Abstract
We studied the endocytosis of fluorescent glycosphingolipid (GSL) analogs in various cell types using pathway-specific inhibitors and colocalization studies with endocytic markers and DsRed caveolin-1 (cav-1). Based on inhibitor studies, all GSLs tested were internalized predominantly (>80%) by a clathrin-independent, caveolar-related mechanism, regardless of cell type. In addition, fluorescent lactosylceramide (LacCer) colocalized with DsRed-cav-1 in vesicular structures upon endocytosis in rat fibroblasts. The internalization mechanism for GSLs was unaffected by varying the carbohydrate headgroup or sphingosine backbone chain length; however, a fluorescent phosphatidylcholine analog was not internalized via caveolae, suggesting that the GSL ceramide core may be important for caveolar uptake. Internalization of fluorescent LacCer was reduced 80–90% in cell types with low cav-1, but was dramatically stimulated by cav-1 overexpression. However, even in cells with low levels of cav-1, residual LacCer internalization was clathrin independent. In contrast, cholera toxin B subunit (CtxB), which binds endogenous GM1, was internalized via clathrin-independent endocytosis in cells with high cav-1 expression, whereas significant clathrin-dependent uptake occurred in cells with low cav-1. Fluorescent GM1, normally internalized by clathrin-independent endocytosis in HeLa cells with low cav-1, was induced to partially internalize via the clathrin pathway in the presence of CtxB. These results suggest that GSL analogs are selectively internalized via a caveolar-related mechanism in most cell types, whereas CtxB may undergo “pathway switching” when cav-1 levels are low.
INTRODUCTION
There is currently a great deal of interest in the study of caveolar-mediated endocytosis because this pathway appears to be important for cell entry and intracellular delivery of some bacterial toxins, viruses, and bacteria as well as some growth factors and other circulating proteins (Schnitzer et al., 1994; Gleizes et al., 1996; Anderson, 1998; Lobie et al., 1999; Okamoto et al., 2000; Shin et al., 2000; Pelkmans et al., 2001; Shubert et al., 2001; Marjomaki et al., 2002). Caveolar endocytosis is a clathrin-independent, dynamin-dependent process (Henley et al., 1998; Oh et al., 1998); however, the molecular machinery that differentiates caveolar endocytosis from other forms of clathrin-independent endocytosis is not yet fully defined. Seminal studies by Orlandi and Fishman (1998) demonstrated that the cholera toxin B subunit (CtxB), which binds to GM1 at the plasma membrane (PM), is internalized by caveolae in several cell types. However, more recent studies have shown that CtxB can be significantly internalized by clathrin-dependent endocytosis in some cell types (Shogomori and Futerman, 2001; Torgersen et al., 2001). Another potential marker for caveolar uptake is labeled albumin, which is reported to be internalized primarily by caveolae (Schnitzer et al., 1994; Shubert et al., 2001).
One well-characterized marker for caveolar internalization is SV40 virus that colocalizes with GFP-tagged caveolin-1 (cav-1) during endocytosis (Pelkmans et al., 2001); however, the intracellular itinerary and slow rate of transport of SV40 virus differs from that of some other cargo internalized via caveolae (Gleizes et al., 1996; Orlandi and Fishman, 1998; Lobie et al., 1999; Sharma et al., 2003). These and other observations suggest the possibility that several forms of caveolar endocytosis may exist (Mineo and Anderson, 2001; Le and Nabi, 2003).
We previously showed that fluorescent (BODIPY-labeled) glycosphingolipid (GSL) analogs (lactosylceramide [LacCer] and globoside) are selectively internalized by a dynamin-dependent, clathrin-independent mechanism in human skin fibroblasts (HSFs) and on the basis of multiple criteria suggested that these lipids were internalized by a “caveolarrelated” process (Puri et al., 2001). This study raised several important questions concerning caveolar endocytosis. First, is selective endocytosis via caveolae a property of other GSLs with different carbohydrate headgroups? Second, what are the biological or biophysical mechanisms (Anderson and Jacobson, 2002; Brown and London, 2000; Edidin, 2003) involved in the selective entry of GSLs into caveolarderived smooth vesicles but not clathrin-coated vesicles? Finally, does selective caveolar endocytosis of GSLs only occur in some cell types (e.g., HSFs) or is it a widely occurring process in mammalian cells? The mechanism for CtxB internalization has been reported to be variable (i.e., clathrin-independent and/or -dependent) depending on cell type and cav-1 expression levels (Shogomori and Futerman, 2001; Torgersen et al., 2001; Sandvig and van Deurs, 2002). This raises the possibility that some GSLs (e.g., GM1) are not internalized primarily via caveolae in some cell types, or alternatively that CtxB perturbs the internalization mechanism of endogenous GM1, and that fluorescent GSL analogs may better reflect the movement of endogenous GSLs. In the present article we sought to resolve these uncertainties by carrying out a detailed study of GSL analog internalization in multiple cell types and by comparing those results to parallel studies using fluorescent CtxB. Our data provide strong evidence that fluorescent GSL analogs of widely varying structure are internalized primarily by a clathrin-independent, cav-1–dependent process. We further show that the mechanism of fluorescent GM1 internalization is altered when bound by CtxB in HeLa cells where cav-1 expression is low. These data suggest that caveolar endocytosis plays a significant role in plasma membrane GSL internalization in multiple cell types.
MATERIALS AND METHODS
Cells and Cell Culture
Normal HSFs were from the Coriell Institute (Camden, NJ). Rat fibroblasts (RFs), HeLa, CHO-K1, Calu-1, and Calu-6 cells were from the American Type Culture Collection (Rockville, MD). MDCK cells were a generous gift from Dr. J.C. Lieske (Mayo Clinic, Rochester, MN). All experiments were performed using monolayer cultures grown to ∼30–50% confluency on acid-etched glass or gridded coverslips for microscopy or in 60-mm dishes for biochemical experiments.
Fluorescent Lipids, Toxins, and Other Reagents
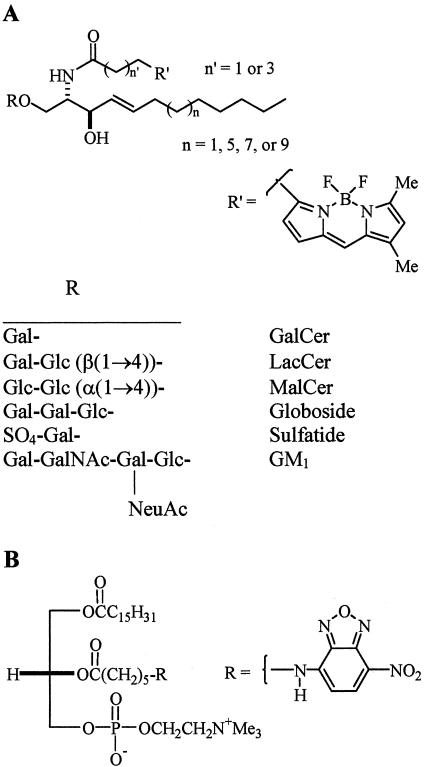
The structures of the various fluorescent lipid analogs are shown in Figure 1. BODIPY-maltosylceramide (MalCer) was synthesized by boron trifluoride mediated glycosylation of d-erythro-2-azido-3-benzoylsphingosine (prepared from d-erythro-C18-sphingosine; Matreya, Pleasant Gap, PA) with per-O-acetylmaltosyl trichloroacetimidate in dichloromethane in the presence of molecular sieves. Hepta-O-acetylmaltosyl trichloroacetimidate was prepared from octa-O-acetylmaltose (Sigma-Aldrich, St. Louis, MO) in two steps: 1) treatment with hydrazine acetate in dimethylformamide, and 2) treatment with trichloroacetonitrile and sodium in dichloromethane. After deprotection of the hepta-O-acetylated GSL with sodium methoxide and reduction of the azide with triphenylphosphine in aqueous tetrahydrofuran, the final product was obtained by N-acylation with the N-hydroxysuccinimidoyl (NHS) ester of 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoic acid (BODIPY-FL-C5, SE; Molecular Probes, Eugene, OR) and purified by column chromatography on silica gel (elution with chloroform/methanol/water 65:35:4 vol/vol/vol), followed by preparative TLC (elution with chloroform/methanol/water 65:25:4 vol/vol/vol). Suspended silica gel was removed by filtration through a Cameo filter (Fisher Scientific, Pittsburgh, PA).
Figure 1.
Structures of fluorescent lipid analogs used in the present study. (A) Various headgroups (R) were attached to BODIPY-ceramide, resulting in BODIPY-GalCer, -LacCer, -MalCer, -globoside, -sulfatide, or -GM1. BODIPY-LacCer analogs were also synthesized using various chain length (C12, C16, C18, or C20) sphingosines or BODIPY-fatty acids (C3 vs. C5 spacer). Fluorescent LacCer bearing an NBD-fatty acid (see B) in place of the BODIPY-fatty acid was also synthesized. (B) Structure of the d-isomer of NBD-labeled PC, a glycerolipid.
C3-BODIPY-LacCer was synthesized as described (Martin and Pagano, 1994), using lyso-LacCer (Matreya) and the NHS ester of 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionic acid (BODIPY-FL, SE; Molecular Probes). C5-BODIPY-LacCer analogs having shorter (C12 or C16) or longer (C20) sphingoid backbones than C3-BODIPY-LacCer (C18) were synthesized from d-erythro-2-azido-3-benzoylsphingosine analogs as described above for BODIPY-MalCer using C12, C16, or C20 D-erythro-sphingosines, custom synthesized by Matreya and BODIPY-FL-C5, SE (Molecular Probes).
C5-BODIPY-fatty acid labeled analogs of galactosylceramide (GalCer), LacCer, globoside, GM1, and sulfatide, and 6-[(7-nitro-benz-2-oxa-1,3-diazol-4-yl)amino]caproic acid labeled-LacCer (NBD-LacCer) were synthesized and purified as described (Martin and Pagano, 1994; Watanabe et al., 1999; Puri et al., 2001). The d-isomer of NBD-phosphatidylcholine (NBD-d-PC) was prepared as described (Martin and Pagano, 1987).
The concentrations of the lipid analogs were determined by measurements of fluorescence intensity relative to known standards. Complexes of the fluorescent lipids with defatted bovine serum albumin (DF-BSA; Sigma-Aldrich) were prepared as described (Martin and Pagano, 1994) and diluted to a final working concentration using 10 mM HEPES-buffered minimal essential medium (pH 7.4) (HMEM). AlexaFluor 488 and 594 (AF488 and AF594)labeled CtxB, transferrin (Tfn), and albumin, and Cascade blue dextran (MW 10 kDa) were from Molecular Probes. Other reagents were from Sigma-Aldrich unless otherwise noted.
Incubation of Cells with Fluorescent Lipids and Various Markers
Cell cultures were washed with ice-cold HMEM (10 mM HEPES-buffered MEM), transferred to a water bath at 10°C, and then incubated with fluorescent lipid/DF-BSA for 30 min to label the PM (Chen et al., 1997). (In one series of experiments, a 0.5 mM ethanolic stock solution of BODIPY-LacCer was prepared, diluted to 2 μM in HMEM, and incubated with the cells as above.) The cells were then washed with cold HMEM and warmed to 37°C for various times to induce endocytosis. After this incubation, the medium was replaced with ice-cold HMEM without glucose containing the inhibitors, 5 mM NaN3 and 50 mM 2-deoxyglucose (HMEM-G+I) and the culture dishes were transferred to a 10°C bath. Fluorescent lipid present at the cell surface was removed by incubating the cells (six times, 10 min each) with 5% DF-BSA in HMEM-G+I at 10°C (Martin and Pagano, 1994; Chen et al., 1997). For CtxB labeling, cells were incubated with 7.5 μg/ml AF594 CtxB for 45 min at 10°C, washed, and further incubated at 37°C for the indicated times. For Tfn labeling, cultures were preincubated in serum-free culture medium for 2 h at 37°C to upregulate the Tfn receptor. Samples were then washed, incubated with 30 μg/ml AF594 Tfn for 45 min at 10°C, washed, and further incubated at 37°C for the indicated times. Excess CtxB or Tfn at the cell surface was removed by acid stripping (30 s at 10°C with HMEM, pH 3.5).
Pharmacological Inhibitors of Endocytosis
Cells were treated with various inhibitors to differentiate clathrin-dependent from clathrin-independent endocytosis as described (Puri et al., 2001). For inhibition of clathrin-dependent endocytosis, samples were pretreated with 8 μg/ml chlorpromazine (CPZ; Gustavsson et al., 1999; Okamoto et al., 2000) or were potassium depleted (Larkin et al., 1983; Hansen et al., 1993); for disruption of caveolar endocytosis, cells were pretreated with 25 μg/ml nystatin (Rothberg et al., 1992) or 200 μM genistein (Aoki et al., 1999; Chen and Norkin, 1999; Liu and Anderson, 1999). The specificity of each inhibitor treatment was evaluated by monitoring the internalization of fluorescent CtxB, albumin, and Tfn as endocytic markers (see Results). Cell viability was >90% for each inhibitor treatment as judged by trypan blue staining.
Transfection Studies
GFP constructs of Eps15 (D3Δ2 [control] and the EH21 mutant EΔ95/295 [dominant negative Eps15]) were from Drs. A. Benmerah and A. Dautry-Varsat (Inserm; Paris); GFP constructs of dynamin 2 (Dyn2ab [control] and Dyn2ab K44A [dominant negative]) were from Dr. M. McNiven (Mayo Clinic). DsRed1-cav-1 was generated from cav-1-GFP (a generous gift from Dr. A. Helenius, Swiss Federal Institute of Technology, Zurich) by removing a BamH1-HindIII fragment containing the cav-1 gene from the cav-1-GFP construct and inserting it into a pDsRed1-C1 expression vector (BD Biosciences Clontech, Palo Alto, CA). Cells were treated with FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) and 2 μg/ml DNA using the manufacturer's protocol. After a 4–6-h treatment, the cells were washed and subsequently cultured for 24–48 h in DMEM containing 10% FBS before treatment with the fluorescent lipids or toxins as above. Transfected cells were detected by GFP- or DsRed fluorescence and the effect on lipid internalization was evaluated as described (Puri et al., 2001; Choudhury et al., 2002).
Colocalization Studies
BODIPY-LacCer with Fluorescent Albumin or Dextran. RFs were incubated for 30 min at 10°C with 2 μM BODIPY-LacCer to label the PM. Cells were then washed and incubated for 2 or 5 min at 37°C in the presence of 30 μg/ml AF594 albumin or 1 mg/ml Cascade blue Dextran. Cells were then washed, back-exchanged, and observed under the fluorescence microscope. In control experiments using cells in which only AF594 albumin (or Cascade blue dextran) was present, no fluorescence was detected in the green microscope channel used to visualize BODIPY-fluorescence.
DsRed-cav-1 with BODIPY-LacCer, Fluorescent Albumin, or Tfn. RFs were transfected with the DsRed-cav-1 construct and 48 h later were pulse-labeled with BODIPY-LacCer, AF488 albumin, or AF488 Tfn as above. Transfected cells were identified by DsRed fluorescence. In control experiments using singly labeled specimens, no overlap between DsRed and either LacCer or AF488 fluorescence was detected under the experimental conditions used.
Colocalization values were calculated using Metamorph image processing software (v4.6; Universal Imaging Corp., Dowingtown, PA) and are based on the overlapping area of green puncta (BODIPY-LacCer, AF488 albumin, or AF488 Tfn) with red puncta (DsRed-cav-1) in doubly labeled specimens.
BODIPY-GM1 Internalization with and without CtxB
HeLa cells were pretreated with nystatin (25 μg/ml) or CPZ (8 μg/ml) in HMEM for 30 min at 37°C. Cells were then washed with ice-cold HMEM, incubated with 1 μM BODIPY-GM1 for 35 min at 10°C, washed, and further incubated for 1 h at 10°C in HMEM with or without 1 μg/ml AF594 CtxB. The cells were then washed, incubated for 5 min at 37°C, acid stripped (see above), and back-exchanged with 5% DF-BSA in HMEM-G+I (2× 2 min at 37°C, followed by 4× 10 min at 10°C). Samples were then observed under the fluorescence microscope. In control experiments using cells in which only AF594 CtxB was present, no fluorescence was detected with the “green” microscope filters under the same exposure conditions used to visualize BODIPY-fluorescence.
Other Procedures
Fluorescence microscopy using an Olympus IX70 fluorescence microscope and quantitative image analysis were performed as described (Puri et al., 2001; Choudhury et al., 2002). For Western blotting, aliquots of cell lysates were solubilized in sample buffer, run on 15% SDS-PAGE gels, and transferred to polyvinylidene difluoride membranes in transfer buffer with 20% methanol. Blots were probed with monoclonal anticav-1 antibody (BD Biosciences PharMingen, San Diego, CA) followed by antimouse horseradish peroxidase secondary antibody (Amersham Life Sciences, Piscataway, NJ).
RESULTS
Endocytosis of Multiple GSL Analogs in Rat Fibroblasts
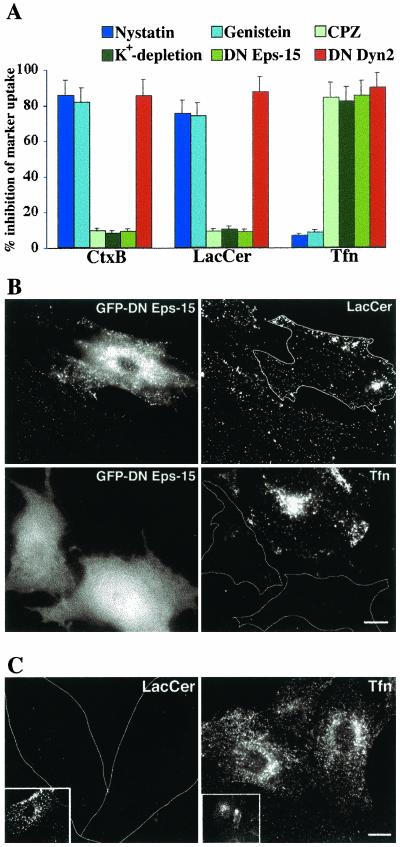
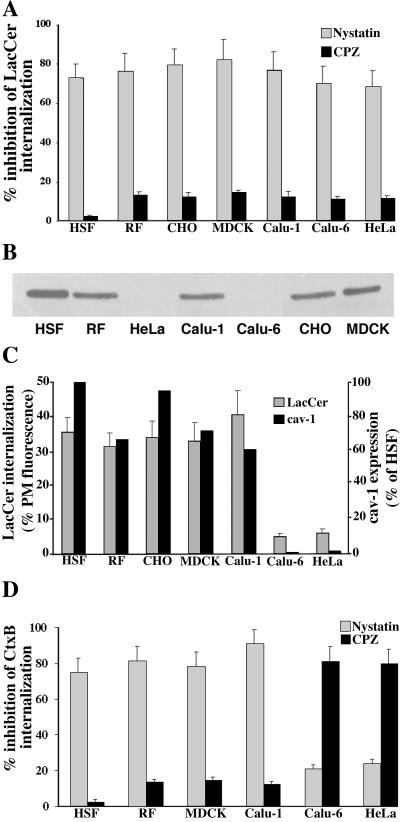
Our previous studies demonstrated that the GSL analogs LacCer and globoside were internalized predominantly via a clathrin-independent caveolar-related mechanism in HSFs (Puri et al., 2001). Thus, it was of interest to further study the internalization of GSL analogs to learn if this was a general phenomenon. We first extensively studied LacCer internalization in RFs. The uptake of BODIPY-LacCer was compared with that of fluorescent Tfn and CtxB, which are selective endocytic markers for clathrin-dependent vs. caveolar endocytosis, respectively. Using a series of pathway-specific pharmacological inhibitors and a DN construct of Eps15, we demonstrated that LacCer internalization in RFs was inhibited ∼80% by nystatin and genistein, but only ∼10% by CPZ, K+-depletion, or DN Eps15 expression, similar to the behavior of CtxB (Figure 2A). In contrast, internalization of fluorescent Tfn was inhibited by CPZ, K+ depletion, or expression of DN Eps15, but was not significantly affected by nystatin or genistein (Figure 2A). Examples of the selective inhibition of Tfn uptake by DN Eps15 and LacCer internalization by nystatin are shown in Figures 2, B and C, respectively. In addition, we found that internalization of Tfn, CtxB, and LacCer were each inhibited by ∼90% in RFs transfected with a DN construct of dynamin (McNiven et al., 2000; Figure 2A). Together, these results demonstrate that in RFs, LacCer internalization occurred by a clathrin-independent mechanism, consistent with our previous studies in HSFs (Puri et al., 2001).
Figure 2.
Differential internalization of endocytic markers and fluorescent lipid analogs in rat fibroblasts. (A) RFs were pretreated with the indicated pharmacological inhibitor or transfected with DN Eps15 or Dyn2 constructs and the internalization (5 min at 37°C) of fluorescent CtxB, Tfn, or BODIPY-LacCer, relative to untreated control samples was quantified by image analysis. Note the similarity of LacCer to CtxB internalization in response to the various treatments. Representative fluorescence micrographs showing the differential internalization (5 min at 37°C) of LacCer vs. Tfn are given in B for RFs transfected with DN Eps15 (white outlines indicate transfected cells) or in C for RFs pretreated with nystatin (white outlines indicate position of cells observed by phase microscopy). Insets show LacCer or Tfn internalization in untreated control cells. Bars, 10 μm.
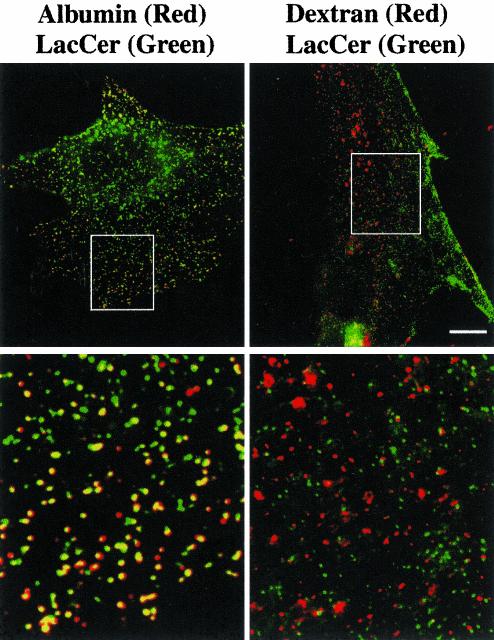
To further characterize the endocytosis of LacCer, we carried out colocalization experiments using labeled albumin, which internalizes via caveolae in some cell types (Shubert et al., 2001). In preliminary studies, we found that internalization (5 min at 37°C) of fluorescent albumin was inhibited by 88.9 ± 6.8% in cells pretreated with nystatin, similar to that seen using LacCer and CtxB (Figure 2A), but was inhibited by only 7.2 ± 1.7% following pretreatment with CPZ. Extensive overlap (>93%) of BODIPY-LacCer and fluorescent albumin was seen after 3 min of internalization at 37°C (Figure 3). We then carried out a parallel experiment using BODIPY-LacCer and fluorescent dextran, which at low concentrations (1 mg/ml) acts as a marker for cdc42-dependent pinocytosis (Sabharanjak et al., 2002). In preliminary studies we found that dextran internalization was virtually unaffected by pretreatment of cells with CPZ (≤2% inhibition) or nystatin (≤5% inhibition), demonstrating that dextran internalization occurred by a mechanism which was distinct from those utilized for endocytosis of Tfn or CtxB. In double-label experiments there was <10% colocalization of LacCer with fluorescent dextran after 5 min of internalization (Figure 3). We also pretreated cells with Clostridium difficile toxin B, an inhibitor of the Rho family GTPases (Aktories et al., 2000), and found that this treatment blocked dextran uptake but had no effect on LacCer internalization (unpublished data).
Figure 3.
Colocalization of BODIPY-LacCer with fluorescent albumin vs. dextran in rat fibroblasts. RFs were incubated with BODIPYLacCer for 30 min at 4°C, washed, and further incubated for 3 min at 37°C with 30 μg/ml AF594-conjugated albumin or 5 min at 37°C with 1 mg/ml Cascade blue dextran. After back-exchange at 10°C to remove any LacCer remaining at the PM (see MATERIALS AND METHODS), the samples were observed under the fluorescence microscope, and separate images were acquired for each fluorophore. Images were rendered in pseudocolor and are presented as overlays: LacCer, green; albumin, red; dextran, red. Note the extensive colocalization of LacCer with albumin but not with dextran. Boxed areas are shown at higher magnification in the corresponding bottom panels. Bar, 10 μm.
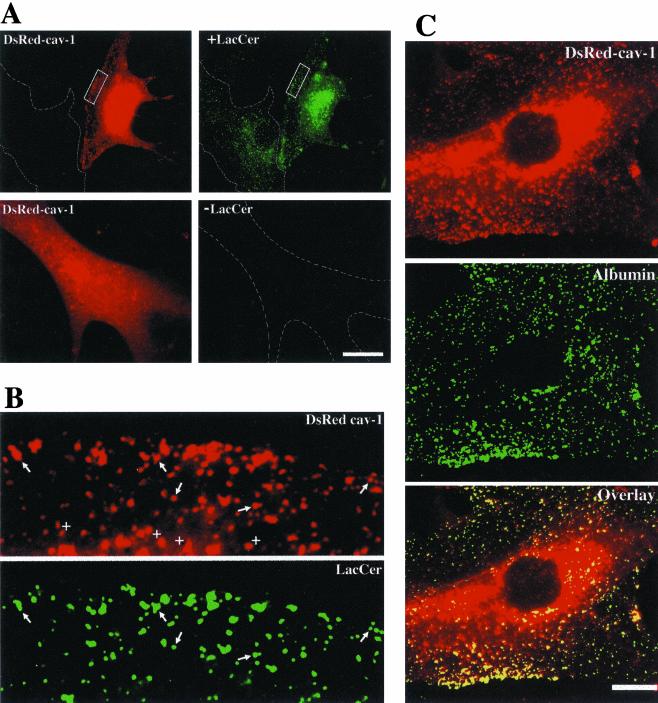
Finally, we carried out colocalization studies in RFs transfected with the DsRed-cav-1 construct. BODIPY-LacCer was internalized for 2 min at 37°C, and images of DsRed-cav-1 (red) and LacCer (green) were acquired. Punctate LacCer fluorescence (green) extensively overlapped with DsRedcav-1 puncta as seen in Figure 4, A and B. Importantly, in adjacent nontransfected cells no red fluorescence from LacCer was detected at these exposure settings. Furthermore, in control samples expressing DsRed-cav-1 but not incubated with BODIPY-LacCer, no fluorescence was detected at green wavelengths (Figure 4A, bottom right panel). Similarly, endocytosed AF488 albumin extensively colocalized with DsRed-cav-1 (Figure 4C), whereas no overlap of DsRedcav-1 was seen in cells incubated with AF488 Tfn.
Figure 4.
DsRed-cav-1 colocalization with BODIPY-LacCer and fluorescent albumin. RFs were transfected with DsRed-cav-1 and 48 h later the distribution of (A and B) BODIPY-LacCer or (C) AF488 albumin after 2 min of internalization at 37°C was examined. Top panels in A show the same field for cav-1 (red) or LacCer (green). Note that in an adjacent nontransfected cell outlined in white, there was no spillover of LacCer fluorescence into the DsRed channel. Bottom panels in (A) show a control experiment using transfected cells without LacCer labeling. Note that no DsRed fluorescence appeared in the green channel at the exposure setting used for LacCer in the doubly labeled specimen. The boxed regions in A are shown at higher magnification in B. Note the similar patterns of punctate fluorescence (e.g., at arrows) for LacCer and cav-1. Quantitative analysis indicated that at least ∼90% of the green puncta colocalized with DsRed-cav-1. However, not all DsRed-cav-1 puncta were positive for LacCer (e.g., see +s). (C) Fluorescence micrographs of a cell expressing DsRed-cav-1 and labeled with AF488 albumin. More than 95% of the AF488 puncta colocalized with DsRed-cav-1. Bars, 10 μm.
Together the results in Figures 2, 3, 4 provide strong evidence that LacCer was internalized via caveolae in RFs, rather than by pinocytosis or other Rho-dependent, nonclathrin endocytic mechanisms.
To investigate the structural determinants of GSLs that result in selective internalization via caveolae, we systematically varied the structure of the fluorescent GSL analogs (see Figure 1) and examined the effect of these variations on the mechanism of analog internalization. To examine the significance of the carbohydrate head group, we used fluorescent analogs of GalCer, globoside, GM1, LacCer, MalCer, and sulfatide. (Fluorescent glucosylceramide was not used because this lipid is internalized from the PM by a combination of endocytic and nonendocytic mechanisms; Martin and Pagano, 1994.) For each of these fluorescent GSL analogs, the fluorescent fatty acid and sphingosine base were identical (n′ = 3, n = 7; Figure 1A). RFs were incubated with each analog in the presence or absence of various inhibitors to differentiate clathrin-dependent from clathrin-independent endocytosis as in Figure 2, and the amount of internalization (at 5 min) was quantified by image analysis. The internalization of each GSL analog (GalCer, MalCer, globoside, sulfatide, and GM1) was substantially inhibited by nystatin (but not by CPZ), similar to BODIPY-LacCer (Table 1), indicating that the specific carbohydrate headgroup structure or the stereochemistry of the glycosidic linkage in the disaccharide moiety of the GSL does not play a signifi-cant role in selective internalization of these GSLs by the clathrin-independent, caveolar mechanism.
Table 1.
Inhibition of lipid analog endocytosis by different biochemical treatments in rat fibroblasts
|
Clathrin-independent internalization
|
Clathrin-dependent internalization
|
|||
|---|---|---|---|---|
| Fluorescent lipid analog | Nystatina | Genisteina | Chlorpromazinea | K+-depletiona |
| % | % | % | % | |
| BODIPY-GalCer | 71.9 ± 9.5 | ND | 10.5 ± 2.8 | ND |
| BODIPY-LacCer | 75.1 ± 6.8 (73.9 ± 8.7)b | 73.9 ± 4.1 | 9.3 ± 1.7 (8.6 ± 1.6)b | 10.4 ± 1.3 |
| BODIPY-MalCer | 76.7 ± 8.2 | ND | 9.2 ± 1.9 | ND |
| BODIPY-Globoside | 82.6 ± 10.5 | 82.0 ± 4.5 | 0.7 ± 0.1 | 7.0 ± 0.8 |
| BODIPY-Sulfatide | 78.2 ± 7.6 | 73.6 ± 9.7 | 9.1 ± 2.2 | ND |
| BODIPY-GM1 | 74.7 ± 8.2 | 69.9 ± 8.9 | 8.8 ± 1.2 | 11.6 ± 2.1 |
| NBD-LacCer | 75.6 ± 6.7 | ND | 15.1 ± 2.4 | ND |
| NBD-D-PC | 20.4 ± 4.4 | ND | 71.3 ± 8.5 | ND |
Cells were pretreated with or without the indicated inhibitor (see MATERIALS AND METHODS) and subsequently incubated with a BSA complex of the indicated lipid analog for 30 min at 10°C to label the PM. The samples were then washed, warmed for 5 min at 37°C to allow endocytosis to occur, and then back-exchanged to remove fluorescent lipid remaining at the PM. Specimens were then observed under the fluorescence microscope and the percent inhibition of internalization of the lipid analog was determined by image analysis of the fluorescently labeled cells. Values are mean ± SD of at least 10 cells in each of the three independent experiments. ND, not determined.
Experiment was performed as in footnote a, except that an ethanolic solution of BODIPY-LacCer was used in place of the BSA complex (see text).
Finally, we carried out a control experiment exactly as above, except that BODIPY-LacCer was introduced into cells from an ethanolic solution rather than from a BSA complex (see MATERIALS AND METHODS). As seen in Table 1, virtually identical results were obtained when the LacCer analog was delivered to RFs using BSA vs. ethanol injection, indicating that the BSA carrier did not influence the LacCer uptake mechanism.
We also examined the importance of hydrophobicity on the mechanism of GSL endocytosis. For these studies, we used BODIPY-LacCer analogs in which the chain length of the sphingosine base (C12 to C20), or fatty acid (C3 vs. C5 spacer) was varied (n = 1,5,7, or 9; n′ = 1 or 3; Figure 1A). Endocytosis of the series of LacCer analogs was studied in RFs as above, but surprisingly none of the modifications in chain length affected the mechanism of LacCer internalization (Table 2). To investigate the possible influence of the BODIPY fluorophore, we studied the internalization of NBD-labeled LacCer and found that its internalization was nystatin-inhibitable and CPZ-insensitive (Table 1), similar to our findings for BODIPY-LacCer (and other BODIPY-GSLs). This demonstrated that the fluorophore (NBD vs. BODIPY) had no apparent influence on the internalization mechanism.
Table 2.
Inhibition of various chain length BODIPY-LacCer analogs in rat fibroblasts
|
% Inhibition
|
||
|---|---|---|
| BODIPY-SL analogs | Nystatin | CPZ |
| C12 So, C5-BODIPY-LacCer | 78.7 ± 11.2 | 9.8 ± 0.9 |
| C16 So, C5-BODIPY-LacCer | 80.8 ± 10.2 | 10.3 ± 1.1 |
| C18 So, C5-BODIPY-LacCer | 75.2 ± 8.9 | 9.3 ± 1.2 |
| C20 So, C5-BODIPY-LacCer | 75.9 ± 6.8 | 8.2 ± 1.2 |
| C18 So, C3-BODIPY-LacCer | 84.3 ± 9.4 | 5.4 ± 0.3 |
Cells were pretreated with or without the indicated inhibitor (see MATERIALS AND METHODS) and subsequently incubated with the indicated BODIPY-SL (see Figure 1 for structures) as described in Table 1. Values are mean ± SD of at least 10 cells in each of the three independent experiments. so, sphingosine.
Finally, we compared NBD-LacCer with NBD-d-PC to study the possible influence of the lipid backbone (ceramide vs. glycerol) on the internalization mechanism. For these studies, it was necessary to use the nonhydrolyzable d-stereoisomer of NBD-PC, because the natural l-isomer is rapidly degraded by cells (Sleight and Pagano, 1984), precluding its use in our endocytosis studies. As shown in Table 1, the endocytosis of NBD-d-PC was found to be predominantly CPZ-inhibitable, suggesting that unlike LacCer, its uptake occurred largely by clathrin-dependent endocytosis. In summary, these structural studies suggest that GSL uptake via caveolae is not selective for a specific carbohydrate headgroup, acyl chain hydrophobicity, or fluorophore substitution; however, comparison with the uptake of NBD-d-PC suggests that the ceramide core of GSLs may play an important role in caveolar endocytosis of GSLs.
LacCer Internalization in Other Cell Types
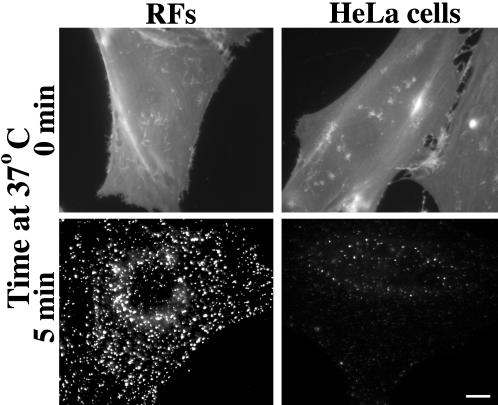
We next extended our studies of BODIPY-LacCer endocytosis to six other cell types in addition to RFs: HSFs, CHO, MDCK, Calu-1, Calu-6, and HeLa cells. In preliminary experiments we incubated each cell type with various concentrations (1–3 μM) of BODIPY-LacCer at 4°C, washed the cells, and quantified the PM fluorescence. This allowed us to adjust the incubation conditions such that the initial labeling of the PM was nearly identical (±10%) for each cell type (e.g., see Figure 5). Cells were then warmed for 5 min at 37°C, and the amount of LacCer internalization from the PM was quantified. Approximately 30–40% of the lipid analog was internalized from the PM in each cell type except in HeLa and Calu-6 cells, where internalization was only ∼5–6% of the initial lipid present at the PM (Figure 6C). An example of this reduced internalization is shown in Figure 5 for HeLa cells vs. RFs. Although the amount of LacCer internalization varied among the different cell types, its internalization was sensitive to nystatin but not to CPZ in each case (Figure 6A), suggesting that LacCer endocytosis was clathrin independent for all the cell types studied. In addition, we carried out colocalization experiments using BODIPY-LacCer and fluorescent albumin, analogous to the experiment shown in Figure 3. Extensive colocalization of the lipid analog and fluorescent albumin was found in all the cell types tested after 3 min of internalization. These experiments were not carried out using HeLa or Calu-6 cells because internalization of LacCer was very low in those cell types.
Figure 5.
BODIPY-LacCer internalization in RFs vs. HeLa cells. Cells were incubated with 2 μM BODIPY-LacCer for 30 min at 10°C, washed and observed immediately (top panels) or warmed for 5 min at 37°C before back-exchange and microscopy (bottom panels). All images were exposed and printed identically. Note that although the PM labeling (0 min) was similar for both cell types, the amount of internalization was greatly reduced in HeLa cells. Bar, 10 μm.
Figure 6.
LacCer and CtxB internalization compared with cav-1 expression in multiple cell types. (A) Cells were pretreated with nystatin or CPZ and the internalization of BODIPY-LacCer was quantified after 5 min at 37°C (see MATERIALS AND METHODS). Values are expressed as percent inhibition relative to untreated control samples. In control experiments (unpublished data), the initial labeling of the PM before internalization was shown to be approximately the same for each cell type. (B) Western blot for Cav-1 in cell lysates prepared from various cell types. The amount of total cell protein was the same (10 μg) for each lane. Low but detectable levels of cav-1 were seen in Calu-6 and HeLa cell extracts using longer exposures. (C) Extent of LacCer internalization (5 min at 37°C) expressed as a percentage of initial PM labeling compared with cav-1 expression levels (relative to HSFs) determined by quantitation of Western blots as in B. (D) Cells were pretreated as in A and then incubated with 7.5 μg/ml AF594-CtxB for 45 min at 10°C, washed, and warmed to 37°C for 5 min. Samples were then washed and acid-stripped, and internalization was quantified as in A. Values in A and D are expressed as percentage of untreated control samples and are mean ± SD of at least 10 cells in each of three independent experiments.
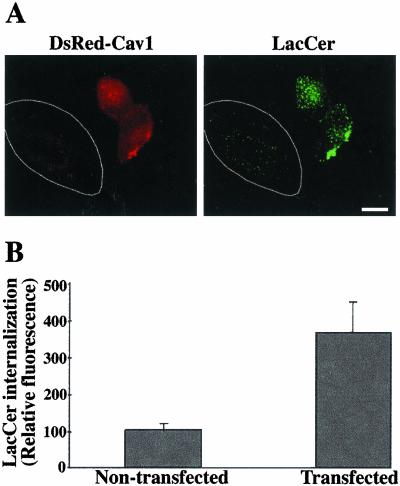
Previous studies have demonstrated that Calu-6 and HeLa cells have low levels of cav-1 mRNA or cav-1 expression relative to other cell types (Racine et al., 1999; Skretting et al., 1999). In the present study we examined cav-1 expression and found that of the seven cell types used, only Calu-6 and HeLa cells had low levels of cav-1, suggesting that the extent of LacCer internalization correlates with cav-1 expression (Figure 6, B and C). To extend this study, Calu-6 and HeLa cells were transfected for 48 h with a construct for DsRedcav-1. The effect on LacCer internalization was then evaluated. Cav-1–transfected cells exhibited dramatically enhanced uptake of LacCer (5 min internalization at 37°C) relative to adjacent nontransfected cells for both HeLa (Figure 7) and Calu-6 cells. In parallel experiments, electron microscopy of DsRed-cav-1–transfected HeLa cells showed numerous uncoated invaginations at the PM and uncoated vesicles in the cytoplasm, whereas almost no such structures were seen in nontransfected cells (unpublished data). These results demonstrate that cav-1 overexpression in HeLa cells stimulates LacCer internalization and promotes the formation of uncoated vesicles.
Figure 7.
Caveolin-1 overexpression stimulates LacCer internalization. HeLa cells were transfected with DsRed-cav-1 for 48 h. Cells were then pulse-labeled with BODIPY-LacCer as in Figure 4. (A) Fluorescence micrograph showing two transfected cells (identified by DsRed fluorescence) and a nontransfected cell in the same field. LacCer was observed at green wavelengths (see MATERIALS AND METHODS). Note the stimulation of LacCer internalization in the transfected cell, relative to the neighboring nontransfected cell outlined in white. Bar, 10 μm. (B) Quantitation of LacCer internalization in transfected vs. nontransfected cells by image analysis. Values are the mean ± SD of at least 10 cells in each of three independent experiments.
Mechanism of CtxB Internalization Varies with Cell Type
We used similar techniques to those described for LacCer to examine the internalization mechanism of CtxB in the cell types used above. (CHO cells were not used because they lack GM1, which is required for CtxB binding.) Cells were incubated with fluorescent CtxB at 4°C to bind endogenous GM1 at the PM, warmed for 5 min at 37°C, washed, acid stripped, and viewed under the fluorescence microscope. Internalization was quantified by image analysis. Nystatin and CPZ pretreatments were used to evaluate, respectively, the relative contributions of clathrin-independent and clathrin-dependent endocytosis to the uptake of CtxB. As shown in Figure 6D, inhibition of CtxB uptake by nystatin or CPZ was variable. Nystatin (but not CPZ) pretreatment significantly inhibited CtxB internalization in RFs, HSFs, MDCK, and Calu-1 cells, whereas CPZ had a significant inhibitory effect in HeLa and Calu-6 cells. This result for CtxB is in striking contrast to that obtained using LacCer whose internalization was inhibited by nystatin (but not CPZ), regardless of the cell type (Figure 6A). Thus, in cell types with low cav-1 expression, CtxB internalization occurred predominantly via clathrin-dependent endocytosis, whereas LacCer internalization remained clathrin independent.
CtxB Alters the Internalization Mechanism of BODIPY-GM1
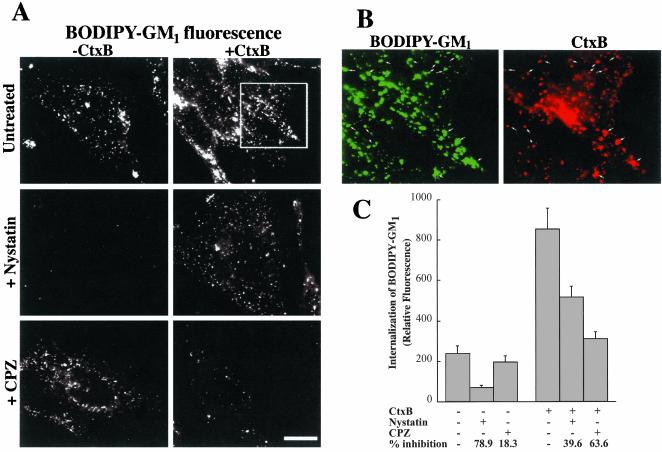
Finally, we tested the possibility that CtxB binding might alter the mechanism of internalization of exogenously added GM1. HeLa cells were incubated with BODIPY-GM1 at 10°C to label the PM, washed, and further incubated for 1 h at 10°C with or without AF594-CtxB. Samples were then warmed at 37°C for 5 min, and the amount of internalization was assessed after acid stripping and back exchange (see MATERIALS AND METHODS). In the absence of bound CtxB, internalization of BODIPY-GM1 was significantly inhibited by pretreatment with nystatin, whereas CPZ treatment had little effect (Figure 8A, top panels, and C). In the presence of bound CtxB, however, two effects were seen. First, the amount of BODIPY-GM1 internalized during 5 min at 37°C was significantly increased over that seen in the absence of CtxB (Figure 8, A and C). In this experiment, the presence of bound CtxB was confirmed by visualization of AF594 fluorescence which was seen to colocalize with the BODIPY-fluorescence (Figure 8B). Second, in the presence of bound CtxB, the relative amount of GM1 uptake which was inhibited by nystatin decreased from 79 to 40%, whereas the fraction which was inhibited by CPZ increased from 18 to 64% (Figure 8C). These results suggest that in HeLa cells, CtxB stimulated the internalization of BODIPY-GM1 mainly by increasing the extent of its uptake via clathrin-dependent endocytosis.
Figure 8.
Effect of CtxB on the internalization of fluorescent GM1 in HeLa cells. (A) Cells were untreated or pretreated with nystatin or CPZ, washed, and incubated with 1 μM BODIPY-GM1 at 10°C to label the PM. Samples were washed and further incubated in HMEM without (–CtxB) or with AF594-CtxB (+CtxB) for 1 h at 10°C. The intensity of BODIPY-fluorescence at the PM was the same (±10%) with or without bound CtxB (unpublished data). The samples were then washed, warmed to 37°C for 5 min, washed, acid-stripped, and back-exchanged to remove CtxB and fluorescent lipid from the PM, and observed under the fluorescence microscope (see MATERIALS AND METHODS). Note that the presence of bound toxin (+CtxB panels) dramatically altered the relative inhibition by nystatin vs. CPZ of BODIPY-GM1 internalization which was viewed at green wavelengths. (B) Colocalization of BODIPY-GM1 with AF594-CtxB after coincubation in the absence of inhibitors as in A. (C) Quantitation of experiment shown in A. Values are mean ± SD of at least 10 cells in each of the three independent experiments. Bars, 10 μm.
DISCUSSION
In the current study we examined the mechanism of internalization of a series of fluorescent GSL analogs and, using multiple criteria, showed that they were endocytosed almost exclusively through a caveolar-related, cav-1–dependent mechanism in RFs and other cell types. The internalization mechanism for these GSLs was unaffected by varying the carbohydrate headgroup or sphingosine backbone chain length, and additional evidence suggested that the ceramide core of the GSLs may be important for caveolar uptake. Although the uptake of the GSL analogs occurred by a nystatin-inhibitable process in cells with both high and low cav-1 expression, this was not the case for fluorescent CtxB used to monitor endogenous GM1 internalization. Rather, its internalization shifted from a clathrin-independent to a clathrin-dependent mechanism in cells with low levels of cav-1. These results suggest that GSLs are selectively internalized through caveolae in many cell types and demonstrate that the fluorescent GSL analogs can be used to reliably monitor caveolar endocytosis. These major points are discussed below.
BODIPY-LacCer Is Internalized by Caveolae
Several pieces of evidence indicate that the BODIPY-LacCer analog was endocytosed through caveolae in RFs. First, we demonstrated that LacCer internalization occurred by a clathrin-independent mechanism using multiple pathway-specific inhibitors as well as a DN construct of Eps15 that blocks clathrin-dependent endocytosis (Figure 2), consistent with our previous results in HSFs (Puri et al., 2001). Second, we showed that the fluorescent LacCer analog colocalized with fluorescently labeled albumin (Figure 3), which is reported to be internalized via caveolae in other cell types (Shubert et al., 2001). Furthermore, both the LacCer analog and fluorescent albumin extensively colocalized with DsRed-cav-1 in RFs at an early stage (2 min) of internalization (Figure 4). Third, the internalized LacCer did not colocalize with fluorescent dextran (Figure 3), a marker for cdc42-dependent pinocytosis. In addition, unlike LacCer uptake, dextran uptake was not inhibitable by nystatin. We also found that C. difficile toxin B, an inhibitor of rho-dependent endocytic mechanisms including the cdc42 pathway (Aktories et al., 2000; Sabharanjak et al., 2002), had no effect on LacCer internalization. In addition, recent studies by Mayor and colleagues have shown that dextran uptake occurs by a dynamin-independent mechanism (Sabharanjak et al., 2002), in contrast to LacCer internalization which was dynamin dependent (Figure 2A). Taken together, our results provide strong evidence that internalization of GSL analogs in RFs occurs via caveolar endocytosis rather than another clathrin-independent endocytic mechanism.
In addition to our studies with RFs, we examined LacCer internalization in HSFs, HeLa, CHO, MDCK, Calu-1, and Calu-6 cells. In each case, LacCer internalization appeared to occur by a similar mechanism to that found for RFs when we examined the effects of biochemical inhibitors (i.e., nystatin vs. CPZ; Figure 6A) or colocalization with labeled albumin. Thus, it seems reasonable to suggest that the mechanism of LacCer internalization does not vary among these cell types and was predominantly via caveolae in each case. However, the amount of LacCer internalization was dramatically reduced in cell types with low levels of cav-1 (HeLa and Calu-6) and could be stimulated in those cell types by overexpression of this protein. These results strongly suggest that the extent of LacCer internalization is closely related to the expression of cav-1 in multiple cell types. Our results are in apparent contrast to those of Nabi and coworkers who found that the overexpression of cav-1 negatively regulates the caveolar-mediated endocytosis of the autocrine motility factor receptor in NIH-3T3 cells (Le et al., 2002). One possible explanation is that there are distinct caveolar-mediated endocytic pathways with different characteristics (Le and Nabi, 2003). In this context, we recently found that BODIPY-LacCer internalized via caveolar-related endocytosis in HSFs is rapidly delivered to early endosomes where it merges with Tfn, a marker for the clathrin pathway (Sharma et al., 2003). In contrast, SV40 virus internalized via caveolae is reported to be transported intracellularly by a slow process that does not include passage through early endosomes (Pelkmans et al., 2001).
Structural Requirements for GSL Internalization
We also attempted to define the molecular features of GSL analogs that result in their selective internalization by caveolae in RFs. To do so, we systematically varied the structure of the fluorescent lipid by modifying its carbohydrate headgroup, chain length of the sphingosine base, or chain length of the fluorescent fatty acid and examined their initial internalization and sensitivity to inhibitor treatments. Fluorescent GalCer, LacCer, MalCer, globoside, GM1, and sulfatide were internalized identically to BODIPY-LacCer (Table 1). Each of these molecules had an identical BODIPY-ceramide backbone and differed only in the saccharide moieties present in the polar headgroup. Of particular interest is the finding that BODIPY-MalCer, whose headgroup is Glc(α1→4)Glc, and LacCer, whose headgroup is Gal(β1→4)Glc, behaved identically despite their different conformations in the vicinity of the carbohydrate/sphingolipid interface, suggesting that the presence of galactose is not required for caveolar endocytosis of the various GSL analogs. To evaluate the possibility that the hydrophobicity of the analog might influence its internalization, we used a series of BODIPY-LacCer analogs in which the chain length of the sphingosine base or fluorescent fatty acid was varied (refer to Figure 1). Alterations in lipid chain length are presumed to affect the hydrophobicity of the analogs (e.g., C12 vs. C20 sphingosine); however, there was no effect on the LacCer internalization mechanism (Table 2). These results suggest that the hydrophobicity of the GSL analogs is not a major determinant for their selective internalization via caveolae.
We also compared the internalization of NBD- vs. BODIPY-labeled LacCer and found that replacing the BODIPY-fluorophore with NBD did not influence the caveolar internalization of the LacCer analogs (Table 1). Finally, we compared the internalization of NBD-LacCer with NBD-d-PC. Unlike the GSL analogs, NBD-d-PC was internalized primarily via clathrin-dependent endocytosis (Table 1). Taken together, these studies suggest that GSL analogs are internalized mainly via caveolae with no specificity toward particular carbohydrate headgroups. However, the sphingoid backbone of the GSLs appeared to be important for selective caveolar endocytosis because the glycerophospholipid, NBD-d-PC, was not internalized by this mechanism. Interestingly, BODIPY-sphingomyelin, which has both a ceramide core (as in GSLs) and a phosphocholine headgroup (as in PC), is internalized approximately equally by both caveolar- and clathrin-mediated endocytosis (Puri et al., 2001).
CtxB (but not LacCer) Internalization Mechanism Varies with Cell Type
We compared the mechanism of internalization of CtxB and LacCer in six different cell types (Figure 6) and found that although LacCer was internalized by a clathrin-independent mechanism in each case, both clathrin-independent (nystatin-sensitive) and clathrin-dependent (CPZ-sensitive) mechanisms could contribute to CtxB uptake to varying extents depending on the cell type. One possible explanation for this is that the internalization mechanism of fluorescent LacCer mimics that of endogenous PM GM1 and that binding of CtxB to GM1 alters its internalization. Alternatively, the presence of the BODIPY- (or NBD-) fluorophore on LacCer could bias its mechanism of internalization as cell type is varied. To test these possibilities, we compared the internalization of BODIPY-GM1 in HeLa cells, with and without bound CtxB (Figure 8). In the absence of bound CtxB, internalization of BODIPY-GM1 was largely nystatin-sensitive (i.e., clathrin-independent and caveolar-related), whereas CPZ (which inhibits clathrin-dependent internalization) had little effect. In contrast, when the same experiment was performed after binding CtxB to the PM, the fraction of GM1 uptake that was inhibited by nystatin decreased, whereas the fraction that was inhibited by CPZ increased (Figure 8). Furthermore, CtxB treatment also increased the total amount of BODIPY-GM1 internalization about fourfold. These results demonstrate that the internalization of the fluorescent GM1 analog was perturbed by CtxB binding, and raise the possibility that internalization of endogenous GM1 might also be affected by the bound toxin in some cell types. We speculate that this perturbation occurs because CtxB binding (five molecules of GM1 bound per molecule of CtxB; Lencer et al., 1999) disrupts the normal interactions of GM1 with neighboring lipids, proteins, and cholesterol that are required for clathrin-independent internalization.
CONCLUSIONS
In summary, the current study demonstrates that fluorescent GSL analogs were internalized via caveolae in multiple cell types and this selective internalization was not affected by simple modifications of the saccharide units making up the GSL headgroup, or by modification of the lipid hydrophobicity. However, evidence was presented that the ceramide core of the GSLs may be an important determinant for their selective caveolar endocytosis. In addition, we show that unlike CtxB, the GSL analogs were internalized via caveolar endocytosis even in cells with low levels of cav-1 expression. These studies suggest that caveolar endocytosis is the major mechanism for the endocytosis of PM GSLs, leading us to speculate that this process may play an important role in the maintenance of PM lipid composition and microdomain structure.
Acknowledgments
This work was supported by National Institutes of Health grant GM22942 to R.E.P. and grant HL16660 to R.B. V.P. was supported by a fellowship from the American Heart Association.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-12-0809. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0809.
Abbreviations used: AF, AlexaFluor; BODIPY, boron dipyrromethenedifluoride; Cav-1, caveolin-1; CPZ, chlorpromazine; CtxB, cholera toxin, B-subunit; DF-BSA, defatted BSA; Eps15, EGFR pathway substrate clone 15; GalCer, galactosylceramide; GM1, ganglioside GM1; GSL, glycosphingolipid; HMEM, 10 mM HEPES-buffered minimal essential medium (pH 7.4); HMEMG+I, HMEM without glucose containing 5 mM NaN3 and 50 mM 2-deoxyglucose; HSFs, human skin fibroblasts; LacCer, lactosylceramide; MalCer, maltosylceramide; NBD, 7-nitrobenz-2-oxa-1,3-diazol-4-yl; PC, phosphatidylcholine; PM, plasma membrane; RFs, rat fibroblasts; SL, sphingolipid; SM, sphingomyelin; So, sphingosine; Tfn, transferrin.
References
- Aktories, K., Schmidt, G., and Just, I. (2000). Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 381, 421–426. [DOI] [PubMed] [Google Scholar]
- Anderson, R.G. (1998). The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225. [DOI] [PubMed] [Google Scholar]
- Anderson, R.G., and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825. [DOI] [PubMed] [Google Scholar]
- Aoki, T., Nomura, R., and Fujimoto, T. (1999). Tyrosine phosphorylation of Caveolin-1 in the endothelium. Exp. Cell Res. 253, 629–636. [DOI] [PubMed] [Google Scholar]
- Brown, D.A., and London, E. (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., Martin, O.C., and Pagano, R.E. (1997). Changes in the spectral properties of a plasma membrane lipid analog during the first seconds of endocytosis in living cells. Biophys. J. 72, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., and Norkin, L.C. (1999). Extracellular Simian Virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246, 83–90. [DOI] [PubMed] [Google Scholar]
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.W., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin, M. (2003). The state of lipids rafts: from model membranes to cells. Annu. Rev. Biophys. Biomolec. Struct. 257–283. [DOI] [PubMed]
- Gleizes, P.E., Noaillac-Depeyre, J., Dupont, M.A., and Gas, N. (1996). Basic fibroblast growth factor (FGF-2) is addressed to caveolae after binding to the plasma membrane of BHK cells. Eur. J. Cell Biol. 71, 144–153. [PubMed] [Google Scholar]
- Gustavsson, J. et al. (1999). Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13, 1961–1971. [PubMed] [Google Scholar]
- Hansen, S.H., Sandvig, K., and van Deurs, B. (1993). Clathrin and HA2 adapters: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley, J.R., Krueger, E.W., Oswald, B.J., and McNiven, M.A. (1998). Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J.M., Brown, M.S., Goldstein, J.L., and Anderson, R.G.W. (1983). Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33, 273–285. [DOI] [PubMed] [Google Scholar]
- Le, P.U., Guay, G., Altschuler, Y., and Nabi, I.R. (2002). Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem. 277, 3371–3379. [DOI] [PubMed] [Google Scholar]
- Le, P.U., and Nabi, I.R. (2003). Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 116, 1059–1071. [DOI] [PubMed] [Google Scholar]
- Lencer, W.I., Hirst, T.R., and Holmes, R.K. (1999). Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450, 177–190. [DOI] [PubMed] [Google Scholar]
- Liu, P., and Anderson, R.G. (1999). Spatial organization of EGF receptor transmodulation by PDGF. Biochem. Biophys. Res. Commun. 261, 695–700. [DOI] [PubMed] [Google Scholar]
- Lobie, P.E., Sadir, R., Graichen, R., Mertani, H.C., and Morel, G. (1999). Caveolar internalization of growth hormone. Exp. Cell Res. 246, 47–55. [DOI] [PubMed] [Google Scholar]
- Marjomaki, V. et al. (2002). Internalization of echovirus 1 in caveolae. J. Virol. 76, 1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, O.C., and Pagano, R.E. (1987). Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells: evidence for a protein-mediated and ATP-dependent process(es). J. Biol. Chem. 262, 5890–5898. [PubMed] [Google Scholar]
- Martin, O.C., and Pagano, R.E. (1994). Internalization and sorting of a fluorescent analog of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J. Cell Biol. 125, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven, M.A., Cao, H., Pitts, K.R., and Yoon, Y. (2000). The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 25, 115–120. [DOI] [PubMed] [Google Scholar]
- Mineo, C., and Anderson, G.W. (2001). Potocytosis. Histochem. Cell Biol. 116, 109–118. [DOI] [PubMed] [Google Scholar]
- Oh, P., McIntosh, D.P., and Schnitzer, J.E. (1998). Dynamin at the neck of cavolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141, 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, Y., Ninomiya, H., Miwa, S., and Masaki, T. (2000). Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J. Biol. Chem. 275, 6439–6446. [DOI] [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001). Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473–483. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Singh, R.D., Dominguez, M., Brown, J.C., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine, C., Belanger, M., Hirabayashi, H., Boucher, M., Chakir, J., and Couet, J. (1999). Reduction of caveolin 1 gene expression in lung carcinoma cell lines. Biochem. Biophys. Res. Commun. 255, 580–586. [DOI] [PubMed] [Google Scholar]
- Rothberg, K.G., Heuser, J.E., Donzell, W.C., Ying, Y.-S., Glenney, J.R., and Anderson, R.G.W. (1992). Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682. [DOI] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Develop. Cell 2, 411–423. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002). Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18, 1–24. [DOI] [PubMed] [Google Scholar]
- Schnitzer, J.E., Oh, P., Pinney, E., and Allard, J. (1994). Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, D.K., Choudhury, A., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564–7572. [DOI] [PubMed] [Google Scholar]
- Shin, J.-S., Gao, Z., and Abraham, N. (2000). Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785–788. [DOI] [PubMed] [Google Scholar]
- Shogomori, H., and Futerman, A.H. (2001). Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J. Biol. Chem. 276, 9182–9188. [DOI] [PubMed] [Google Scholar]
- Shubert, W., Frank, P.G., Razani, B., Park, D.S., Chow, C.-W., and Lisanti, M.P. (2001). Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619–48622. [DOI] [PubMed] [Google Scholar]
- Skretting, G., Torgersen, M.L., van Deurs, B., and Sandvig, K. (1999). Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J. Cell Sci. 112, 3899–3909. [DOI] [PubMed] [Google Scholar]
- Sleight, R.G., and Pagano, R.E. (1984). Transport of a fluorescent phosphatidylcholine analog from the plasma membrane to the Golgi apparatus. J. Cell Biol. 99, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen, M.L., Skretting, G., van Deurs, B., and Sandvig, K. (2001). Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114, 3737–3742. [DOI] [PubMed] [Google Scholar]
- Watanabe, R., Asakura, K., Rodriguez, M., and Pagano, R.E. (1999). Internalization and sorting of plasma membrane sphingolipid analogues in differentiating oligodendrocytes. J. Neurochem. 73, 1375–1383. [DOI] [PubMed] [Google Scholar]