Abstract
Cortactin is an F-actin binding protein that activates actin-related protein 2/3 complex and is localized within lamellipodia. Cortactin is a substrate for Src and other protein tyrosine kinases involved in cell motility, where its phosphorylation on tyrosines 421, 466, and 482 in the carboxy terminus is required for cell movement and metastasis. In spite of the importance of cortactin tyrosine phosphorylation in cell motility, little is known regarding the structural, spatial, or signaling requirements regulating cortactin tyrosine phosphorylation. Herein, we report that phosphorylation of cortactin tyrosine residues in the carboxy terminus requires the aminoterminal domain and Rac1-mediated localization to the cell periphery. Phosphorylation-specific antibodies directed against tyrosine 421 and 466 were produced to study the regulation and localization of tyrosine phosphorylated cortactin. Phosphorylation of cortactin tyrosine 421 and 466 was elevated in response to Src, epidermal growth factor receptor and Rac1 activation, and tyrosine 421 phosphorylated cortactin localized with F-actin in lamellipodia and podosomes. Cortactin tyrosine phosphorylation is progressive, with tyrosine 421 phosphorylation required for phosphorylation of tyrosine 466. These results indicate that cortactin tyrosine phosphorylation requires Rac1-induced cortactin targeting to cortical actin networks, where it is tyrosine phosphorylated in hierarchical manner that is closely coordinated with its ability to regulate actin dynamics.
INTRODUCTION
Cell movement involves coordination of multiple signaling pathways regulating cell-substratum adhesion and actin cytoskeletal dynamics (Mitchison and Cramer, 1996; Webb et al., 2002). Initiation of motility requires cell polarization and formation of the leading edge at the migratory front. The main “organelle” at the leading edge responsible for motility is the lamellipodium, formed as a result of nascent polymerization and remodeling of the cortical actin cytoskeleton (Small et al., 2002). Lamellipodia formation requires activation of the small GTPase Rac1, which occurs after engagement and activation of several growth factor and adhesion receptors (Hall, 1998).
Rac1 signals regulating lamellipodia formation are mediated in part through multiple pathways that activate members of the WAVE/Scar protein family (Miki et al., 2000; Eden et al., 2002). WAVE/Scar proteins interact with F- and G-actin (Suetsugu et al., 2001), and with actin-related protein complex 2/3 (Arp2/3) complex, the molecular “machine” that nucleates the formation of branched actin networks (Cooper et al., 2001; Higgs and Pollard, 2001). Interaction of WAVE/Scar proteins with Arp2/3 complex leads to enhanced Arp2/3 complex actin nucleation activity, resulting in increased cortical actin polymerization and lamellipodia protrusion (Borisy and Svitkina, 2000). Rac1 targets WAVE/Scar along with several other actin-binding proteins that regulate actin dynamics in lamellipodia (Miki et al., 1998, Mishima and Nishida, 1999; Kessels et al., 2000).
Lamellipodia adhere through integrin-based adhesion complexes with the extracellular matrix. Adhesion complexes within lamellipodia (focal “contacts”) are formed concurrently with actin networks in response to Rac1 (Nobes and Hall, 1995) and share many of the same proteins that are found in focal “adhesions,” larger, distally occurring cell-substratum contact sites regulated by RhoA (Ridley and Hall, 1992). Assembly and regulation of focal contacts and adhesions requires the activity of several tyrosine kinases, including Src (Craig and Johnson, 1996). Rac1 activation translocates Src into lamellipodia (Fincham et al., 1996), where its kinase activity is required for proper lamellipodia and focal contact formation (Timpson et al., 2001; Prasad et al., 2002), indicating that Src substrates in lamellipodia are important in regulating lamellipodia dynamics.
Several Src substrates associated with the actin cytoskeleton are not components of cell-substratum adhesion complexes (Brown and Cooper, 1996). One of these is cortactin, a multidomain protein enriched within lamellipodia (Wu et al., 1991). Cortactin interacts with Arp2/3 complex (Weed et al., 2000; Uruno et al., 2001) and F-actin (Wu and Parsons, 1993), interactions that are required for Rac1-induced cortactin translocation to the cortical actin network (Weed et al., 2000; Di Ciano et al., 2002). Cortactin stimulates Arp2/3 actin nucleation activity and prevents disassembly of Arp2/3-F-actin networks (Weaver et al., 2001; Uruno et al., 2001), suggesting that cortactin plays a direct role in lamellipodia protrusion and integrity.
Multiple signaling pathways that activate Rac1 also lead to cortactin tyrosine phosphorylation (Weed and Parsons, 2001). Src phosphorylates murine cortactin on three tyrosine residues (421, 466, and 482) within a proline-rich carboxyl-terminal domain (Huang et al., 1998), residues that are also phosphorylated by the kinases Fer (Kapus et al., 2000) and c-Met (Crostella et al., 2001). Phosphorylation of cortactin tyrosines 421, 466, and 482 is required for efficient cell motility in several cell types, indicating that cortactin tyrosine phosphorylation plays an important role in cell migration (Huang et al., 1998; Crostella et al., 2001). However, the structural and temporal requirements, as well as the intracellular signaling pathways regulating cortactin tyrosine phosphorylation are unknown.
We have examined the domain requirements for cortactin tyrosine phosphorylation and determined that phosphorylation of cortactin by Src requires the Arp2/3 and F-actin binding domains. Site-specific antibodies against cortactin phosphotyrosine 421 and 466 indicate that phosphorylation of these residues occurs in a hierarchical manner, is dependent on targeting to the cortical actin network and is regulated by the activation state of Rac1. Tyrosine phosphorylated cortactin is enriched in lamellipodia. We propose that cortactin tyrosine phosphorylation is coordinated with actin remodeling, where it may serve to integrate and transduce signals involved in cell migration.
MATERIALS AND METHODS
DNA Constructs
The FLAG-tagged cytomegalovirus-driven cortactin expression construct pcDNA3FLAG2AB cortactin (FLAG-FL) (Du et al., 1998) was used as the template for production of cortactin constructs containing tyrosine-to-phenylalanine point mutations of codons 421, 466, and 482. Mutation of individual residues was conducted by site-directed mutagenesis by using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's protocol. All primers were designed as described previously (Huang et al., 1998), synthesized by Invitrogen (Carlsbad, CA) and high-performance liquid chromatography purified. Constructs with more than one mutation were created by successive rounds of mutagenesis. Mutation of each codon was confirmed individually by DNA sequencing. FLAG-N-term (NT) and FLAG-C-term (CT) cortactin constructs were as described previously (Weed et al., 2000). The FLAG-C-term construct with triple tyrosine to phenylalanine mutations at codons 421, 466, and 482 (CT-T) was generated as a KpnI-EcoRI fragment as described previously (Weed et al., 2000) by using FLAG-FL with the three phenylalanine mutations (FL-T) as a template, and subcloned into KpnI-EcoRI digested pcDNA3FLAG2AB (Devarajan et al., 1997).
FLAG-tagged coronin1 was generated by polymerase chain reaction (PCR) amplification of the coronin1 cDNA in pCR2.1 (Okumura et al., 1998) with the 5′ primer containing a KpnI restriction endonuclease site and the 3′ primer containing a stop codon followed by an EcoRI site. The amplified cDNA was digested with KpnI and EcoRI and subcloned into KpnI-EcoRI digested pcDNA3FLAG2AB. For production of the coronin1-cortactin C-term hybrid construct (FLAG-coronin1-CT), a KpnI-BamHI PCR fragment spanning full-length coronin1 cDNA lacking the stop codon was ligated in frame with a BamHI-EcoRI fragment from pRK5myc-C-term (Weed et al., 2000) and KpnI-EcoRI digested pcDNA3FLAG2AB. pRK5myc, pRK5myc Rac1N17, and pRK5myc Rac1L61 were described previously (Lamarche et al., 1996). All PCR-generated constructs were verified by DNA sequencing.
Antibodies and Western Blotting
Anti-cortactin antibodies 4F11, anti-C-term, and anti-N-term have been described previously (Wu and Parsons, 1993; Weed et al., 1998). Anti-FLAG M5 and anti-FLAG M2 affinity resin were purchased from Sigma-Aldrich (St. Louis, MO). Anti-phosphotyrosine RC-20, anti-activated epidermal growth factor receptor (EGFR), and pan-reactive EGFR antibodies were purchased from BD Transduction (San Diego, CA). Anti-phosphotyrosine 4G10 (direct conjugate to horseradish peroxidase) and anti-Rac1 monoclonal antibodies (mAbs) were purchased from Upstate Biotechnology (Lake Placid, NY). The anti-cmyc epitope mAb 9E10, anti-SRC2, and anti-Src N16 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin was purchased from Oncogene Sciences (San Diego, CA).
Phosphorylation site-specific antibodies recognizing mouse cortactin phosphorylated on tyrosine 421 (anti-cortactin pY421) or tyrosine 466 (anti-cortactin pY466) were manufactured and are marketed by BioSource International (Camarillo, CA). These rabbit polyclonal antibodies were generated by immunization of 13-mer peptides surrounding the sites of phosphorylation for both pY421 and pY466 and were purified using both negative and positive affinity purification methods to optimize specificity for the targeted phosphoepitope. Specificity was demonstrated using peptide competition assays (our unpublished data) and through the analysis of site-directed mutants for each tyrosine as shown in Figure 3, A and B. Anti-Src pY418 was purchased from Biosource International. Secondary antibodies coupled to horseradish peroxidase were purchased from Amersham Biosciences (Piscataway, NJ) and Pierce Chemical (Rockford, IL). Secondary antibodies coupled to AlexaFlour 488, 594, and 647, and phalloidin conjugated to AlexaFlour 594 were from Molecular Probes (Eugene, OR).
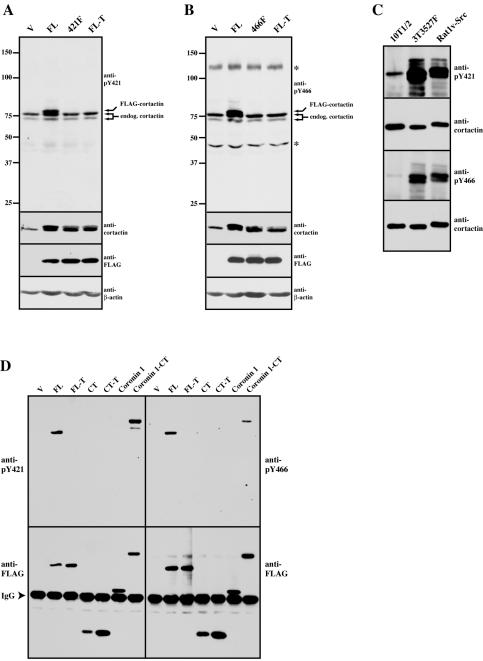
Figure 3.
Specificity and characterization of phosphospecific antibodies against cortactin pY421 and pY466. (A) Specificity of the anti-pY421 cortactin antibody. Cell lysate (50 μg) from 10T1/2 fibroblasts transfected with empty FLAG vector (V), full-length wild-type cortactin (FL), full-length cortactin with a Y-F mutation at codon 421 (421F), and full-length cortactin 421F/466F/482F (FL-T) were resolved by 8% SDS-PAGE and Western blotted for anti-pY421 cortactin as indicated. The blot was then successively stripped and reprobed with 4F11 (anti-cortactin), M5 (anti-FLAG), and anti-β-actin antibodies as indicated. The positions of the endogenous and FLAG-cortactin detected by anti-pY421 are indicated on the right. The position of molecular weight markers is shown on the left. (B) Specificity of the anti-pY466 cortactin antibody. 10T1/2 fibroblasts were transfected and lysates assayed as in A except a FLAG-tagged full-length cortactin with a Y-F mutation at codon 466 was used in place of the Y421F mutant (466F). Asterisks (*) denoting the positions of cross-reactive bands at Mr 125 and 45 kDa are shown on the right. (C) Detection of cortactin tyrosine 421 and 466 phosphorylation in response to Src transformation. Cell lysates (500 μg) from 10T1/2 fibroblasts (10T1/2); NIH 3T3 fibroblasts expressing an avian Y527F mutant of c-Src (3T3 527F) and Rat1 fibroblasts expressing avian v-Src (Rat1v-Src) were immunoprecipitated with 4F11, washed, and subjected to 8% SDS-PAGE. Cortactin tyrosine phosphorylation was assayed by Western blotting with anti-cortactin pY421 (top) and anticortactin pY466 (third panel). Blots were stripped and reprobed with 4F11 to verify the presence of precipitated cortactin (second and fourth panels). (D) Phosphorylation of cortactin tyrosines 421 and 466 in the coronin1-cortactin C termini chimera. C3H 10T1/2 fibroblasts were transfected and recombinant proteins immunoprecipitated as in Figure 2B. Phosphorylation of tyrosine 421(anti-pY421) (left) and 466 (anti-pY466) (right) was analyzed by Western blotting with the indicated phosphospecific antibodies. Blots were then stripped and reprobed with M5 (anti-FLAG) to verify expression of the FLAG constructs (bottom).
Western blotting was performed as described previously (Kanner et al., 1990) with 5% bovine serum albumin (Fisher Scientific, Pittsburgh, PA) used for blocking and blotting with phospho-tyrosine–specific antibodies and 5% nonfat dry milk for all other antibodies. Primary antibodies were used at the flowing concentrations or dilutions: 4F11 (0.2 μg/ml), anti-N-term or anti-C-term (1 μg/ml), M5 (5 μg/ml), 4G10 (1:2000), RC-20 (1:1000), ant-cortactin pY421 (0.05–0.1 μg/ml), anti-cortactin pY466 (0.05–0.5 μg/ml), anti-activated EGFR and pan-reactive EGFR (1:2500), anti-Src pY418 (0.5 μg/ml), anti-SRC2 and Src N16 (1:100), anti-Rac1 (1:2000), and anti-β-actin (1:5000). Primary antibodies were detected with the appropriate horseradish peroxidase-conjugated secondary (1:10,000) and immunoreactive bands were visualized using a chemiluminescent substrate (SugperSignal West Pico; Pierce Chemical). Blots were stripped by incubation in 62.5 mM Tris-HCl (pH 6.8), 2% SDS, and 100 mM 2-mercaptoethanol at 50°C for 40 min and washed extensively before blocking and reblotting.
Cell Culture and Transfections
C3H 10T1/2, 5Hd47, and NeoR1 fibroblast cell lines were a gift from Sarah Parsons (University of Virginia, Charlottesville, VA). COS-1 cells were a gift from Michael Webber (University of Virginia). NIH 3T3 527F and Rat1 v-src-transformed fibroblasts were a gift from Steve Anderson (University of Colorado Health Sciences Center, Denver, CO). Cells were cultured with 10% fetal calf serum (Hyclone Laboratories, Logan, UT) in DMEM supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin (Mediatech, Herndon, VA). NeoR1 and 5Hd47 cells were maintained in the presence of 400 μg/ml G-418 sulfate (Mediatech). Transfection of FLAG-cortactin and myc-Rac constructs in C3H 10T1/2, 5Hd47, and COS-1 cells was conducted as described previously (Weed et al., 2000) by using the Polyfect reagent (QIAGEN, Valencia, CA) and 4 μg of total DNA. For immunofluorescence studies, transfected cells were plated onto coverslips coated with 40 μg/ml fibronectin (Sigma-Aldrich) and processed as described previously (Weed et al., 1998). For the production of motile cells, C3H 10T1/2 cells were plated onto glass coverslips and grown to confluence for 48 h. Monolayers were scraped with a plastic pipette tip and cells were cultured for 4 h before fixation and processing for immunofluorescence microscopy. For spreading cells, C3H 10T1/2 cells were trypsinized, washed, and plated onto fibronectin-coated coverslips for 30 min as described previously (Weed et al., 1998) and Rat1 v-src cells were trypsinized and plated onto glass coverslips overnight before fixation and immunolabeling. NeoR1 cells were grown to confluence, starved for 18 h with DMEM/0.5% fetal bovine serum, trypsinized, and plated onto coverslips in DMEM for 2 h. Cells were treated with 20 ng/ml epidermal growth factor (EGF) (Upstate Biotechnology) for the times indicated before fixation and processing for immunofluorescence microscopy.
Immunofluoresence Microscopy
Cells were fixed and immunolabeled as described previously (Weed et al., 1998) except that immunofluorescence buffer (Schafer et al., 1998) was substituted for phosphate-buffered saline (PBS). Epitope-tagged constructs were detected with M5 (5 μg/ml) or 9E10 (1: 1000). Endogenous cortactin was detected with either anti-N-term (1 μg/ml) or 4F11 (0.25 μg/ml). Anti-cortactin pY421 was used at 1–2 μg/ml. AlexaFlour 594 phalloidin (Molecular Probes) was used at 1:1000. Primary monoclonal antibodies were detected with anti-mouse AlexaFlour 594 for double-labeling and anti-mouse AlexaFlour 647 for triple-labeling experiments; rabbit primary antibodies were detected with anti-rabbit AlexaFlour 488. All secondary antibodies were used at 1 μg/ml. Labeled coverslips were mounted with the AntiFade kit (Molecular Probes), and cells were viewed using a Nikon E600 epifluorescence microscope equipped with a Plan Aprochromat 60× objective lens. Images were acquired with a Spot RT charge-coupled cooled device (Diagnostic Instruments, Sterling Heights, MI) driven by native software and were processed on a Macintosh computer with Adobe Photoshop software. A minimum of 90 cells were evaluated for each condition, with at least 85% of the cells demonstrating the represented effects except where noted.
Cell Lysis and Immunoprecipitation
For evaluation of anti-cortactin pY421 and 466 antibodies, 10T1/2 cells transfected with pcDNA3FLAG2AB, FLAG-FL, FLAG-421F, FLAG-466F, or FLAG-FL-T were washed in PBS containing 1 mM NaVO4 and lysed in ice-cold modified radioimmunoprecipitation assay buffer (RIPA) (Du et al., 1998) supplemented with protease inhibitor cocktail (1:200; Sigma-Aldrich). Lysates were clarified by centrifugation at 14,000g for 10 min, and 50 μg of total cell protein was resolved by SDS-PAGE and assayed by Western blotting. EGF-mediated cortactin tyrosine phosphorylation was evaluated by treatment of serum-starved NeoR1 cells with 5 ng/ml EGF for the indicated time periods. Cells were lysed in modified RIPA, and 100 μg of protein was analyzed by Western blotting with anti-pY421, anti-pY466, 4F11, anti-activated EGFR, and anti-EGFR antibodies; 150 μg of protein was used for Src analysis
Cells expressing FLAG-cortactin constructs were lysed in modified RIPA, and 1 mg of each clarified lysate was incubated with 25 μl of FLAG M2 affinity resin (Sigma-Aldrich) for 2 h at 4°C. Immune complexes were collected by centrifugation, washed twice with modified RIPA, separated by SDS-PAGE, and Western blotted with antibodies as described. For precipitation of endogenous cortactin, 25 μl of ImmunoPure Plus immobilized protein A agarose beads (50% slurry in PBS; Pierce Chemical) were precharged with 7.5 μg of rabbit anti-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) in 500 μl of PBS for 2 h. Beads were washed twice with PBS, resuspended in 500 μl of PBS and incubated with 5 μg of 4F11 overnight. After washing the beads twice with PBS, cortactin was immunoprecipitated from modified RIPA lysates (500 μg of protein) of C3H 10T1/2, NIH 3T3 527F, and Rat1 v-src cells by incubation with 4F11-charged beads for 2 h. Immune complexes were processed for Western blotting as described above. Protein concentrations were determined using the DC Protein Assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
In Vitro Kinase Assays
For analysis of cortactin phosphorylation by Src, C3H 10T/12 cells transfected with FLAG-cortactin constructs were lysed in modified RIPA and immunoprecipitated with FLAG M2 resin as described. Immune complexes were washed twice with modified RIPA buffer, once with PBS containing 2 M KCl, and once with kinase buffer (50 mM HEPES, pH 7.3, 5 mM MnCl2). Complexes were incubated in kinase buffer containing 10 μCi of [γ-32P]ATP (PerkinElmer Life Sciences, Boston, MA) with or without 1 U of purified Src (Upstate Biotechnology) in a final volume of 50 μl. Reactions were incubated at 30°C for 10 min, stopped with an equal volume of hot 2× SDS-PAGE sample buffer, resolved by SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Amersham Biosciences). After autoradiography, membranes were stripped as described above, exposed to x-ray film overnight to ensure removal of all radiolabel, and then blotted with anti-N-term and C-term cortactin antibodies.
RESULTS
The Amino-Terminal Domain Is Required for Tyrosine Phosphorylation of the Cortactin Carboxyl Terminus
Previous work has demonstrated that Src phosphorylates murine cortactin primarily on tyrosines 421, 466, and 482 within the carboxyl-terminal proline-rich domain (Figure 1A; Huang et al., 1998). To investigate the extent of phosphorylation of each individual tyrosine in response to c-Src activation in vivo, a panel of all possible combinatorial mutations of tyrosines 421, 466, and 482 to phenylalanine were produced in a FLAG-tagged wild-type cortactin expression construct by successive rounds of site directed mutagenesis. Wild-type FLAG-cortactin (FL) and mutant constructs were transfected into 5Hd47 fibroblasts, a nontransformed variant of C3H 10T1/2 cells that exhibits 10-fold c-Src overexpression (Wilson and Parsons, 1990). As shown in Figure 1B, FL (wild-type) cortactin gave a robust signal when immunoprecipitated and analyzed for tyrosine phosphorylation by Western blotting with the pan-phosphotyrosine antibody 4G10. The single mutation of tyrosine 421 (421F) to phenylalanine resulted in a significant reduction in cortactin tyrosine phosphorylation compared with FL cortactin, whereas single mutations of either tyrosine 466 or tyrosine 482 to phenylalanine (466F and 482F, respectively) had less of an effect, with phosphotyrosine levels similar to that of FL cortactin. Cortactin constructs containing the pairwise mutant combinations 421F/466F and 421F/482F exhibited diminished phosphotyrosine levels similar to that of 421F, whereas the 466F/482F construct was still abundantly phosphorylated. A cortactin construct containing tyrosine-to-phenylalanine mutation of all three sites (421F/466F/482F) resulted in further (but not complete elimination of) detectable phosphotyrosine levels. In agreement with previous results (Huang et al., 1998), these data indicate that codons 421, 466, and 482 are the tyrosine residues on murine cortactin phosphorylated by c-Src, with tyrosine 421 being the primary site of Src-mediated cortactin phosphorylation in vivo.
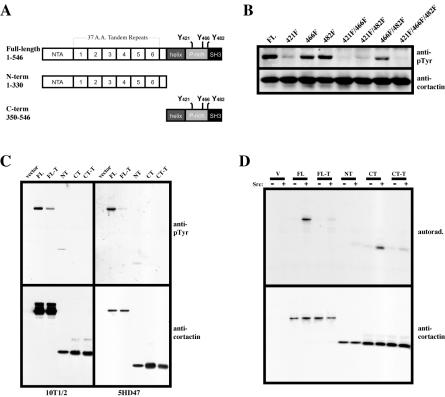
Figure 1.
Tyrosine phosphorylation of cortactin requires the amino-terminal domain. (A) Diagram of cortactin expression constructs. The cortical targeting domain (N-term) consists of the amino-terminal acidic (NTA) Arp2/3 binding site and the F-actin binding site within the fourth 37 amino acid tandem repeat. The position of the three tyrosine residues phosphorylated by Src and other kinases within the proline-rich carboxyl-terminal domain (C-term) are shown. Numbers represent amino acids encoded by each construct. (B) Differential tyrosine phosphorylation of cortactin tyrosine point mutants in vivo. Individual and serial point mutants (Y-F) representing all possible mutational combinations of the three mapped Src phosphorylation sites were introduced into a cytomegalovirus-driven FLAG-tagged full-length cortactin expression construct and transfected into 5Hd47 fibroblasts. After 18 h, cells were lysed and recombinant cortactin proteins were immunoprecipitated with anti-FLAG agarose beads. Immune complexes were resolved by 8% SDS-PAGE and analyzed for tyrosine phosphorylation by Western blotting with 4G10 (anti-pTyr). The blot was then stripped and reprobed with 4F11 (anti-cortactin) to verify the equal presence of FLAG-cortactin proteins. The codon positions of mutated tyrosine to phenylalanine residues are indicated at the top. FL, full-length, wild-type cortactin. (C) Tyrosine phosphorylation of cortactin domains. 10T1/2 and 5Hd47 cells were transfected with either empty FLAG-vector (vector), full-length wild-type cortactin (FL), full-length 421F/466F/482F cortactin (FL-T), cortactin N-term (NT), cortactin C-term (CT), or cortactin C-term 421F/466F/482F (CT-T) domains. Cells were lysed and FLAG-cortactin proteins were analyzed for phosphotyrosine content as in B (top). Blots were then stripped and the presence of cortactin domains verified by blotting with a mixture of anti-N-term and anti-C-term antibodies (bottom). Note the greater amount of full-length cortactin protein analyzed from the 10T1/2 versus the 5Hd47 line. (D) Src phosphorylation of cortactin domains. FLAG-tagged cortactin domains analyzed in C were immunoprecipitated from transfected 10T1/2 cells and subjected to in vitro kinase assay analysis with [γ-32P]ATP in the absence (–) or presence (+) of 1 U of purified Src. Phosphorylated FLAG-cortactin proteins were visualized by autoradiography (top) after 10% SDS-PAGE and transfer to polyvinylidene difluoride membranes. The blot was stripped and the presence of FLAG-cortactin proteins was determined by Western blotting as in C (bottom). The results are indicative of at least three independent experiments.
In vitro, Src directly phosphorylates a recombinant cortactin polypeptide encoding the proline-rich carboxyl-terminal domain (Huang et al., 1998). To determine if Src-mediated phosphorylation of cortactin requires the presence of additional cortactin domains besides the proline-rich region in vivo, FLAG-tagged cortactin constructs, including constructs encoding either the amino-(N-term) or carboxyl (C-term)-cortactin domains (Figure 1A) were transfected into C3H 10T1/2 and 5Hd47 fibroblasts, and the overexpressed cortactin fusion proteins were immunoprecipitated with anti-FLAG resin. Tyrosine phosphorylation of the expressed cortactin proteins was determined by Western blotting with RC-20 (Figure 1C, top) and expression of each cortactin peptide was verified by immunoblotting with anticortactin antibodies (Figure 1C, bottom). While full-length, wild-type cortactin (FL) was tyrosine phosphorylated when expressed in C3H 10T1/2 cells, its phosphorylation was significantly enhanced when expressed in 5Hd47 fibroblasts (Figure 1C; note lower expression levels of FL in 5Hd47). Expression of FLAG-cortactin 421F/466F/482F (FL-T) displayed diminished but dectable tyrosine phosphorylation compared with wild-type cortactin when expressed in either C3H 10T1/2 or 5Hd47 cells. Interestingly, FLAG-N-term (NT) displayed a low but consistently reproducible (n = 3) level of tyrosine phosphorylation in C3H 10T1/2 cells that was not elevated in 5Hd47 cells. Surprisingly, expression of FLAG-C-term (CT), which contains the tyrosine residues targeted by Src, was not detectably tyrosine phosphorylated (Figure 1C), as was a FLAG-C-term construct with the triple tyrosine to phenylalanine mutations at 421, 466 and 482 (CT-T). The lack of tyrosine phosphorylation in the CT construct suggested that Src-mediated phosphorylation of the cortactin carboxyl-terminal domain requires the presence of the cortactin amino terminus.
To verify that the lack of phosphorylation of the carboxyl-terminal domain was not due to misfolding and subsequent lack of recognition by Src, FLAG-cortactin constructs were expressed in C3H 10T1/2 cells, immunoprecipitated with anti-FLAG resin, and washed with 2 M KCl to remove associated proteins. Immune complexes were incubated with purified Src and [γ-32P]ATP, and resolved by SDS-PAGE. Autoradiography (Figure 1D, top) demonstrated efficient phosphorylation of FLAG-FL and the FLAG-CT, with little phosphorylation of either FLAG-FL-T, FLAG-NT, or FLAG-CT-T. Stripping and probing of the membrane with anti-cortactin antibodies confirmed precipitation of the FLAG-cortactin proteins (Figure 1D, bottom). These data support the conclusion that the amino-terminal cortactin domain is tyrosine phosphorylated independent of Src activity and that phosphorylation of the C-terminal cortactin domain by Src requires the presence of the cortactin amino terminus.
Tyrosine Phosphorylation of the Cortactin Carboxyl Terminus Requires Localization to the Cell Cortex
The amino-terminal cortactin domain is required for Rac1-mediated targeting of cortactin to the cell periphery, a process that involves direct association with F-actin and the Arp2/3 complex (Weed et al., 2000). To determine whether the inability of the cortactin C-terminal domain to become tyrosine phosphorylated required Rac1-mediated localization of the cortactin carboxyl-terminal domain to the cell cortex, a FLAG-tagged construct was created where the C-terminal cortactin domain was fused in frame to the C termini of the unrelated actin-binding and lamellipodia protein coronin1 (Figure 2A). Coronin1 also targets to the cortical actin cytoskeleton in a Rac1-dependent manner and directly binds Arp2/3 complex but is not tyrosine phosphorylated (Mishima and Nishida, 1999; Humphries et al., 2002). We selected this strategy over examining cortactin tyrosine phosphorylation after direct perturbation of the actin cytoskeleton with cytochalasian D or latrunculin B because these drugs lead to robust Src activation, potentially resulting in aberrant cortactin tyrosine phosphorylation (Lock et al., 1998). To determine whether the coronin1-cortactin C-term (coronin1-CT) construct restored the ability of the cortactin carboxyl-terminal domain to become tyrosine phosphorylated, COS-1 cells were transfected with FLAG-coronin1 and FLAG-coronin1-CT, along with FLAG-FL, FLAG-CT, and FLAG-CT-T as controls. FLAG-tagged proteins were immunoprecipitated and tyrosine phosphorylation of recombinant proteins was analyzed by Western blotting with RC-20. Both FLAG-FL and FLAG-coronin1-CT were tyrosine phosphorylated, whereas FLAG-CT, FLAG-CT-T, and FLAG-coronin1 were not (Figure 2B, top). The blot was stripped and precipitation of the FLAG-tagged proteins was verified by immunoblotting with anti-FLAG M5 (Figure 2B, bottom). These data indicate that fusion of the cortactin carboxy terminus to coronin1 restores the ability of the carboxyl-terminal cortactin domain to become tyrosine phosphorylated.
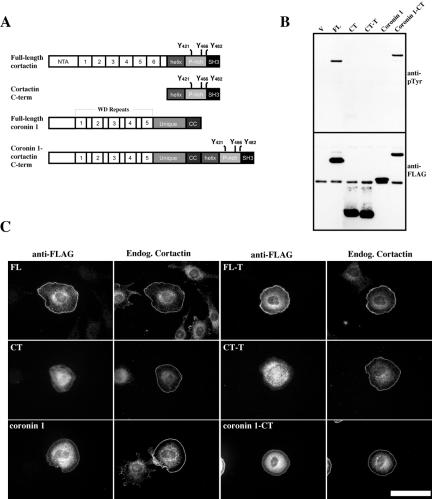
Figure 2.
Cortical targeting of the cortactin C terminus in a coronin1-cortactin hybrid protein restores the ability of the cortactin C-terminal domain to become tyrosine phosphorylated. (A) Diagrammatic representation of FLAG-tagged full-length wild-type cortactin, cortactin C terminus, coronin1, and coronin1-cortactin CT chimeric constructs. (B) Tyrosine phosphorylation of the cortactin C terminus in a coronin-cortactin chimera. COS-1 cells were transfected with either empty FLAG-vector (V), full-length wild-type cortactin (FL), full-length cortactin 421F/466F/482F (FL-T), N-term (NT), C-term (CT), C-term 421F/466F/482F (CT-T), full-length coronin1 (coronin 1), and full-length coronin1 fused to the cortactin C-term (coronin1-CT). After transfection, cells were lysed and recombinant proteins immunoprecipitated with anti-FLAG agarose. Proteins were resolved by SDS-PAGE and Western blotted with RC-20 (anti-pTyr) (top). The blot was stripped and recombinant proteins were detected with M5 (anti-FLAG) (bottom). (C) Rac-mediated targeting of coronin1 constructs and colocalization with cortactin. 10T1/2 cells were cotransfected with myc-L61Rac and various FLAG-tagged cortactin, coronin or coronin-CT-cortactin constructs as indicated. After transfection, cells were trypsinized, plated on fibronectin-coated coverslips, and allowed to spread for 1 h before fixation. Cells were double labeled with M5 (anti-FLAG) and polyclonal cortactin anti-Nterm antibodies (endog. cortactin). Bar, 50 μM.
To determine whether the FLAG-coronin proteins targeted to the cell periphery in response to Rac1 activation, C3H 10T1/2 cells were cotransfected with constitutively active myc-RacL61 and FLAG-FL, FL-T, CT, CT-T, coronin1, and coronin1-CT. After transfection, cells were trypsinized and plated on fibronectin-coated coverslips. Under these conditions, C3H 10T1/2 cells expressing RacL61 exhibit a rounded morphology, and cortactin localization at the cell periphery is dependent on the presence of the amino-terminal domain (Weed et al., 2000). In agreement with these previous results, FLAG-FL and FL-T accumulated at the cell cortex with endogenous cortactin, whereas FLAG-CT and FLAG-CT-T failed to localize at the periphery and remained cytoplasmic (Figure 2C). FLAG-coronin1 and FLAG-coronin1-CT also localized with cortactin at the cortex, although a large amount of both of these proteins was found in the cytoplasm (Figure 2C). In all cases, endogenous cortactin exhibited efficient cortical localization. Together, these data suggest that Rac1-induced localization of cortactin to the cell cortex is required for tyrosine phosphorylation of the carboxyl-terminal cortactin domain.
Production and Specificity of Phosphorylation-specific Antibodies against Phosphorylated Tyrosine 421 and Tyrosine 466 of Murine Cortactin
Tyrosine 421 and 466 are the primary residues phosphorylated by c- and v-Src in murine cortactin (Figure 1B; Huang et al., 1998). To facilitate the analysis of these sites and to determine whether these sites are phosphorylated in response to Rac1-mediated cortical targeting, we developed antibodies specific to phosphotyrosine 421 and 466 of murine cortactin. To confirm the specificity of each antibody, C3H 10T1/2 cells were transfected with empty FLAG vector, FLAG-FL, FLAG-FL-T, and either FLAG-FL with an individual tyrosine-to-phenylalanine mutation at codon 421 for anti-pY421 analysis (421F) or at codon 466 for anti-pY466 analysis (466F) (Figure 3, A and B, respectively). Total cell lysates were immunoblotted with either anti-pY421 (Figure 3A) or anti-pY466 cortactin antibodies (Figure 3B). The anti-pY421 cortactin antibody detected endogenous cortactin as a doublet that migrated between 72 and 78 kDa by SDS-PAGE in lysates from cells expressing the empty FLAG vector (V), FLAG-421F, and FL-T. However, an additional band migrating at ∼80 kDa was detected in lysates from cells transfected with FLAG-FL (Figure 3A), indicating that the anti-pY421 cortactin antibody selectively recognizes cortactin phosphorylated on tyrosine 421. Similar results were obtained with the anti-pY466 cortactin antibody, although two additional cross-reactive bands were observed at ∼125 and 47 kDa (Figure 3B). Nevertheless, the anti-pY466 antibody is specific for the cortactin epitope because it also detected FLAG-FL but failed to recognize either FLAG-466F or FLAG-FL-T (Figure 3B). The additional band present in the FL lanes of the anti-421 and 466 blots is FLAG-FL because this band is due to the slightly slower electrophoretic mobility caused by the presence of the FLAG-tag in the recombinant protein (Weed et al., 2000). Stripping and reprobing of the blots with the anti-cortactin mAb 4F11 resulted in a tight cortactin doublet in lysates containing FLAG-tagged cortactin proteins (Figure 3, A and B), with the recombinant FLAG-cortactin proteins constituting the slower migrating form of the two bands because this band was absent in lysates from cells transfected with the empty FLAG vector (V). Immunoblotting of lysates with the anti-M5 mAb (anti-FLAG) directly verified the expression of the FLAG-cortactin proteins, and equivalent loading of protein amounts was confirmed by stripping and immunoblotting for β-actin (anti-β-actin) (Figure 3, A and B). Collectively, these results show that the anti-pY421 and anti-pY466 cortactin antibodies specifically recognize their respective phosphoepitopes.
To determine that the anti-phosphospecific cortactin antibodies detect tyrosine phosphorylation of cortactin at codons 421 and 466 as a result of Src activation, cortactin was immunoprecipitated from murine fibroblasts with normal levels of c-Src activity (C3H 10T1/2), fibroblasts expressing a tyrosine-to-phenylalanine–activating mutation in c-Src (3T3 527F) and Rat1 fibroblasts expressing v-Src (Rat1 v-Src). A substantial increase in cortactin phosphorylation was observed on tyrosine 421 and tyrosine 466 in cortactin from 3T3 527F and Rat1 v-Src fibroblasts compared with C3H 10T1/2 cells after immunoblotting with anti-pY421 and anti-pY466 antibodies (Figure 3C). Stripping and reprobing of the blots with 4F11 indicated that equivalent amounts of cortactin were assayed (Figure 3C), with the slower migrating form of cortactin from Rat1 v-Src cells attributable to the larger size of cortactin in rat versus murine cells (Ohoka and Takai, 1998). These results indicate that the anti-pY421 and anti-pY466 cortactin antibodies detect Src-mediated phosphorylation of murine cortactin at tyrosine 421 and 466 and that these antibodies also detect phosphorylation of rat cortactin on these residues as expected from sequence analysis of both phosphorylation sites (Figure 8).
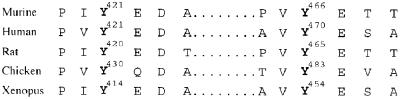
Figure 8.
Conservation of cortactin tyrosine 421 and 466 phosphorylation sites in vertebrate species. Alignment of sequences surrounding tyrosine 421 and 466 of murine cortactin (GenBank accession no. U03184) with homologous regions from human (M98343), rat (AF054619), chicken (M73705), and Xenopus (AB027611) cortactin. Phosphorylated and predicted tyrosine residues are numbered and in bold.
Given the specificity of the anti-pY421 and pY-466 cortactin antibodies, we sought to determine whether these sites were in part responsible for the tyrosine phosphorylation within the cortactin carboxy terminus observed in the cortical targeting of the FLAG-coronin1-CT construct (Figure 2B). C3H 10T1/2 cells were transfected with either empty FLAG vector, FLAG-FL, FLAG-FL-T, FLAG-NT, FLAG-CT, FLAG-CT-T, FLAG-coronin1, or FLAG-coronin1-CT. Recombinant proteins were immunoprecipitated with anti-FLAG resin, and tyrosine phosphorylation of FLAG-tagged proteins was analyzed by Western blotting with anti-pY421 and anti-pY466 cortactin antibodies. As shown in Figure 3D, FLAG-FL and coronin1-CT were the only proteins exhibiting phosphorylation of tyrosine 421 and 466. All recombinant proteins were precipitated as determined by anti-FLAG M5 immunoblotting. Phosphorylation of tyrosine 421 and tyrosine 466 in the coronin1-CT fusion protein therefore indicates that these two residues in the cortactin carboxy terminus contribute to the tyrosine phosphorylation observed in this construct in Figure 2B.
Phosphorylation of Tyrosine 421 in Murine Cortactin Is Required for Tyrosine 466 Phosphorylation
Several Src substrates containing multiple phosphorylation sites can be differentially regulated with respect to each other (Guappone et al., 1998; Schaller and Schaefer, 2001; Ruest et al., 2001). To determine whether phosphorylation of tyrosine 421 and 466 in cortactin occurs in an independent or interdependent manner in response to Src activation in vivo, 5Hd47 fibroblasts were transfected with the panel of FLAG-tagged cortactin constructs containing all possible tyrosine-to-phenylalanine mutational combinations of codons 421, 466, and 482 (Figure 1B). Expressed proteins were immunoprecipitated with anti-FLAG resin, and tyrosine 421 and 466 phosphorylation was analyzed by Western blotting with anti-pY421 and anti-pY466 cortactin antibodies (Figure 4). As expected, the anti-pY421 cortactin antibody detected all proteins where tyrosine 421 was not mutated to phenylalanine (FL, 466F, 482F, and 466F/482F), whereas cortactin constructs containing phenylalanine mutations at codon 421 (421F, 421F/466F, 421F/482F, and 421F/466F/482F) were not detected by anti-pY421 cortactin (Figure 4). Interestingly, the anti-pY466 antibody only detected the FL and 482F proteins, failing to detect constructs where tyrosine 466 was intact in the context of a phenylalanine mutation at codon 421 (421F and 421F/482F) as well as constructs with phenylalanine present at codon 466 (466F, 421F/466F, 466F/482F, and 421F/466F/482F) (Figure 4). This result indicates that the presence of a phosphorylated tyrosine residue at position 421 is required for phosphorylation of tyrosine 466 in murine cortactin in vivo, and that phosphorylation of tyrosine 421 and 466 is independent of tyrosine 482 phosphorylation.
Figure 4.
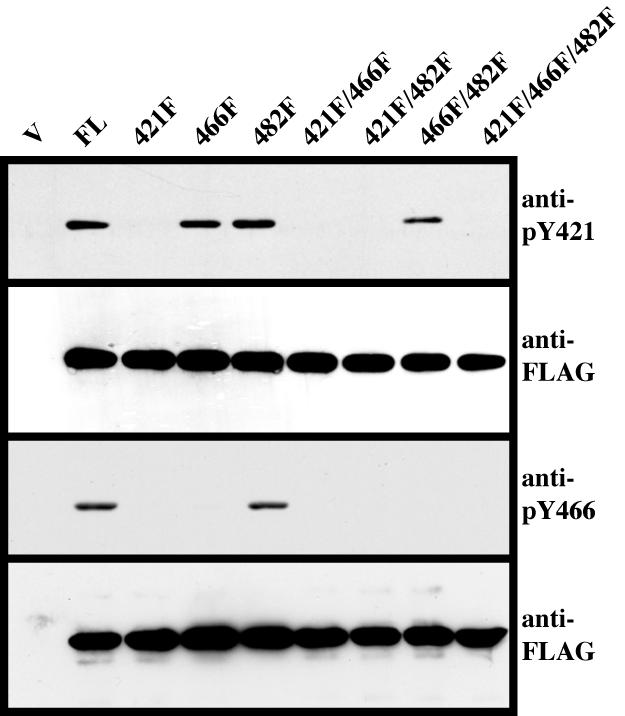
Phosphorylation of cortactin tyrosine 421 is required for tyrosine 466 phosphorylation. 5HD47 fibroblasts were transfected with Y-F point mutant FLAG-cortactin constructs as described in Figure 1B. Cells were lysed and FLAG-tagged proteins were immunoprecipitated with anti-FLAG resin. Immune complexes were washed, subjected to 8% SDS-PAGE, and analyzed for cortactin tyrosine phosphorylation at codon 421 (anti-pY421) (top) and codon 466 (anti-pY466) (third panel). Blots were then stripped and reprobed with M5 (anti-FLAG) to verify expression of the FLAG constructs (second and fourth panels). Blots are representative of results obtained from three independent experiments.
Localization of pY421 Cortactin with Cortical Actin Structures
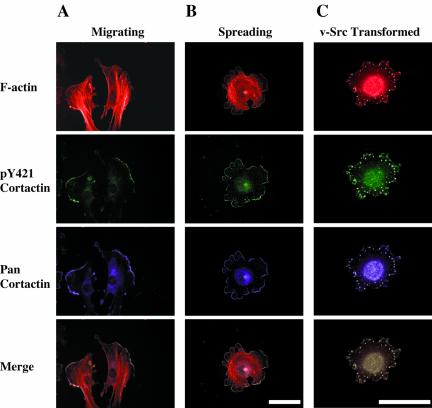
In nontransformed cells, cortactin localizes with the cortical F-actin network within lamellipodia and is also concentrated in a cytoplasmic pool near the perinuclear region (Wu and Parsons, 1993). In Src-transformed fibroblasts, cortactin redistributes into cell-substratum contacts known as podosomes (Wu et al., 1991). To determine the subcellular localization of tyrosine phosphorylated cortactin in normal and transformed cell types, C3H 10T1/2 and Rat1 vSrc fibroblasts were cultured in the presence of serum and immunolabeled with anti-pY421 cortactin (Figure 5). In both motile (Figure 5A) and spreading (Figure 5B) C3H 10T1/2 cells, anti-pY421 cortactin labeling was concentrated primarily with F-actin and cortactin within lamellipodia, with labeling of the perinuclear region observed to a lesser extent relative to the levels of total cortactin in the cytoplasm. Anti-pY421 cortactin exhibited strong labeling of podosomes in Rat 1 v-Src cells that also overlapped with F-actin and cortactin (Figure 5C). Overlaying of individual images for F-actin, pY421 cortactin, and total (pan) cortactin indicated that pY421 cortactin is enriched with the pool of cortactin that is associated with cortical actin in lamellipodia and podosomes (Figure 5, A–C). The anti-pY466 antibody failed to specifically label cortactin in cells (our unpublished data), presumably due to the cross-reactivity of this antibody with other cellular proteins (Figure 3B).
Figure 5.
Cortactin phosphorylated at tyrosine 421 is enriched in the leading edge of lamellipodia and in podosomes. Motile (A), spreading (B) 10T1/2 fibroblasts, and Rat1v-Src (C) cells were fixed and labeled with phalloidin (F-actin), anti-phosphotyrosine 421 cortactin (pY421 cortactin) and 4F11 (cortactin). Image sets were overlaid (merge) to demonstrate coincident localization of cortical F-actin, pY421 cortactin, and total cortactin (white). Bars, 50 μM.
EGF Stimulates Phosphorylation of Cortactin on Tyrosine 421 and Tyrosine 466
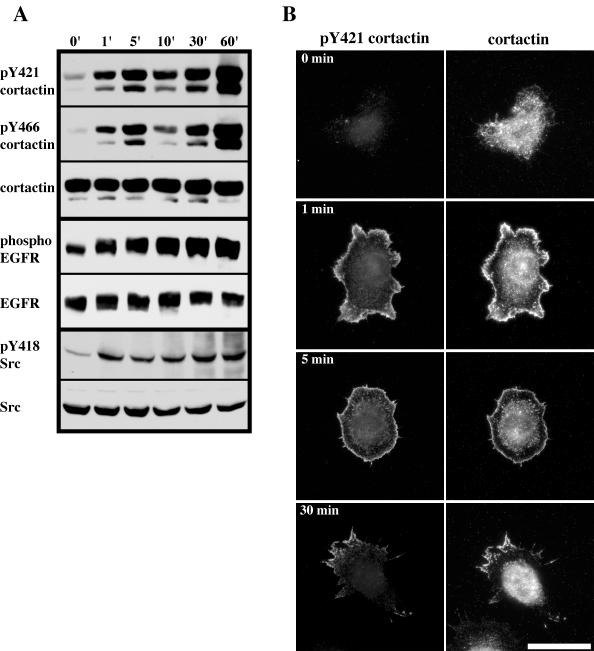
EGF is known to stimulate cortactin tyrosine phosphorylation in a Src-dependent manner (Maa et al., 1992). To determine whether tyrosine 421 and tyrosine 466 of cortactin are phosphorylated in response to EGF, serum-starved C3H 10T1/2 cells with 40-fold overexpression of EGFR (NeoR1; Maa et al., 1995) were treated with EGF and lysed at various time points over a 1-h period. Tyrosine phosphorylation of cortactin was subsequently analyzed by Western blotting of total cell extracts with anti-pY421 and anti-pY466 cortactin antibodies (Figure 6A). Serum-starved NeoR1 cells exhibited low levels of pY421 and pY466 phosphorylation that was elevated by EGF treatment by 1 min, increased after 5 min, decreased at 10 min, and increased from 30 min to 1 h (Figure 6A). The biphasic response of cortactin tyrosine phosphorylation in NeoR1 cells in response to EGF is similar to that observed in cells overexpressing c-Src (Maa et al., 1992) and in cells treated with fibroblast growth factor 1 (Zhan et al., 1993). The changes in tyrosine 421 and 466 phosphorylation were not due to fluctuations in cortactin protein levels, because stripping and reprobing of the blots with 4F11 did not indicate a change in cortactin amounts over the course of the experiment (representative cortactin blot shown in Figure 6A). Immunoblotting of lysates from stimulated NeoR1 cells at the same time points with antibodies against activated EGF receptor (phospho-EGFR) and Src (pY418 Src) indicated that both enzymes attained rapid (within 1 min) and sustained activation over the course of the experiment (Figure 6A). These data indicate that tyrosine 421 and 466 are phosphorylated in response to EGF treatment in NeoR1 cells with kinetics similar to that in other cell systems and that the time course of cortactin tyrosine 421 and tyrosine 466 phosphorylation parallels the activation of both EGF receptor and Src.
Figure 6.
Phosphorylation of cortactin tyrosine 421 and 466 induced by EGF. (A) Time course of EGF-stimulated cortactin tyrosine 421 and 466 phosphorylation. Confluent NeoR1 fibroblasts were serum starved for 18 h and then stimulated with 5 ng/ml EGF for the indicated times (top). After stimulation, cells were lysed and 100 μg of total cell protein was analyzed by Western blotting for phosphorylation of cortactin tyrosine 421 (pY421 cortactin) and tyrosine 466 (pY466 cortactin). Blots were stripped and reprobed with 4F11 (cortactin) to confirm equal cortactin levels (representative blot from one time course is shown). Activation of EGFR and Src were evaluated by Western blotting of either 100 μg (EGFR) or 150 μg (Src) of cell lysate from each time point with antibodies recognizing phosphorylated EGFR (phospho-EGFR) or Src (pY418 Src). The total levels of EGFR (EGFR) and Src (Src) were determined by Western blotting of lysates with pan-reactive antibodies. Blots are representative of results obtained from four independent experiments. (B) Distribution of pY421 cortactin during EGFR activation. Confluent, serum-starved NeoR1 cells were trypsinized and plated onto fibronectin-coated coverslips for 2 h in serum-free media. Cells were then stimulated with EGF (20 ng/ml) for the indicated times, fixed, and labeled with anti-pY421 (pY421 cortactin) and 4F11 (cortactin) antibodies. Bar, 50 μM.
In addition to Src, EGF also activates Rac1, which leads to cortical actin polymerization that results in the formation of membrane ruffles and lamellipodia (Ridley et al., 1992; Bailly et al., 1999). Because pY421 cortactin localizes to lamellipodia with cortical F-actin in cells cultured in serum (Figure 5, A and B) the subcellular distribution of both cortactin and pY421 cortactin in NeoR1 cells was examined after EGF treatment (Figure 6B). Serum-starved NeoR1 cells (0 min) were largely devoid of pY421 cortactin, with only faint labeling present in the cytoplasm where the majority of the cortactin pool was localized (Figure 6B). Serum starved cells treated for 1 min with 20 ng/ml EGF demonstrated nearly exclusive labeling of pY421 cortactin within broad membrane ruffles, with very little staining of the perinuclear region in spite of the presence of abundant cytoplasmic cortactin (Figure 6B). A similar pattern was observed after 5 min of EGF treatment, although the membrane ruffles containing cortactin and pY421 cortactin were narrower (Figure 6B), reminiscent of the phenotype exhibited by cells overexpressing activated Rac1 (Figure 2B). By 30 min, the majority (60%) of cells were similar to the phenotype observed after 5 min of EGF stimulation. However, a significant number (34%) of cells exhibited a polarized phenotype indicative of motility, with a clearly defined leading and trailing edge. In these cells, pY421 cortactin was enriched almost exclusively within leading edge lamellipodia as well as in the trailing edge, with little labeling of the cytoplasm despite the presence of an abundant cortactin pool in this region (Figure 6B). These data indicate that pY421 cortactin selectively localizes to membrane ruffles and lamellipodia in response to EGF receptor activation.
Phosphorylation of Cortactin Tyrosine 421 and Tyrosine 466 Requires Rac1 Activity
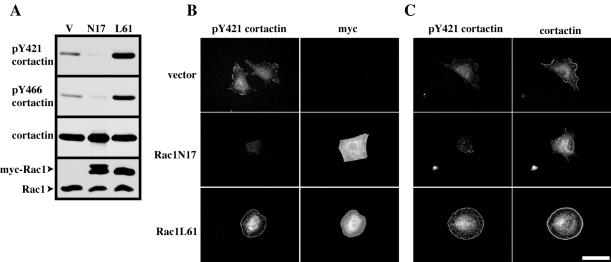
Translocation of cortactin from the cytoplasm into membrane ruffles and lamellipodia requires Rac1 activity (Weed et al., 1998). Because pY421 cortactin was selectively localized to these regions as a result of EGF treatment in NeoR1 cells, we examined whether phosphorylation of cortactin tyrosine 421 and tyrosine 466 was regulated by the activation state of Rac1. C3H 10T1/2 cells were cotransfected with FLAG-FL cortactin and with empty myc vector (V), dominant negative myc-Rac1T17N (N17), or constitutively active myc-Rac1Q61L (L61). FLAG-FL cortactin was immunoprecipitated with anti-FLAG resin, and tyrosine phosphorylation of cortactin was assayed by immunoblotting with anti-pY421 and anti-pY466 antibodies. FLAG-FL cortactin was phosphorylated at basal levels on tyrosine 421 and tyrosine 466 when coexpressed with the myc vector. However, phosphorylation of FLAG-FL cortactin tyrosines 421 and 466 was inhibited when expressed with myc-Rac1N17 (Figure 7A). Conversely, expression of myc-RacL61 enhanced FLAG-FL cortactin tyrosine 421 and 466 phosphorylation compared with that of cells transfected with vector alone (Figure 7A). Expression of myc-Rac proteins was verified by immunoblotting of cell lysates with an anti-Rac1 mAb, which detects both endogenous Rac1 as well as the slower migrating myc-Rac1 proteins (Figure 7A). These results indicate that the activity of Rac1 regulates phosphorylation of cortactin tyrosine 421 and 466.
Figure 7.
Rac activation is required for efficient phosphorylation of cortactin tyrosine 421 and 466. (A) The activation state of Rac1 modulates phosphorylation of cortactin tyrosine 421 and 466. 10T1/2 fibroblasts were cotransfected with FLAG-tagged full-length wild-type cortactin and either empty myc vector (V), myc-Rac1 T17N (N17), or myc-Rac1 Q61L (L61). After transfection, cells were lysed, FLAG-cortactin immunoprecipitated with anti-FLAG resin and cortactin tyrosine phosphorylation evaluated at tyrosine 421 (pY421 cortactin) and 466 (pY466 cortactin) by Western blotting. A representative blot after stripping and reblotting with 4F11 (cortactin) is shown to confirm precipitation of FLAG-cortactin. All blots are representative of three independent experiments. Expression of myc-Rac1 constructs was evaluated after Western blotting of 50 μg of cell lysate with an anti-Rac1 antibody. Arrows denote the positions of endogenous and myc-tagged Rac1 as indicated. (B) Localization of pY421 cortactin at the cell periphery requires Rac1 activation. C3H 10T1/2 fibroblasts transfected with either empty myc vector (vector), myc-Rac1 T17N (Rac1N17) or myc-Rac1 Q61L (Rac1L61) were trypsinized and plated onto fibronectin coated coverslips in serum-free media for 90 min. After fixation, cells were immunolabeled labeled with both anti-pY421 (pY421 cortactin) and 9E10 (myc) (B) or with anti-pY421 (pY421 cortactin) and 4F11 (cortactin) (C) antibodies. Bar, 50 μM.
To evaluate the subcellular distribution and degree of cortactin tyrosine phosphorylation in response Rac1 activity in vivo, C3H 10T1/2 cells transfected with empty myc vector (vector), myc-Rac1N17 and myc-Rac1L61were trypsinized, plated onto fibronectin, and allowed to spread for 2 h. Cells were immunostained with anti-pY421 cortactin and anti-myc 9E10 (Figure 7B) or anti-pY421 cortactin and anti-cortactin 4F11 (Figure 7C). In cells transfected with myc vector, pY421 cortactin localized at the perinuclear region and within lamellipodia with endogenous cortactin (Figure 7, B and C) as observed previously (Figure 5, A and B). Expression of myc-Rac1N17 inhibited the localization of cortactin to the cell periphery and resulted in the loss of pY421 immunolabeling from the cell cortex as well as significantly reducing level of pY421 labeling in the cytoplasm (Figure 7, B and C). In contrast, myc-RacL61 expression resulted in the translocation of cortactin to the cell cortex (Figure 7C) along with increased pY421 cortactin immunolabeling that localized at both the cell periphery and the perinuclear region (Figure 7, B and C). These results suggest that Rac1-induced translocation of cortactin to the cell periphery is required for phosphorylation of cortactin tyrosine 421
DISCUSSION
Previous work has established that tyrosines 421, 466, and 482 in murine cortactin as the central residues targeted by Src, Fer and c-Met kinases (Huang et al., 1998; Kapus et al., 2000; Crostella et al., 2001). Although phosphorylation of these residues is required for efficient cell motility (Huang et al., 1998; Crostella et al., 2001), the spatial and temporal requirements for cortactin tyrosine phosphorylation were previously unknown. The results presented herein demonstrate that phosphorylation of tyrosine residues in the carboxyl-terminal cortactin domain requires the cortactin amino terminus. Site-specific antibodies against phosphotyrosine 421 and 466, two sites of Src-mediated cortactin tyrosine phosphorylation, demonstrate that both of these tyrosine residues are phosphorylated in response activation of Src, EGFR, and Rac1. Western blot analysis of cortactin point mutants indicates that tyrosine 421 phosphorylation is required for phosphorylation of tyrosine 466. Cortactin phosphorylated on tyrosine 421 is enriched in lamellipodia and podosomes where it colocalizes with cortical F-actin. Phosphorylation of tyrosine 421 and 466 is dependent on activation of Rac1. Collectively, our results indicate that tyrosine phosphorylation of cortactin requires Rac1-mediated targeting to the cortical actin network within lamellipodia, where it is tyrosine phosphorylated at codons 421 and 466 in a hierarchical manner. We propose that phosphorylation of cortactin at tyrosine 421 and 466 serves to integrate tyrosine kinase-based signals with signaling pathways that contribute to actin cytoskeletal assembly and lamellipodia formation.
In agreement with previous results (Huang et al., 1998), expression of cortactin tyrosine point mutants in c-Src–overexpressing cells indicates that tyrosines 421, 466, and 482 are phosphorylated in vivo as a result of Src activity, with tyrosine 421 as the primary site of phosphorylation. Although codons 421, 466, and 482 are phosphorylated by Src, mutation of these three sites to phenylalanine does not completely abolish cortactin tyrosine phosphorylation. An additional tyrosine at codon 485 has been reported to be weakly phosphorylated by Src in vitro (Huang et al., 1998). Of the 27 tyrosine residues in murine cortactin, 12 are clustered within the carboxy terminus (Miglarese et al., 1994) with the remaining residues distributed throughout the amino terminus. Our analysis indicates that the amino terminal cortactin domain is tyrosine phosphorylated in a manner independent from Src and is phosphorylated at a low stoichiometry and/or at a single site. Tyrosine phosphorylation of the amino-terminal domain is significantly enhanced after treatment of cells with several growth factors (our unpublished data) pointing to a potential physiological role. Although the significance of this is currently unclear, one possibility is that tyrosine phosphorylation of the cortactin amino terminus may modulate its interaction with Arp2/3 complex and/or F-actin networks.
The availability of phosphorylation site-specific antibodies against tyrosine 421 and 466 in murine cortactin allows for the specific analysis of these phosphorylation sites. Oncogenic transformation by v-Src results in increased cortactin tyrosine phosphorylation (Kanner et al., 1990; Wu et al., 1991), and our results by using site-specific antibodies indicate that tyrosines 421 and 466 are phosphorylated in v-Src–transformed cells. Analysis of tyrosine point mutants with site-specific phosphorylation antibodies indicates that phosphorylation of tyrosine 421 is required for phosphorylation of tyrosine 466 (Figure 4), a conclusion consistent the results shown in Figure 1B by using a general anti-phosphotyrosine antibody. The amino acid sequence distal to tyrosine 421 (421YEDA424) conforms to the consensus binding site for Src homology 2 (SH2) domains (Songyan et al., 1993) and is conserved in all vertebrate cortactins identified to date (Figure 8). Src coimmunoprecipitates with cortactin in multiple cell systems (Zhan et al., 1994; Okamura and Resh, 1995; van Damme et al., 1997), and the SH2 domain of Src can associate with tyrosine-phosphorylated cortactin (Okamura and Resh, 1995). Treatment of Csk–/– fibroblasts with the SH2 inhibitory compound AP22408 inhibits cortactin tyrosine phosphorylation (Violette et al., 2001), pointing to a direct requirement for Src SH2 association with cortactin as a precursor event for cortactin tyrosine phosphorylation. These results, together with our observations suggests that cortactin is phosphorylated by Src in a sequential manner involving initial phosphorylation of tyrosine 421, potentially creating a binding site for the Src SH2 domain that would allow for Src association and subsequent phosphorylation of tyrosine 466. The involvement of tyrosine 482 in such a processive mechanism will require the development of phosphorylation specific antibodies against this site.
Cortactin tyrosine phosphorylation occurs concurrently with a multitude of signaling events that induce remodeling of the cortical actin cytoskeleton (Weed and Parsons, 2001 and references therein). Our data herein suggest that the ability of cortactin to regulate the actin cytoskeleton is structurally coupled with phosphorylation of tyrosine residues in the carboxy terminus. The inability of the carboxyl terminal cortactin domain to become tyrosine phosphorylated when expressed alone suggests that localization and association with F-actin and/or Arp2/3 complex at the cell periphery mediated by the amino terminus is required for phosphorylation of this domain. The ability of coronin1, a cortical actin and Arp2/3 complex binding protein implicated in lamellipodia formation (Mishima and Nishida, 1999; Humphries et al., 2002) to rescue tyrosine phosphorylation of the cortactin carboxy terminus supports a model whereby tyrosine phosphorylation of the carboxy terminus requires targeting of cortactin to and association with Arp2/3 complex and the cortical actin cytoskeleton, although the possibility exists that an actin-binding amino terminus (from either cortactin or coronin1) is required for proper conformational folding of the carboxy terminus and subsequent recognition by Src and other tyrosine kinases in vivo.
The enrichment of cortactin phosphorylated on tyrosine 421 in lamellipodia and podosomes, dynamic sites of actin remodeling, supports a possible role for cortactin tyrosine phosphorylation in regulating actin dynamics. Although the binding of cortactin to F-actin is not influenced by Src phosphorylation (Wu and Parsons, 1993), previous work has suggested that phosphorylation of Src by cortactin down-regulates the ability of cortactin to bundle actin filaments (Huang et al., 1997), although other groups have not detected efficient bundling in the presence of cortactin (Weaver et al., 2001). Cortactin exists as a monomer (Weaver et al., 2002) and possesses a single F-actin binding site (Weed et al., 2000), making the mechanism by which cortactin bundles F-actin unclear. Another possibility is that tyrosine phosphorylation of cortactin may influence the cortical actin cytoskeleton by creating binding sites for SH 2/3 adaptor molecules involved in actin remodeling. Tyrosine phosphorylated cortactin interacts with the SH2 domains of Nck and Crk (Okamura and Resh, 1995), two adaptor proteins that couple phosphotyrosine signals through interactions with their SH2 domains to Rac1 and Arp2/3 complex mediated actin reorganization by SH3 domain-mediated interactions (reviewed in Li et al., 2001a; Feller, 2001). Phosphorylation of cortactin at tyrosine 421 and/or 466 may therefore serve as an indirect mechanism for regulating the cortical cytoskeleton in addition to the direct ability of the cortactin amino terminus to regulate Arp2/3 complex actin nucleation activity.
In agreement with our findings, a recent report has suggested that Rac1 activation enhances cortactin translocation in cells exposed to hyperosmotic conditions (Di Ciano et al., 2002). This group reported that Rac1N17 inhibited cortactin tyrosine phosphorylation in their system, although the level of inhibition was not to the extent of that observed in our report. This may be due to differences specific to hyperosmotic shock and the implicated kinase Fer (Kapus et al., 2000), which differs from the analysis preformed in our fibroblast system, as well as the phosphorylation of other tyrosine residues on cortactin that may not be regulated by the activation state of Rac1. In addition to its association with the cortical actin cytoskeleton, cortactin phosphorylated on tyrosine 421 was also present to a lesser extent within the cytoplasmic cortactin pool in motile and spreading cells (Figure 5, A and B), and this cytoplasmic distribution was enhanced in cells expressing Rac1 L61 (Figure 7, B and C). Di Ciano et al. (2002) speculate that tyrosine phosphorylation of cortactin may serve to stimulate the release of cortactin from Arp2/3 networks as a result of hyperosmolarity, and the presence of cytoplasmic cortactin phosphorylated on tyrosine 421 in fibroblasts supports such a hypothesis. However, serum-starved cells treated with EGF, a potent Rac1 activator, led to the rapid and specific accumulation of cortactin phosphorylated on tyrosine 421 at the cell cortex, with little phosphorylated cortactin present in the cytoplasm (Figure 6B). Although our biochemical and localization data strongly suggest that Rac1 activation is the major determinant in the phosphorylation of cortactin tyrosine 421 and 466 in fibroblasts, the differential effects between EGF and serum in regulating the levels of cytoplasmic cortactin phosphorylated on tyrosine 421 points to the potential participation of additional signaling pathways contributing to cortactin tyrosine phosphorylation.
In addition to their localization in lamellipodia, both cortactin and Src are present at significant levels in perinuclear vesicular compartments within the cytoplasm, where their distribution demonstrates a degree of overlap (Maa et al., 1992; Okamura and Resh, 1995). Previous work demonstrating the requirement for Rac1 activity in the translocation of cortactin and Src to lamellipodia (Weed et al., 1998; Timpson et al., 2001) combined with the data presented in this report suggests that a possible mechanism for Src-mediated cortactin tyrosine phosphorylation may involve Rac1-induced targeting of nonphosphorylated cortactin and Src from cytoplasmic compartments to the cortical actin cytoskeleton. This would allow for the compartmentalization of cortactin and Src in lamellipodia, where subsequent juxtaposition of cortactin to activated Src would result in the processive phosphorylation of cortactin on tyrosines 421, 466, and 482. The requirement for phosphorylation of cortactin on these tyrosine residues in normal cell migration (Huang et al., 1998; Crostella et al., 2001) and tumor cell metastasis (Li et al., 2001b) highlights the importance of cortactin tyrosine phosphorylation in regulating normal and neoplastic cell movement. By controlling the subcellular localization of cortactin and Src, Rac1 may serve to integrate actin cytoskeletal signaling pathways through cortactin by regulating Arp2/3 complex activity with transmembrane receptor and nonreceptor tyrosine phosphorylation pathways used during lamellipodia extension and subsequent cell motility.
Acknowledgments
We thank Scott Lewis (BioSource International) for technical support, Andrew Kinley and Andrei Karginov (University of Virginia) for early assistance, Sarah Parsons and Mike Cox (University of Virginia) for helpful discussions, and the laboratory of Matt Thomas (Washington University, St. Louis, MO) for coronin1 cDNA. This work was supported by National Institutes of Health grant DE14364, a seed grant from the University of Colorado Cancer Center, and by start up funds from the University of Colorado School of Dentistry to S.A.W. J.T.P. acknowledges the support of National Institutes of Health grants CA-40042, CA-29243, and GM-38542. For specific questions about the phosphorylation of site-specific antibodies to cortactin pY421 and pY466, please contact Erik Schaefer (eschaefer@qcb.com) or Lynda Zorn (lzorn@qcb.com)
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-11-0753. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0753.
Abbreviations used: Arp2/3, actin related protein 2/3; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; pY, phosphotyrosine; RIPA, radioimmunoprecipitation assay; SH2/3, Src homology 2/3.
References
- Bailly, M., Macaluso, F., Cammer, M., Chan, A., Segall, J.E., and Condeelis, J.S. (1999). Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J. Cell Biol. 145, 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy, G.G., and Svitkina, T.M. (2000). Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104–112. [DOI] [PubMed] [Google Scholar]
- Brown, M.T., and Cooper, J.A. (1996). Regulation, substrates and functions of Src. Biochem. Biophys. Acta 1287, 121–149. [DOI] [PubMed] [Google Scholar]
- Cooper, J.A., Wear, M.A., and Weaver, A.M. (2001). Arp2/3 complex: advances on the inner workings of a molecular machine. Cell 107, 703–705. [DOI] [PubMed] [Google Scholar]
- Craig, S.W., and Johnson, R.P. (1996). Assembly of focal adhesions; progress, paradigms, and portents. Curr. Opin. Cell Biol. 8, 74–85. [DOI] [PubMed] [Google Scholar]
- Crostella, L., Lidder, S., Williams, R., and Skouteris, G.G. (2001). Hepatocyte growth factor/scatter factor-induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene 20, 3735–3745. [DOI] [PubMed] [Google Scholar]
- Devarajan, P., Stabach, P.R., Dematteis, M.A., and Morrow, J.S. (1997). Na, K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin-Darby canine kidney cells. Proc. Natl. Acad. Sci. USA 94, 10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano, C., Nie, Z., Szaszi, K., Lewis, A., Urono, T., Zhan, X., Rotstein, O.D., Mak, A., and Kapus, A. (2002). Osmotic stress-induced remodeling of the cortical cytoskeleton. Am. J. Physiol. 283, C850–C865. [DOI] [PubMed] [Google Scholar]
- Du, Y., Weed, S.A., Xoing, W.-C., Marshall, T.D., and Parsons, J.T. (1998). Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol. Cell. Biol. 18, 5838–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden, S., Rohatgi, R., Podtelejnikov, A.V., Mann, M., and Kirschner, M.W. (2002). Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793. [DOI] [PubMed] [Google Scholar]
- Feller, S.M. (2001). Crk family adaptors-signalling complex formation and biological roles. Oncogene 20, 6348–6371. [DOI] [PubMed] [Google Scholar]
- Fincham, V.J., Unlu, M., Brunton, V.G., Pitts, J.D., Wyke, J.A., and Frame, M.C. (1996). Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J. Cell Biol. 135, 1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guappone, A.C., Weimer, T., and Flynn, D.C. (1998). Formation of a stable src-AFAP-110 complex through either an amino-terminal or a carboxy-terminal SH2-binding motif. Mol. Carcinog. 22, 110–119. [DOI] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Higgs, H.N., and Pollard, T.D. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676. [DOI] [PubMed] [Google Scholar]
- Huang, C., Liu, J., Haudenschild, C.C., and Zhan, X. (1998). The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273, 25770–25776. [DOI] [PubMed] [Google Scholar]
- Huang, C., Ni, Y., Wang, T., Gao, Y., Haudenschild, C.C., and Zhan, X. (1997). Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 13911–13915. [DOI] [PubMed] [Google Scholar]
- Humphries, C.L., Balcer, H.I., D'Agostino, J.L., Winsor, B., Drubin, D.G., Barnes, G., Andrews, B.J., and Goode, B.L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner, S.B., Reynolds, A.B., Vines, R.R., and Parsons, J.T. (1990). Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc. Natl. Acad. Sci. USA 87, 3328–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus, A., Di Ciano, C., Sun, J., Zhan, X., Kim, L., Wong, T.W., and Rotstein, O.D. (2000). Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. The role of Fyn and FER kinases. J. Biol. Chem. 275, 32289–32298. [DOI] [PubMed] [Google Scholar]
- Kessels, M.M., Engqvust-Goldsein, A.E., and Drubin, D.G. (2000). Association of mouse actin-binding protein 1 (mAbp1/SH3P7), a src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol. Biol. Cell 11, 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche, N., Tapon, N., Stowers, L., Burbelo, P.D., Aspenstrom, P., Bridges, T., Chant, J., and Hall, A. (1996). Rac and cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87, 519–529. [DOI] [PubMed] [Google Scholar]
- Li, W., Fan, J., and Woodley, D.T. (2001a). Nck/Dock: an adaptor between cell surface receptors and the actin cytoskeleton. Oncogene 20, 6403–6417. [DOI] [PubMed] [Google Scholar]
- Li, Y., Tondravi, M., Liu, J., Smith, E., Haudenschild, C.C., Kaczmarek, M., and Zhan, X. (2001b). Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 61, 6906–6911. [PubMed] [Google Scholar]
- Lock, P., Abram, C.L., Gibson, T., and Courtneidge, S.A. (1998). A new method for isolating tyrosine kinase substrates used to identify Fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 17, 4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa, M.-C., Leu, T.H., McCarley, D.J., Schatzman, R.C., and Parsons, S.J. (1995). Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA 92, 6981–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa, M.-C., Wilson, L.K., Moyers, J.S., Vines, R.R., Parsons, J.T., and Parsons, S.J. (1992). Identification and characterization of a cytoskeleton-associated, epidermal growth factor sensitive pp60c-src substrate. Oncogene 7, 2429–2438. [PubMed] [Google Scholar]
- Miglarese, M.R., Mannion-Henderson, J., Wu, H., Parsons, J.T., and Bender, T.P. (1994). The protein tyrosine kinase substrate cortactin is differentially expressed in murine B lymphoid tumors. Oncogene 9, 1989–1997. [PubMed] [Google Scholar]
- Miki, H., Suetsugtu, K., and Takenawa, T. (1998). WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, H., Yamaguchi, H., Suetsugu, S., and Takenawa, T. (2000). IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408, 732–735. [DOI] [PubMed] [Google Scholar]
- Mishima, M., and Nishida, E. (1999). Coronin localizes to leading edges and is involved in cell spreading and lamellipodium extension in vertebrate cells. J. Cell Sci. 112, 2833–2842. [DOI] [PubMed] [Google Scholar]
- Mitchison, T.J., and Cramer, L.P. (1996). Actin-based cell motility and cell locomotion. Cell 84, 371–379. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Okmura, H., and Resh, M.D. (1995). p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J. Biol. Chem. 270, 26613–26618. [DOI] [PubMed] [Google Scholar]
- Ohoka, Y., and Takai, Y. (1998). Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells 3, 603–612. [DOI] [PubMed] [Google Scholar]
- Okumura, M., Kung, C., Wong, S., Rodgers, M., and Thomas, M.L. (1998). Definition of family of coronin-related proteins conserved between humans and mice: close genetic linkage between coronin-2 and Cd45-asociated protein. DNA Cell Biol. 17, 779–787. [DOI] [PubMed] [Google Scholar]
- Prasad, N., Topping, R.S., and Decker, S.J. (2002). Src family tyrosine kinases regulate adhesion-dependent tyrosine phosphorylation of 5′-inositol phosphatase SHIP2 during cell attachment and spreading on collagen I. J. Cell Sci. 115, 3807–3815. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., and Hall, A. (1992). The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Ruest, P.J., Shin, Nah-Young, Polte, T.R., Zhang, Xiaoe, and Hanks, S.K. (2001). Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 21, 7641–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D.A., Welch, M.D., Machesky, L.M., Bridgman, P.C., Meyer, S.M., and Cooper, J.A. (1998). Visualization and molecular analysis of actin assembly in living cells. J. Cell Biol. 143, 1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, M.D., and Schaefer, E.M. (2001). Multiple stimuli induce tyrosine phosphorylation of the Crk-binding sites of paxillin. Biochem. J. 360, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, J.V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120. [DOI] [PubMed] [Google Scholar]
- Songyan, Z., et al. (1993). SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–78. [DOI] [PubMed] [Google Scholar]
- Suetsugu, S., Miki, H., Yamaguchi, H., Obinata, T., and Takenawa, T. (2001). Enhancement of branching efficiency by the actin filament-binding activity of N-WASP/WAVE2. J. Cell Sci. 114, 4533–4542. [DOI] [PubMed] [Google Scholar]
- Timpson, P., Jones, G.E., Frame, M.C., and Brunton, V.G. (2001). Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11, 1836–1846. [DOI] [PubMed] [Google Scholar]
- Uruno, T., Liu, J., Zhang, P., Fan, Y., Egile, C., Li, R. Mueller, S.C., and Zhan, X. (2001). Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3, 259–266. [DOI] [PubMed] [Google Scholar]
- van Damme, H., Brok, H., Schuuring-Scholtes, E., and Schuuring, E. (1997). The redistribution of cortactin into cell-matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and post-translational modification. J. Biol. Chem. 272, 7374–7380. [DOI] [PubMed] [Google Scholar]
- Violette, S.M., et al. (2001). Bone-targeted Src SH2 inhibitors block Src cellular activity and osteoclast-mediated resorption. Bone 28, 54–64. [DOI] [PubMed] [Google Scholar]
- Weaver, A.M., Heuser, J.E., Karginov, A.V., Lee, W.-I., Parsons, J.T., and Cooper, J.A. (2002). Interaction of cortactin and N-WASp with Arp2/3 complex. Curr. Biol. 12, 1270–1278. [DOI] [PubMed] [Google Scholar]
- Weaver, A.M., Karginov, A.V., Kinley, A.W., Weed, S.A., Yan, L., Parsons, J.T., and Cooper, J.A. (2001). Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11, 370–374. [DOI] [PubMed] [Google Scholar]
- Webb, D.J., Parsons, J.T., and Horwitz, A.F. (2002). Adhesion assembly, disassembly and turnover in migrating cells - over and over and over again. Nat. Cell Biol. 4, E97–E100. [DOI] [PubMed] [Google Scholar]
- Weed, S.A., and Parsons, J.T. (2001). Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418–6434. [DOI] [PubMed] [Google Scholar]
- Weed, S.A., Du, Y., and Parsons, J.T. (1998). Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111, 2433–2444. [DOI] [PubMed] [Google Scholar]
- Weed, S.A., Karginov, A.V., Schafer, D.A., Weaver, A.M., Kinley, A.W., Cooper, J.A., and Parsons, J.T. (2000). Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, L.K., and Parsons, S.J. (1990). Enhanced EGF mitogenic response is associated with enhanced tyrosine phosphorylation of specific cellular proteins in fibroblasts overexpressing c-src. Oncogene 5, 1471–1480. [PubMed] [Google Scholar]
- Wu, H., and Parsons, J.T. (1993). Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 120, 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., Reynolds, A.B., Kanner, S.B., Cines, R.R., and Parsons, J.T. (1991). Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Biol. Cell 11, 5113–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, X., Hu, X., Hampton, B., Burges, W.H., Friesel, R., and Maciag, T. (1993). Murine cortactin is phosphorylated in response to fibroblast growth factor-1 on tyrosine residues late in the G1 phase of the BALB/c 3T3 cell cycle. J. Biol. Chem. 268, 24427–24431. [PubMed] [Google Scholar]
- Zhan, X., Plourde, C., Hu, X., Friesel, R., and Maciag, T. (1994). Association of fibroblast growth factor receptor-1 with c-Src correlates with association between c-Src and cortactin. J. Biol. Chem. 269, 20221–20224. [PubMed] [Google Scholar]