a positive control. (C) The UBA domain of Vsm1 is not required for binding to Sso. WT cells overproducing either native Vsm1 (Vsm1) or Vsm1 lacking its UBA domain (ΔUBA) were lysed and subjected to IP by using anti-Vsm1 abs. Proteins were detected using anti-Sso and -Vsm1 abs. TCL (100 μg/lane).
To verify this, we constructed a mutant lacking the UBA domain (Vsm12-402). This mutant should not bind ubiquitin and is unlikely to participate in degradative processes (Clarke et al., 2001). We overexpressed both native and mutant forms of VSM1 in sec9-4 cells and tested their growth at semirestrictive temperatures (Figure 8B). We found that the Vsm1 deletion mutant repressed the growth of sec9-4 cells better than native Vsm1, suggesting that UBA domain is not necessary for inhibition.
Finally, we tested the binding of native Vsm1 and Vsm12-402 to the Sso t-SNAREs in sec9-4 cells. We found that both forms of Vsm1 bound equally to Sso, suggesting that the UBA domain does not mediate SNARE interactions (Figure 8C). This lends credence to the idea that any role of Vsm1/Ddi1 in protein degradation is irrelevant to its function on the secretory pathway.
DISCUSSION
SNARE phosphorylation may be an important way to modulate protein trafficking and secretion. Previous work from our laboratory identified the Sso t-SNAREs as targets for PKA activity (Marash and Gerst, 2001). Phosphorylation of Sso1 at serine-79 inhibited the interaction with its SNARE partners, resulting in an inhibition in exocytosis. This effect was observed in cells lacking the Snc exocytic v-SNAREs, as well as in cells expressing the full complement of SNAREs, suggesting that the effect of PKA phosphorylation on SNARE complex assembly is physiologically relevant. Ongoing work has shown that t-SNARE phosphorylation is not exclusive to the exocytic process but that it is a general feature of secretory pathway, such as endocytosis (Gurunathan et al., 2002) and ER-Golgi transport (our unpublished data). We have found that the Tlg t-SNAREs, which operate on the endocytic and endosomal transport routes, are also phosphorylated by PKA, which inhibits SNARE assembly and endocytosis (Gurunathan et al., 2002).
Although a role for phosphorylation in the regulation of SNARE assembly is obvious, the precise mechanism by which it controls complex formation in vivo is not fully resolved. Herein, we demonstrate that phosphorylation may control the ability of the Sso t-SNARE to form complexes with Sec9, by modulating the interaction between Sso and the Vsm1 SNARE regulator. Sso binds directly to Vsm1 (Figure 1), a v-SNARE–binding protein and negative regulator of exocytosis (Lustgarten and Gerst, 1999). Phosphorylation of Sso1 on serine-79 by PKA increases its affinity for Vsm1 (Figures 2, 3, and 5), leading to a dramatic reduction of bound Sec9 (Figures 5 and 6A). This was shown using either phosphorylated Sso1 or an aspartate substitution at position 79. Correspondingly, substitution of serine-79 with alanine inhibited Vsm1 binding to Sso in vivo (Figures 4, A and B, and 6A). Loss of Sec9 binding to phosphorylated (or pseudophosphorylated) Sso was concommitant with an increase in Vsm1 binding (Figures 5 and 6A). This suggests that t-SNARE phosphorylation inhibits the formation of SNARE complexes not only through a change in affinity (Marash and Gerst, 2001) but also through the recruitment of accessory factors (this study). Thus, the function of SNARE regulators, such as Vsm1, may be to control the availability of SNAREs to enter into SNARE complexes.
Vsm1 binding to Sso requires the NH2 terminus of the t-SNARE, because deletion of this regulatory region blocks the interaction. Yet, the NH2 terminus alone binds less Vsm1 than full-length Sso (Figure 1B), suggesting that presence of the COOH-terminal SNARE binding domain yields a structure that is better recognized by Vsm1. Because Vsm1 seems to bind Sso that is not assembled into SNARE complexes (Figures 4C and 5) and displaces Sec9 (Figures 1C and 5), it is likely that Vsm1 interacts preferably with the closed and inactive conformation of Sso, rather than with the active open conformation. This idea is partially supported by tests using specific open conformation mutants of Sso, which bind less Vsm1 than native Sso (Figure 4D). In fact, the lower level of phosphorylation (and loss of Vsm1 binding) seen with the alanine-79 mutant (Figure 4, A and B) correlated well with the rescue of sec9-4 cells (Figure 6C) and greatly enhanced function of an open form of the Sso t-SNARE (Figure 6B). Thus, it may be that dephosphorylation of serine-79 stabilizes the open conformation of Sso (Figure 6C) and leads to Sec9 binding, whereas phosphorylation favors the closed conformation, and leads to Vsm1 binding. More work will be required to verify these points.
This work suggests that PKA positively regulates the Sso–Vsm1 interaction. This is consistent with previous data showing that t-SNARE phosphorylation regulates the binding of accessory factors. For example, casein kinase II phosphorylation of syntaxin-4 decreased its affinity for SNAP-25, but increased the affinity for synaptotagmin (Risinger and Bennett, 1999). Because PKA-dependent phosphorylation of Sso1 greatly increased Vsm1 binding in vitro (Figures 2, A and C, 3, and 5), we assumed that dephosphorylation would result in its release in vivo. Indeed, CAPP activation by the ceramide analog C2-ceramide resulted in the release of Vsm1 from Sso (Figure 4B). Interestingly, dissociation of the LMA1 SNARE regulator from Vam3 depends upon the activity of the Glc7 phosphatase (PP1). Inhibition of PP1 by the phosphatase inhibitor microcystin LR blocks the release of LMA1 from Vam3 and subsequent vacuolar fusion (Peters et al., 1999; Wickner and Haas, 2000). Therefore, a precedent for phosphorylation/dephosphorylation in the binding and release of SNARE regulatory factors exists, and to which Vsm1 adheres.
Because Vsm1 seems to bind to uncomplexed Sso t-SNAREs (Figures 4C and 5) and interacts with the NH2 terminus of Sso (Figure 1B), it suggests that the inhibitory effect of Vsm1 is exerted before SNARE assembly. This interpretation agrees with a previous finding showing that the overexpression of VSM1 affects only sec9-4, but not sec9-7, yeast (Lustgarten and Gerst, 1999). The Sec9-4 mutant protein is deficient in its ability to enter into SNARE complexes at restrictive temperatures, whereas Sec9-7 forms complexes but is unable to confer secretion (Rossi et al., 1997). Because both Vsm1 and Sso are evenly distributed over the plasma membrane (Brennwald et al., 1994; Lustgarten and Gerst, 1999), it suggests that the inhibitory action of Vsm1 (i.e., preventing the association of Sec9 with Sso) takes place there. Our estimates reveal that cells have ∼250,000 molecules of Sso and 120,000 of Vsm1, of which ∼40% coprecipitates with Sso (our unpublished data) and 20% with Snc (Lustgarten and Gerst, 1999). Thus, Vsm1 is likely to regulate Sso function all over the plasma membrane and not just at the site of exocytosis. Similar to Vsm1, Munc18 inhibits SNARE assembly in vitro and dissociates from syntaxin after assembly occurs (Garcia et al., 1994; Hata et al., 1993; Pevsner et al., 1994). This suggests that Munc18, like Vsm1, restricts SNARE partnering by preventing the t-SNAREs from assembling into binary complexes. However, Sec1 in yeast is bound to SNARE complexes at the site of vesicle fusion and may enhance their assembly (Carr et al., 1999; Peng and Gallwitz, 2002). Thus, Sec1 and Vsm1 seem to have very different functions.
Overall, Vsm1 seems to be both a v- and t-SNARE regulator that controls SNARE assembly. Because deletion of VSM1 in snc2Δ cells only slightly enhances their growth and has no effect upon snc null cells (our unpublished data), we suggest that the assembly of SNARE complexes is regulated by other, perhaps redundant, factors. This is supported by gene knockout studies of other SNARE regulators. For example, the knockout of synaptophysin does not significantly affect neurotransmission in mice (McMahon et al., 1996). In addition, deletion of LMA1 subunits in yeast does not affect cell growth or viability (Xu et al., 1997). Thus, some SNARE regulators either have a minor role in SNARE assembly or are redundant with other proteins that regulate complex formation. In conclusion, we have built upon previous findings showing that PKA-dependent phosphorylation of t-SNAREs regulates their ability to assemble into functional SNARE complexes (Marash and Gerst, 2001; Gurunathan et al., 2002). Herein, we propose that one role for t-SNARE phosphorylation is to recruit Vsm1, a negative regulator of secretion (Figure 9, see model). Binding of Vsm1 to Sso may prevent the formation of binary Sso–Sec9 SNARE complexes, which ultimately regulates the formation of functional trans SNARE complexes. Thus, SNARE phosphorylation adds another layer of complexity into the regulation of membrane fusion in eukaryotes.
Figure 9.
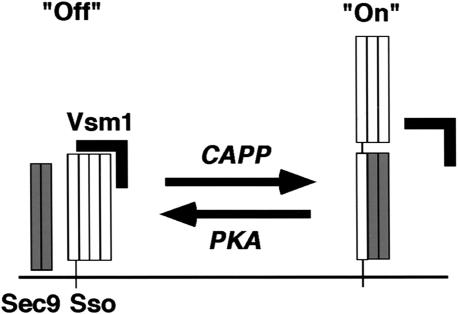
A possible model for Vsm1 regulation of Sso t-SNARE availability. In yeast, Sso exists in either a closed inactive conformation (Off) or an open active conformation, which binds its Sec9 t-SNARE partner (On). Phosphorylation of Sso by PKA recruits Vsm1, stabilizes the closed conformation of the t-SNARE, and inhibits Sec9 binding. Dephosphorylation of Sso by a CAPP releases Vsm1, stabilizes the open conformation of the t-SNARE, and leads to Sec9 binding.
Acknowledgments
We thank Hagai Abeliovich, David Banfield, Pat Brennwald, Fred Hughson (Princeton University, Princeton, NJ), Gabrielle Fischer von Mollard, Sirkka Keranen, Mary Munson, Peter Novick (Yale University, New Haven, CT), and Michael Wigler (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) for the generous gifts of antibodies and other reagents. J.E.G. is supported by grants from the Israel Science Foundation and the Minerva Foundation, Germany. J.E.G. holds the Henry Kaplan Chair in Cancer Research.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-12-0804. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0804.
References
- Bertolaet, B.L., Clarke, D.J., Wolff, M., Watson, M.H., Henze, M., Divita, G., and Reed, S.I. (2001). UBA domain of DNA damage-inducible proteins interacts with ubiquitin. Nature 8, 417–422. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., and James, D.E. (2001). Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 20, 3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald, P., Kearns, B., Champion, K., Keranen, S., Bankaitis, V., and Novick, P. (1994). Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79, 245–258. [DOI] [PubMed] [Google Scholar]
- Carr, C.M., Grote, E., Munson, M., Hughson, F.M., and Novick, P.J. (1999). Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, D.J., Mondesert, G., Segal, M., Bertolaet, B.L., Jensen, S., Wolff, M., Henze, M., and Reed, S.I. (2001). Dosage suppressors of pds1 implicate ubiquitin-associated domains in checkpoint control. Mol. Cell. Biol. 21, 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve, A., Protopopov, V., and Gerst, J.E. (1995). Yeast synaptobrevin homologs are modified posttranslationally by the addition of palmitate. Proc. Natl. Acad. Sci. USA 92, 5987–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, E.P., Gatti, E., Butler, M., Burton, J., and De Camilli, P. (1994). A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc. Natl. Acad. Sci. USA 91, 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst, J.E. (1999). SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol. Life Sci. 55, 707–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan, S., Marash, M., Weinberger, A., and Gerst, J.E. (2002). t-SNARE phosphorylation regulates endocytosis. Mol. Biol. Cell 13, 1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, Y., Slaughter, C.A., and Sudhof, T.C. (1993). Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366, 347–351. [DOI] [PubMed] [Google Scholar]
- Lin, R.C., and Scheller, R.H. (2000). Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 16, 19–49. [DOI] [PubMed] [Google Scholar]
- Lustgarten, V., and Gerst, J.E. (1999). Yeast VSM1 encodes a v-SNARE binding protein that may act as a negative regulator of constitutive exocytosis. Mol. Cell. Biol. 19, 4480–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marash, M., and Gerst, J.E. (2001). t-SNARE dephosphorylation promotes SNARE assembly and exocytosis in yeast. EMBO J. 20, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, H.T., Bolshakov, V.Y., Janz, R., Hammer, R.E., Siegelbaum, S.A., and Sudhof, T.C. (1996). Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc. Natl. Acad. Sci. USA 93, 4760–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson, M., and Hughson, F.M. (2002). Conformational regulation of SNARE assembly and disassembly in vivo. J. Biol. Chem. 277, 9375–9381. [DOI] [PubMed] [Google Scholar]
- Nicholson, K.L., Munson, M., Miller, R.B., Filip, T.J., Fairman, R., and Hughson, F.M. (1998). Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat. Struct. Biol. 5, 793–802. [DOI] [PubMed] [Google Scholar]
- Peng, R., and Gallwitz, D. (2002). Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 157, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, C., Andrews, P.D., Stark, M.J., Cesaro-Tadic, S., Glatz, A., Podtelejnikov, A., Mann, M., and Mayer, A. (1999). Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters, C., Bayer, M.J., Buhler, S., Andersen, J.S., Mann, M., and Mayer, A. (2001). Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Pevsner, J., Hsu, S.C., and Scheller, R.H. (1994). n-Sec 1, a neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA 91, 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov, V., Govindan, B., Novick, P., and Gerst, J.E. (1993). Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell 74, 855–861. [DOI] [PubMed] [Google Scholar]
- Risinger, C., and Bennett, M.K. (1999). Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem. 72, 614–624. [DOI] [PubMed] [Google Scholar]
- Rossi, G., Salminen, A., Rice, L.M., Brunger, A.T., and Brennwald, P. (1997). Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem. 272, 16610–16617. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and Warren, G. (1994). Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 4, 220–233. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., Fasshauer, D., Jahn, R., and Brunger, A.T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Verhage, M., et al. (2000). Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869. [DOI] [PubMed] [Google Scholar]
- Waters, M.G., and Hughson, F.M. (2000). Membrane tethering and fusion in the secretory and endocytic pathways. Traffic 1, 588–597. [DOI] [PubMed] [Google Scholar]
- Weber, T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T.H., and Rothman, J.E. (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Wickner, W., and Haas, A. (2000). Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem. 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Xu, Z., Mayer, M., Muller, E., and Wickner, W. (1997). A heterodimer of thioredoxin and IB2 cooperates with Sec18 (NSF) to promote yeast vacuole inheritance. J. Cell Biol. 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Sato, K., and Wickner, W. (1998). LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell 93, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Yang, B., Steegmaier, M., Gonzalez, L.C., and Scheller, R.H. (2000). nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 148, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]