Abstract
Many mycobacteria are intramacrophage pathogens that reside within nonacidified phagosomes that fuse with early endosomes but do not mature to phagolysosomes. The mechanism by which mycobacteria block this maturation process remains elusive. To gain insight into whether fusion with early endosomes is required for mycobacteria-mediated inhibition of phagosome maturation, we investigated how perturbing the GTPase cycles of Rab5 and Rab7, GTPases that regulate early and late endosome fusion, respectively, would affect phagosome maturation. Retroviral transduction of the constitutively activated forms of both GTPases into primary murine macrophages had no effect on Mycobacterium avium retention in an early endosomal compartment. Interestingly, expression of dominant negative Rab5, Rab5(S34N), but not dominant negative Rab7, resulted in a significant increase in colocalization of M. avium with markers of late endosomes/lysosomes and increased mycobacterial killing. This colocalization was specific to mycobacteria since Rab5(S34N) expressing cells showed diminished trafficking of endocytic tracers to lysosomes. We further demonstrated that maturation of M. avium phagosomes was halted in Rab5(S34N) expressing macrophages supplemented with exogenous iron. These findings suggest that fusion with early endosomes is required for mycobacterial retention in early phagosomal compartments and that an inadequate supply of iron is one factor in mycobacteria's inability to prevent the normal maturation process in Rab5(S34N)-expressing macrophages.
INTRODUCTION
The mechanism by which mycobacteria inhibit this phagosome maturation process remains poorly defined. Progress along these lines has been hindered primarily by the lack of efficient transfection protocols that allow for exogenous protein expression in macrophages. Nevertheless, an attractive hypothesis to explain the block in mycobacteria-phagosome maturation entails mycobacterial manipulation of the host cell machinery so as to limit fusion between early and late endosomes with phagosomes and thereby prevent phagosome maturation.
The Rab proteins are members of the Ras-superfamily of low-molecular-weight GTPases that cycle between their active GTP and inactive GDP bound forms and function as molecular switches to promote vesicle fusion (Martinez and Goud, 1998). Rab5 and Rab7 are two members of the Rab family that facilitate early and late endosome fusion, respectively (Feng et al., 1995; Li, 1996; Vitelli et al., 1997). The GTPase-defective mutants of these Rab proteins are constitutively active and accelerate vesicle fusion, whereas the GDP-bound Rab mutants, which are incapable of nucleotide exchange, limit vesicle fusion and function as dominant-negatives mutants. More recently, it has been reported that Rab5 also regulates fusion of endosomes with phagosomes in an analogous manner to endosome-endosome fusion and thus may play an important role in the phagosome maturation process (Duclos et al., 2000).
We have examined the effect of perturbing the GTPase cycles of Rab5 and Rab7 to define the importance of early and late endosome fusion events during phagosome maturation in primary macrophages infected with M. avium 101. This pathogenic strain of M. avium, originally isolated from an AIDS patient, is retained within an early phagosome compartment in infected murine macrophages (Xu et al., 1994). Using a retroviral transduction system, we were able to successfully express wild-type, constitutively active, or dominant negative forms of Rab5 and Rab7 in primary murine bone marrow macrophages. Our studies described here address whether constitutively active Rab5 or Rab7 would facilitate the transport of the mycobacteria to a late endosome/lysosome compartment and whether limiting endosome fusion with the mycobacteria phagosome, through the expression of dominant negative Rab mutants, results in altered trafficking of the mycobacteria. We show that fusion of phagosomes with early endosomes and an adequate iron supply are important for mycobacteria to halt the phagosome maturation process.
MATERIALS AND METHODS
Bone Marrow Macrophage Isolation and Culturing
Bone marrow macrophages (BMMs), used in all experiments were isolated from 6–10-week-old BALB/c mice as previously described (Bohlson et al., 2001). The isolated macrophages were cultured on 100-mm nontissue culture plates in DMEM (GIBCO BRL, Grand Island, NY) supplemented with 20 mM HEPES (Fisher Scientific, Fair Lawn, NJ), 10% fetal bovine serum (FBS; GIBCO BRL), 100 U/ml penicillin and 100 μg/ml streptomycin (BioWhittaker, Walkersville, MD), 2 mM l-glutamine, and 20% L-cell supernatant as a source of macrophage colony-stimulating factor (BMM medium). The macrophages were used 7–10 d after isolation or frozen after 7 d of culture in freezing media (50% DMEM, 40% FBS, and 10% endotoxin-tested dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). Thawed or fresh macrophages were cultured on nontissue culture plates for 3–7 d and then replated at ∼2 × 105 cells per glass coverslip (Fisher Scientific, Pittsburg, PA) in 6-well tissue culture plates (Corning Incorporated, Corning, NY). Cells were allowed to adhere for 18–24 h before treatments with bacteria and virus. All tissue culture reagents were found to be negative for endotoxin contamination by the E-Toxate assay (Sigma).
M. avium Culturing and FITC Labeling
To generate fluorescein isothiocyanate (FITC)-labeled M. avium 101 stocks, the bacteria were passaged through a mouse to ensure virulence. A single colony was used to produce frozen stocks. Before freezing, cultures were pelleted and washed with Hanks' buffered saline solution (HBSS; GIBCO) supplemented with 1% bovine serum albumin (BSA; ICN Biochemicals Inc., Aurora, OH). For some experiments, M. avium was thawed and immediately heat-killed by incubating the mycobacteria at 85°C for 30 min. Plating the treated M. avium confirmed that >99% of the mycobacteria was incapable of growth. Cultures were resuspended in boric acid buffer, pH 9.2, containing 1.5 mg/ml FITC powder (Sigma) dissolved in DMSO (Sigma) and incubated at 37°C for 2 h. Cultures were washed with HBSS supplemented with 1% BSA to remove any residual buffer and pelleted. FITC-labeled cultures were resuspended and frozen as described (Bohlson et al., 2001). Frozen stocks were quantified by serial dilution.
Complement Opsonization
Appropriate concentrations of mycobacteria were suspended in macrophage culture media containing 10% horse serum (GIBCO) as a source of complement components (Bohlson et al., 2001) and incubated for 2 h at 37°C. The complement opsonized M. avium was directly added to BMMs at a 10:1 bacilli-to-macrophage ratio for all infection experiments, and the infections were performed as described (Roach and Schorey, 2002).
Generation of Retroviral Expression Plasmids and Ecotrophic Retrovirus
The retroviral expression plasmids were constructed by subcloning cDNAs encoding human wild-type Rab5, Rab5(Q79L), Rab5(S34N), and canine wild-type Rab7, Rab7(Q67L), and Rab7(S22N) (generously provided by Dr. C. D'Souza-Schorey, University of Notre Dame, Notre Dame, IN) into the retroviral plasmid pLZRS-IRES-NEO (generously provided by Dr. J. Collard, NKI, Amsterdam, Netherlands). The vector pLZRS-IRES-NEO encodes a multicloning site, followed by an IRES (internal ribosome entry site) sequence, the neomycin resistance gene (NEO), and oriP (origin of replication of the Epstein-Barr virus; Michiels et al., 2000). Expression plasmids containing the cDNA of the various Rabs were tagged at the 5′ end with a sequence encoding a 10-amino acid peptide from hemagglutinin (HA; Palacios et al., 2001). The Rab5s, originally in pCDNA 3.1, were removed by XbaI digestion and subcloned into a pCDNA3.1(–) containing the HA tag coding sequence to create an inframe fusion between the HA tag and the Rab5. These HA-Rab5 constructs were sequenced to confirm the correct reading frame and point mutations.
The pCDNA-HA-Rab5s were digested with ClaI and NotI and the HA-Rab5 coding sequences were cloned into the BstBI and NotI sites of pLZRS-IRES-NEO. The PCR-based amplification of the Rab7s used a 5′ primer (ATCGATATGTACCCATATGACGTTCCAGACTACGCGATGACCTCTAGGAAGAAAGTG), which contained a ClaI restriction site, the HA epitope sequence, and 21 base pairs of the Rab7 N-terminal sequence and a 3′ primer (GCGGCCGCACTCTGTGCTCTGCTCTCAC),which contained a NotI restriction site, followed by the complement of the Rab7 C-terminal sequence. The PCR product was subcloned into the pGEM expression vector (Promega, Madison, WI) using the PCR-generated adenosine overhangs. The HA-Rab7 constructs were sequenced to confirm the proper reading frame and DNA sequence. ClaI- and NotI-digested HA-Rab7 coding sequence was cloned into the ClaI and BstBI sites of pLZRS-IRES-NEO. These pLZRS Rab clones were transfected into the Phoenix (φNX) cell line and used for generating the ecotrophic retrovirus as described elsewhere (Michiels et al., 2000). In brief, φNX cells were seeded onto 6-well tissue culture dishes (Corning Inc.) and transfected using standard calcium phosphate procedure. Cells were incubated in culture media for 24 h, the medium was replaced, and virus was collected after 48 h. Virus-containing supernatants were stored at –80°C for up to 6 months and were subjected to no more than one freeze-thaw cycle.
Transduction of BMMs
BMMs on glass coverslips were infected with complement opsonized M. avium 101 as indicated above before transduction with the Rab-containing retrovirus. One milliliter of freshly thawed retrovirus-containing supernatant mixed with 1% DOTAP liposomal transfection reagent (Boehringer Mannheim, Indianapolis, IN) was added to the BMMs. Retroviral infection was allowed to proceed for 4 h at 37°C. Cells were gently washed in phosphate-buffered saline (PBS) and then incubated in normal growth media. Forty-eight hours after incubation, the cells were fixed and processed for antibody staining.
Antibody Staining and Immunofluorescence Labeling
The immunofluorescence staining and confocal microscopy were conducted as previously described (Boshans et al., 2000). Briefly, infected cells were fixed in 2% paraformaldehyde (Sigma) in PBS. Fixed cells were permeabilized with 0.02% Triton X-100 (Sigma), blocked with 0.2% BSA and 0.02% gelatin (Sigma), and washed with PBS, 1% BSA. The anti-mouse mAb against HA (Covance, Berkeley, CA), the anti-rat mAb against Lamp1 (1D4B; The Developmental Hybridoma Bank, University of Iowa) and the anti-rabbit polyclonal antibody against transferrin receptor (Santa Cruz Biotechnology, Santa Cruz, CA) were used at a 1:100 dilution in PBS, 1% gelatin. All secondary antibodies were obtained from Chemicon International (Temecula, CA) and used at a dilution of 1:600 in PBS, 1% gelatin.
Fluorescent Dextran Uptake by Macrophages
Fluid phase ingestion of Dextran by macrophages was conducted as previously described (Racoosin and Swanson, 1993; Thilo et al., 1995). Briefly, 10,000 molecular weight, lysine fixable Texas-red Dextran (Molecular Probes, Eugene, OR) was added to cells at a concentration of 1.5 mg/ml. Cells were incubated at 25°C for 5 min, washed extensively with PBS, and incubated at 37°C, 5% CO2 for 30 min to chase the dextran to the lysosome. Cells were fixed and processed for immunofluorescence as described above.
Iron Loading Assays
For the 72-h time point, 100 μM ferric ammonium citrate (FAC; Sigma) was added to the culture media of M. avium–infected BMMs. These infected BMMs were incubated in the presence of FAC for 24 h before viral transduction. The FAC was maintained in the culture media throughout the remaining 48-h incubation. For the 48-h time point, 100 μM FAC was added to the opsonization media of the M. avium in an attempt to preload the mycobacteria with iron before macrophage infection. BMMs were infected with the iron-loaded bacteria followed immediately by the viral transduction. FAC was again maintained in the culture media throughout the remaining 48-h incubation. For experiments using transferrin-linked FAC, apotransferrin (Sigma) was incubated with FAC at a 2:1 ratio overnight at 4°C and concentrated in an Amicon concentrator (Amicon, Beverly, MA) followed by two washes of the retentate as described (Olakanmi et al., 2000).
Gallium Treatment
Mycobacteria and macrophages were treated with Gallium as previously described (Olakanmi et al., 2000). Briefly, glass coverslips were added to each well of a 6-well tissue culture plate (Corning Inc.), and BMMs were plated at 2 × 105 cells per well. Cells were allowed to adhere for 18–24 h. To limit the amount of available iron to the BBMs, cells were cultured in media containing DMEM and supplemented with 1% FBS for 24 h before treatment with bacteria and gallium. M. avium was opsonized in the presence of 500 μM gallium. BMMs in DMEM, 1% FCS were infected with the gallium-loaded bacteria. The infections were performed as described above. Gallium at 500 μM was added to the BMM growth media during the infection. Cells were then fixed and stained as described above.
Mycobacteria Killing Assay
BMMs, 1 × 106, on glass coverslips were infected with complement opsonized M. avium 101 at a 30:1 bacilli-to-macrophage ratio and were left untreated or transduced with Rab5 WT, Rab5(S34N) or Ev-Neo–containing retrovirus as described above. Immediately postviral transduction, coverslips containing infected, transduced, or nontransduced cells were either removed from the well and lysed for 2 min with 200 μl of 1% IGEPAL (Sigma) in PBS (initial infection load) or incubated for 2, 5, 8, or 12 d in fresh BMM media before lysis. Supernatants were collected at each time point and spun at 14,000 × g for 10 min to pellet any released bacilli, and the pellet was pooled with the cell lysis. The M. avium was quantitated by serial dilution on Middlebrook 7H10/OADC (Bector Dickinson, Sparks, MD) agar plates.
Statistical Analysis
Data were analyzed by a one-tailed Student's t test. Statistical significance was assumed at p < 0.05. For all immunofluorescence n = 3 or greater and error bars represent SD.
RESULTS
Expression of Rab5 and Rab7 in Primary Murine Bone Marrow–derived Macrophages
A disruption of Rab5 or Rab7 function presents an attractive hypothesis to explain the halt in phagosome maturation in cells infected with mycobacteria. The difficulty with this type of analysis has been the expression of Rab (or other) proteins in macrophage cell lines and, even more problematic, in primary macrophages. Here we have used a “second generation” retroviral expression system (Michiels et al., 2000) to successfully transduce Rab5, Rab7, and mutants thereof, into primary murine BMMs.
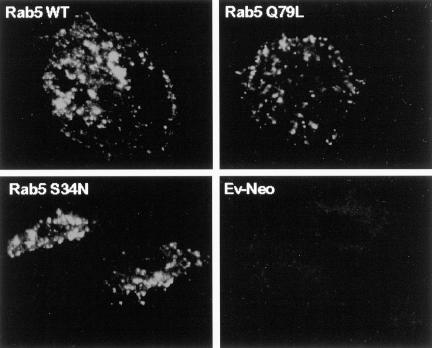
As described in MATERIALS AND METHODS, HA-tagged wild-type and mutant Rab5 and Rab7 genes were subcloned into the retroviral expression vector pLZRS-IRES-NEO. Plasmids were transfected into the ecotrophic packaging cell line, Phoenix (φNX), for generation of recombinant ecotropic virus capable of infecting rodent cells, as previously described (Michiels et al., 2000). Mouse BMMs were infected with recombinant virus and 48 h postinfection, expression of Rab proteins was assessed by immunofluorescence microscopy. We first examined the distribution of wild-type Rab5. As shown in Figure 1, confocal microscopy of the transduced BMMs showed a punctate staining pattern characteristic of endosome distribution (Zerial, 1993; Woodman, 2000b). Only a low background staining pattern was detected under identical conditions when BMMs were infected with “empty” retrovirus containing the pLZRS vector alone. Using an approach similar to that described above, we examined the distribution of the activated and dominant negative Rab5 mutants, Rab5(Q79L) and Rab5(S34N), respectively. Both Rab5 mutants exhibited a punctate “endosome-like” staining pattern (Figure 1). Furthermore, in all cases, BMMs expressing the recombinant Rab5s ranged from 85 to 100%.
Figure 1.
HA-Rab5 expression in transduced murine macrophages. BMMs were transduced with HA-tagged Rab5 WT (A), Rab5(Q79L) (B), or Rab5(S34N) (C). The expression and distribution of Rab5 in transduced BMMs was determined by confocal immunofluorescence microscopy using a mAb specific to the HA tag. Rab5-transduced BMMs show a characteristic punctate pattern. Background levels of HA staining were determined by using the anti-HA antibody on BMMs transduced with untagged Ev-Neo (D) and visualizing the macrophages under conditions identical to those used with the HA-Rab–transduced BMMs. Image bar, 16 μm. Confocal sections, 0.5 μm.
Next, we generated recombinant viruses expressing HA-tagged wild-type Rab7 and its activated and dominant negative mutants, Rab7(Q67L) and Rab7(S22N), respectively. Rab7 and its mutants also exhibited a punctate staining pattern characteristic of late endosomes and showed expression efficiency similar to that with Rab5 (our unpublished results).
Colocalization of M. avium with Rab5 and Rab 7 in Transduced Macrophages
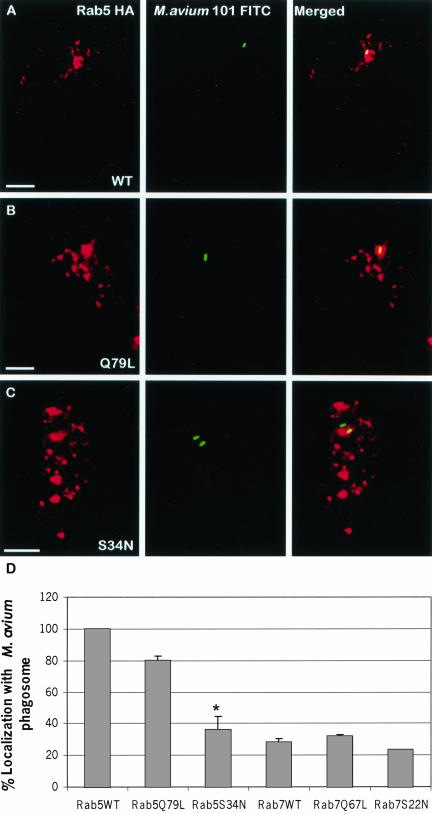
To determine if phagosomes containing mycobacteria acquired Rab5 and Rab7 proteins, we first infected BMMs with FITC-labeled M. avium 101 followed by infection with retrovirus encoding HA-tagged wild-type Rab5 and Rab7. Fortyeight hours postinfection, the distribution of mycobacteria and Rab proteins were visualized by confocal immunofluorescence microscopy. As shown in Figure 2, almost all of the mycobacteria-containing phagosomes were positive for Rab5 (Figure 2, A and D), whereas significantly fewer were positive for Rab7 (our unpublished results and Figure 2E). This is in agreement with previous reports indicating that live pathogenic mycobacteria are retained within an early phagosome compartment which contain Rab5 but not Rab7 (Deretic et al., 1997).
Figure 2.
Colocalization of M. avium 101 with Rab5 but not Rab7 in transduced BMMs. BMMs were infected with M. avium 101 and with retrovirus encoding for HA-tagged Rab5 WT (A), Rab5(Q79L) (B), and Rab5(S34N) (C). Transduced macrophages were labeled for HA-tagged Rab proteins (red), and colocalization with the FITC-labeled bacilli (green) was determined by confocal immunofluorescence microscopy. Coincident staining appears yellow in the merged images. The number of mycobacterial phagosomes (n = 50 per experiment) that colocalize with the HA-Rab proteins were determined and expressed as the mean ± SD for three separate experiments (D). Significant differences were observed between Rab5(S34N) and Rab5 WT in their colocalization with the M. avium phagosome. *p < 0.0001. Image bar, 16 μm. Confocal sections, 0.5 μm.
Next we examined the distribution of mycobacteria relative to constitutively active or dominant negative forms of Rab5 in transduced BMMs. As indicated in Figure 2 there was a significant difference between the number of mycobacteria phagosomes that were positive for Rab5(Q79L), the GTP-bound, compared with Rab5(S34N), the GDP-bound, mutant (Figure 2, B, C, and D). As observed for wild-type Rab5, Rab5(Q79L) was present on the mycobacterial phagosome. In marked contrast, only 38% of the mycobacteria stained positive for Rab5(S34N). The decreased overlap of dominant negative Rab5 with the mycobacteria phagosome may result from decreased fusion of endosomes with phagosomes, as has been previously described in Rab5(S34N)-expressing cells (Woodman, 2000a). We also examined infected BMMs for the distribution of FITC-labeled M. avium with constitutively active (Q67L) and dominant-negative (S22N) Rab7. As shown in Figure 2D only a limited number of mycobacterial phagosomes stained positive for the Rab 7 mutant proteins.
Mycobacterial Trafficking in Macrophages Expressing Wild-type or Mutant Rab5 or Rab7
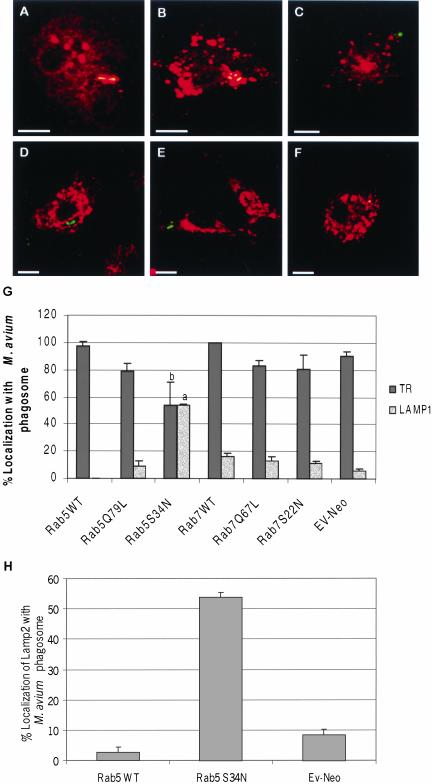
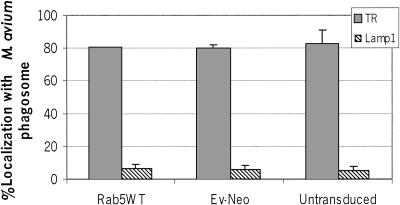
The previous experiments suggest that the mycobacteria were retained within an early phagosomal compartment in BMMs expressing WT or Rab5(Q79L). To further define the mycobacterial cellular localization, we used confocal immunofluorescence microscopy to determine if the transferrin receptor (TR) or the lysosome-associated membrane protein 1 (LAMP1) localized to the mycobacterial phagosome in Rab5 transduced BMMs. As predicted, in wild-type and Rab5(Q79L)-expressing cells we observed ∼80–95% of the mycobacterial phagosomes to stain positive for the transferrin receptor, an early/recycling endosome marker, whereas only ∼10–15% contained detectable LAMP1, a late endosome/lysosome marker (Figure 3A-B, D-E, and G). This suggests that an increased level of active Rab5 in BMMs is not sufficient to force the mycobacterial phagosome through the maturation process. In contrast, the mycobacterial phagosomes in BMMs expressing the dominant negative Rab5 showed limited TR staining and increased LAMP1 staining compared with cells expressing Rab5 WT (Figure 3, C, F, and G). LAMP2, another late endosome/lysosome marker, also colocalized with M. avium 101 in BMMs expressing Rab5(S34N; Figure 3H). These studies suggest that expression of Rab5(S34N) results in increased transport of M. avium to a late endosome or lysosome compartment. This finding was unexpected because Rab5(S34N) has previously been shown to reduce the rate of ligand trafficking (McCaffrey et al., 2001). However, we did observe other features characteristic of the Rab5 mutants, including enlarged and fragmented TR-positive endosomes in Rab5(Q79L) and Rab5(S34N) expressing BMMs (Figure 3, B and C, respectively).
Figure 3.
Trafficking of M. avium 101 to a LAMP1-positive, TR-negative compartment in macrophages expressing Rab5(S34N). BMMs expressing HA-tagged Rab5 WT (A and D), Rab5(Q79L) (B and E), or Rab5(S34N) (C and F) were stained for TR (red, A–C) or LAMP1 (red, d–F), and the amount of colocalization with M. avium 101 (green) was determined by confocal immunofluorescence microscopy. Coincident staining appears yellow in the merged images. The number of mycobacterial phagosomes (n = 50 per experiment) that stained positive for TR or LAMP1 (G) or LAMP2 (H) was quantified. Similar TR and LAMP1 staining experiments were completed using Rab7-transduced BMMs, and the results were quantified (G). The data are presented as the mean ± SD from three separate experiments. Comparison between Rab5(S34N)- and Rab5 WT–expressing macrophages indicate significant differences in the colocalization between the mycobacterial phagosome and endosome/lysosome markers. (a) p = 0.0348, (b) p < 0.0001. Image bar, 16 μm. Confocal sections, 0.5 μm.
Similar staining studies were done with wild-type and mutant Rab7s. Unlike the Rab5 results, we observed no significant differences in transduced BMMs between vector alone, wild-type, Q67L and S22N Rab7 in the TR and LAMP1 staining of the mycobacteria phagosome (Figure 3G).
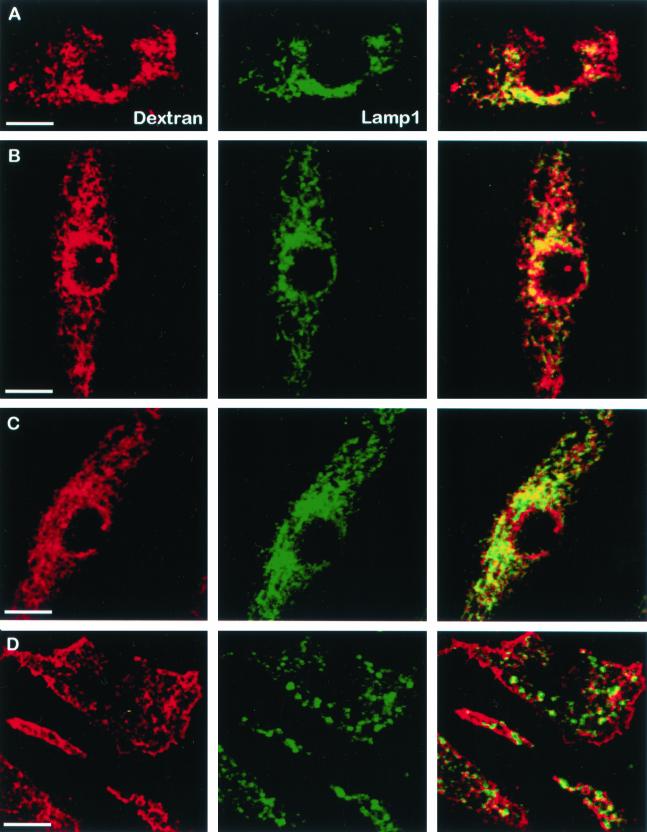
To further compare our system with published data, we evaluated the transport of the fluid phase marker dextran through the endocytic pathway in Rab5-transduced BMMs. The murine BMMs were retrovirally infected with Rab5 WT, Q79L and S34N expression constructs and then treated with Texas Red–labeled dextran as described in the MATERIALS AND METHODS. As predicted, dextran was rapidly transported to a LAMP1-positive compartment in BMMs expressing WT or Rab5(Q79L; Figure 4, B and C). However, in BMMs expressing Rab5(S34N), colocalization of dextran with LAMP1 was markedly diminished compared with control cells (Figure 4, A and D). This indicates that the dominant-negative Rab5-expressing BMMs are limited in their endocytosis and trafficking, as predicted from previous studies in cells expressing Rab5(S34N; Barbieri et al., 1994; Li et al., 1994). Thus, although expression of Rab5(S34N) markedly diminished fluid phase uptake and transport, it stimulated M. avium transport to a late endosome/lysosome compartment.
Figure 4.
Minimal colocalization between dextran and LAMP1 in Rab5(S34N)-expressing macrophages. BMMs were infected with retrovirus containing the pLZRS constructs: Ev-Neo (A), Rab5 WT (B), Rab5(Q79L) (C), and Rab5(S34N) (D). Transduced BMMs were incubated with 1.5 μg of Texas-Red–labeled dextran for 5 min, washed, and incubated for an additional 30 min with fresh BMM media to chase the dextran to the lysosome. BMMs were then fixed, permeabilized, and stained for LAMP1 (green). Coincident staining appears yellow in the merged images. Data shown is representative of three separate experiments. Image bar, 16 μm. Confocal sections, 0.5 μm.
M. avium within Rab5 S34N-expressing Macrophages Showed Decreased LAMP1 Staining in Cells Preloaded with Free Iron But Not Transferrin-bound Iron
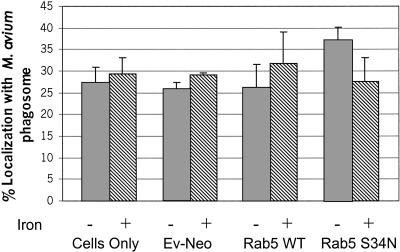
To further investigate the findings described above, we tested the hypothesis that a limitation in iron acquisition by M. avium, due to decreased fusion of mycobacteria phagosomes with early endosomes, is responsible for the acquisition of late endosome markers in Rab5(S34N)-expressing BMMs. Iron is required for mycobacterial growth (De Voss et al., 1999; Lounis et al., 2001). Mycobacteria have evolved high-affinity siderophores, exochelins, which function to bind extracellular iron and mycobactins whose role is to transport iron into the mycobacteria (Raghu et al., 1993). Recent studies by Schlesinger and colleagues demonstrate that inhibition of iron acquisition by intracellular M. tuberculosis in human BMMs results in increased killing of the mycobacteria (Olakanmi et al., 2000). In these studies they also showed that intracellular M. tuberculosis was accessible to radiolabeled iron added to the macrophage culture media. Together, these data suggest that intracellular mycobacteria can obtain iron through the macrophage and that this iron acquisition is required for their intracellular survival.
To test our hypothesis, we examined the effect of supplementing Rab5(S34N)-transduced BMMs with exogenous iron. Previous studies have shown that nontransferrin-bound iron can be absorbed by the human monocyte-like cell line THP-1 (Scaccabarozzi et al., 2000). Additional studies with rat hepatocytes indicate that Fe-citrate can be taken in by facilitated diffusion (Baker et al., 1998). Furthermore, using 59Fe-chloride we observed that the BMMs can absorb iron (our unpublished results). Therefore, we investigated how “iron-loading” the M. avium would affect its trafficking in Rab5(S34N)-expressing BMMs. In dominant-negative Rab5-expressing cells, incubated with 100 μM Fe-citrate, there was a significant decrease in the number of M. avium phagosomes staining positive for LAMP1 compared with non–iron-treated macrophages (Figure 5A). The addition of Fe-bound transferrin to Rab5(S34N)-transduced BMMs using the same protocol indicated for Fe-citrate had no affect on M. avium transport to a LAMP1-positive compartment (Figure 5B). This is likely due to limited uptake of transferrin-bound iron in cells expressing dominant-negative Rab5 (Stenmark et al. 1994) and the already high concentration of transferrin-bound iron in fetal calf serum.
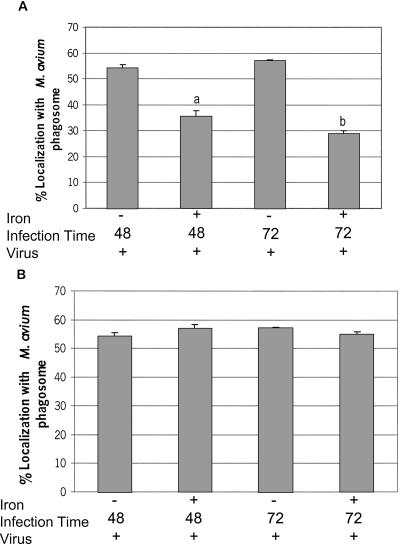
Figure 5.
The addition of Fe-citrate to Rab5(S34N)-expressing macrophages significantly decreases the number of LAMP1-positive M. avium 101 phagosomes. The M. avium 101-infected BMMs expressing the Rab5(S34N) mutant were treated with 100 μM Fe-citrate (A) or with 100 μM of iron complexed to transferrin (B) for either 48 or 72 h before fixing and staining the macrophages for LAMP1. As controls some Rab5(S34N)-expressing BMMs infected with M. avium 101 were left untreated. The degree of colocalization between the mycobacterial phagosome and LAMP1 was determined as described in Figure 3. The data are presented as the mean ± SD from three separate experiments. Comparison between iron-treated and untreated transduced macrophages. (a) p = 0.0022, (b) p = 0.0006.
Iron is known to have various effects on host cell activities including increased expression of TNF-α mRNA and TNF-α secretion in PMA-differentiated THP-1 cells (Scaccabarozzi et al., 2000). Therefore, we tested whether the influence of iron on mycobacterial localization was specific to BMMs expressing Rab5(S34N) or whether it had a general effect on mycobacterial trafficking. As shown in Figure 6, we observed minimal LAMP1 but strong TR staining of M. avium phagosomes in nontransduced BMMs or macrophages transduced with pLZRS or Rab5 WT in the presence of Fe-citrate, similar to our earlier findings (see Figure 3). This indicates that treatment of BMMs with Fe-citrate is not affecting the positioning of the mycobacteria within the endocytic pathway unless the BMMs are expressing the dominant negative Rab5.
Figure 6.
Addition of Fe-citrate to macrophages transduced with Rab5 WT or Ev-Neo had no effect on M. avium trafficking. BMMs infected with M. avium 101 were left untreated or retrovirally transduced with the Rab5 WT or Ev-Neo pLZRS constructs. The infected BMMs were incubated with Fe-citrate as described in Figure 5. The cells were fixed, permeabilized, and stained for either LAMP1 or TR. The number of mycobacterial phagosomes (n = 50 per experiment) that colocalize with LAMP1 or TR was determined and expressed as the mean ± SD for three separate experiments.
Further support for iron having a direct effect on mycobacteria comes from our experiments using gallium, a group IIIA metal. Gallium (Ga) can be absorbed by macrophages in a transferrin-dependent and -independent manner and can substitute for iron in many biomolecular complexes (Chitambar and Zivkovic, 1987; Olakanmi et al., 1994). However, Ga is unable to undergo redox cycling and therefore disrupts the function of many iron-binding proteins such as ribonucleotide reductase, thus affecting DNA replication (Chitambar et al., 1988). Olakanmi and colleagues found that addition of Ga to M. tuberculosis–infected human macrophages resulted in increased killing of the mycobacteria, which was likely preceded by increased phagosome-lysosome fusion (Olakanmi et al., 2000). We found that addition of 500 μM Ga-citrate to M. avium–infected macrophages resulted in a significant increase in colocalization between LAMP1 and mycobacteria compared with infected control BMMs (29.8 ± 80% of M. avium phagosomes staining positive for LAMP1 in Ga-treated BMMs compared with 8.0 ± 1.1% in untreated infected cells; mean ± SD from three separate experiments). These results support our hypothesis that an adequate iron concentration within the M. avium phagosome is required for mycobacteria to maintain its block of the phagosome maturation process.
Previous studies have demonstrated that killed mycobacteria show increased trafficking to late endosome/lysosome compartments compared with live bacilli (Clemens and Horwitz, 1995), suggesting that metabolic activity is required for mycobacteria to maintain itself within an early phagosome compartment. Therefore we would predict that addition of iron would not affect transport of dead mycobacteria through the phagosome maturation process in Rab5(S34N)-expressing BMMs. As shown in Figure 7, colocalization between LAMP1 and heat-killed M. avium in macrophages expressing dominant-negative Rab5 was not effected by the addition of iron. Further, expression of Rab5(S34N) in BMMs did not result in increased transport of heat-killed M. avium to a LAMP1-positive compartment compared with nontransduced, pLZRS, or Rab5 WT transduced BMMs, indicating that the effect of dominant negative Rab5 on M. avium transport is limited to live, metabolically active mycobacteria.
Figure 7.
Addition of Fe-citrate had no effect on the colocalization of LAMP1 with phagosomes containing heat-killed M. avium in macrophages expressing Rab5(S34N). BMMs infected with heat-killed M. avium 101 were left untreated or retrovirally transduced with Rab5 WT, Rab5(S34N), or Ev-Neo pLZRS. The infected BMMs were incubated with Fe-citrate for 48 h as described in Figure 5. The fixed and permeabilized cells were stained for LAMP1. The number of mycobacterial phagosomes that colocalized with LAMP1 were quantitated and expressed as the mean ± SD from three separate experiments.
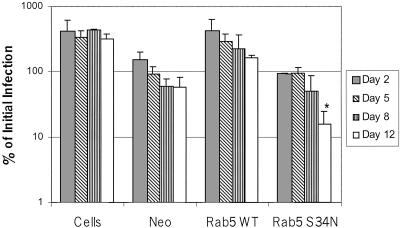
As noted above, limiting accessibility of phagocytosed M. tuberculosis to iron resulted in decreased mycobacteria viability (Olakanmi et al., 2000). To determine if this was also the case for BMMs expressing Rab5(S34N), we infected transduced and control BMMs with M. avium 101 and defined colony counts overtime (6 h to 12 d). As shown in Figure 8, there was a significant decrease in colony-forming units isolated from Rab5(S34N)-expressing BMMs over the 12-d infection period. This was not observed for the Rab5 WT or pLZRS-transduced BMMs or for nontransduced cells, indicating that expression of Rab5(S34N) in BMMs results in decreased M. avium viability. This is likely due, at least in part, to decreased accessibility of M. avium to iron in these transduced cells.
Figure 8.
Killing of M. avium 101 in macrophages expressing Rab5(S34N). Triplicate wells of BMMs were infected with M. avium and either left untreated or retrovirally transduced with the Rab5 WT, Rab5(S34N), or Ev-Neo pLZRS constructs. One set of infected BMMs were harvested immediately after the viral transduction and this served as the initial infection load (i.e., 100%). Infected BMMs were also harvested after 2, 5, 8, and 12 d. Bacilli from lysed BMMs were quantitated by serial dilution and expressed, for each set of transductions, as percent of initial infection load ±SD. Initial infection loads were: 3.95 × 106 for nontransduced cells, 7.44 × 106 for Ev-Neo, 3.68 × 106 for Rab5 WT, and 1.13 × 107 for Rab5(S34N)-transduced BMMs. A representative of two experiments is presented. *p = 0.035.
DISCUSSION
Pathogenic mycobacteria reside and replicate within the phagosomal compartment of macrophages. The mycobacteria containing phagosome resists fusion with late endosomes and lysosomes, and this inhibition is critical for the mycobacterium's intracellular survival (Clemens, 1996; Deretic and Fratti, 1999; Fratti et al., 2001). However, studies indicate that mycobacterial phagosomes are capable of limited membrane fusion and acquire various plasma membrane and endosomal markers including the transferrin receptor and the ganglioside GM1 (Russell et al., 1996). Together, the published data suggest that phagosomes containing pathogenic mycobacteria are selective in their fusion with other membrane vesicles and do not mature to form a phagolysosome. How mycobacteria modify the phagosome maturation/membrane fusion process remains elusive. However, recent data suggest that the mycobacteria actively block phagosome maturation by manipulation of the host cell endosome machinery. Specifically, recent studies by Fratti et al. (2001) have demonstrated that phagosomes containing mycobacteria or mycobacterial products fail to recruit EEA1, an effector molecules important in phagosomal biogenesis. Additionally, mycobacterial phagosomes contain a degraded form of cellubrevin, a molecule also implicated in phagosome maturation (Fratti et al., 2002).
Our ability to dissect the mechanism by which mycobacteria inhibit the phagosome maturation process would be facilitated by introducing various molecules involved in the vesicle fusion process and then determine their effect on mycobacterial trafficking. However, introducing genes into primary macrophages, the host cell for mycobacteria, has been problematic. We have used a second generation retrovirus transduction system to introduce genes into murine bone marrow–derived macrophages (Michiels et al., 2000). This system has a number of advantages: 1) the genes introduced into the macrophage are inserted into the chromosome, maintaining increased stability of the DNA compared with episomally replicating plasmids, 2) expression levels using retroviral systems are typically 2–3-fold higher than endogenous protein and therefore problems associated with marked protein overexpression are not observed, and 3) we were able to consistently obtain >85% of the primary macrophages to express recombinant protein by this method.
In our study we addressed whether Rab5 and Rab7 are targets of the mycobacteria in modulating the phagosome maturation process by introducing these proteins in their wild-type, constitutively active and dominant negative forms into murine BMMs. The small-molecular-weight GT-Pases Rab5 and Rab7 are important in trafficking material through the endocytic pathway. Rab5 functions in both endocytosis from the plasma membrane and homotypic fusion between early endosomes (Li, 1996; Somsel Rodman and Wandinger-Ness, 2000). Rab7 regulates transport from the early endosome to the late endosome (Feng et al., 1995; Mohrmann and van der Sluijs, 1999). Previous studies, using the murine macrophage cell line J774, indicate that M. bovis BCG phagosomes retain Rab5 but fail to acquire Rab7 (Via et al., 1997). Our studies with primary murine macrophages also showed M. avium phagosomes to retain wild-type or constitutively active Rab5 and exclude wild-type or constitutively active Rab7. This suggests that increased expression of activated Rab5 or Rab7 does not alter the trafficking of the M. avium significantly. This was supported by the colocalization of the M. avium phagosome with the transferrin receptor but not LAMP1, indicating retention of the mycobacteria in an early endosome/recycling compartment.
An interesting result from our studies was the augmented LAMP1 and LAMP2 staining of the M. avium phagosome in macrophages expressing the dominant negative Rab5, indicating an increased transport to a late endosome/lysosome compartment. This was specific since expression of dominant negative Rab7 in BMMs had no effect on M. avium trafficking compared with nontransduced cells. Our results were unexpected as previous studies have shown decreased endocytosis of transferrin receptor in Rab5(S34N)-expressing cells (Stenmark et al., 1994). Expression of Rab5(S34N) also decreases transport of endocytosed material to a lysosomal compartment as indicated by a 50% reduction in LDL and EGF degradation in transfected HeLa cells (McCaffrey et al., 2001). Our results from the dextran uptake experiments also suggest a role for Rab5(S34N) in the endocytic process because colocalization between intracellular LAMP1 and Texas Red–labeled dextran was significantly diminished in Rab5(S34N)-expressing BMMs compared with control cells. However, these results may be due primarily to the importance of Rab5 in the endocytic process rather then its role in the maturation of early endosomes to late endosomes and fusion with lysosomes. This is supported by our data with the heat-killed mycobacteria where we observed no difference in trafficking of the dead M. avium to a LAMP1-positive compartment between WT and dominant negative Rab5-expressing BMMs.
Insight into a potential explanation for the increased transport of M. avium to a LAMP1- and LAMP2-positive compartment in Rab5(S34N)-expressing macrophages came from our TR localization studies. As expected, there was a significant decrease in TR staining of the M. avium phagosome in Rab5(S34N)-expressing macrophages compared with controls. Therefore, a decrease in transferrin bound iron within the M. avium phagosome would be expected. Mycobacteria, like many pathogenic bacteria, are exquisitely sensitive to iron deprivation (Lounis et al., 2001). Analysis of the M. tuberculosis genome suggests that iron is an obligate cofactor for at least 40 different enzymes (De Voss et al., 1999). It is involved, when complexed with heme, in electron transport and oxygen metabolism (De Voss et al., 1999). Nonheme iron is also a cofactor for proteins involved in amino acid and pyrimidine biosynthesis as well as enzymes such as ribonucleotide reductase involved in DNA synthesis and superoxide dismutase (Edwards et al., 2001). However, whether iron serves as a cofactor for mycobacterial enzymes required for blocking the phagosome maturation process has yet to be determined.
An important aspect of the host defense against bacterial pathogens is restriction of available iron. Natural resistance associated membrane protein 1 (Nramp1) is a well-known example of a protein whose activity is associated with resistance to various intracellular pathogens including Leishmania, Salmonella, and Mycobacterium (Vidal et al., 1995; Bellamy, 1999). Nramp1 is expressed in macrophages and its expression is upregulated with IFN-γ treatment, an important cytokine involved in controlling intramacrophage pathogens (Govoni et al., 1995; Atkinson et al., 1997). Functional studies with different Nramp homologues suggest that this class of protein functions to transport divalent cations, including iron, across membranes (Forbes and Gros, 2001). Therefore, a likely role for Nramp1 is to pump iron out of the phagosome, thus limiting the amount of iron available for the pathogenic mycobacteria (Forbes and Gros, 2001).
There are also clinical data showing a strong correlation between iron overload in patients and enhanced risk of death due to tuberculosis (Murray et al., 1978). Animal studies suggest that iron may be a limiting factor during a mycobacterial infection, because mice fed an iron-rich diet have a higher M. avium infection load than mice fed a normal iron diet (Dhople et al., 1996). Furthermore, studies by Schaible et al. (2002) indicate that the increased susceptibility of β-2 Microglobulin null mice to M. tuberculosis infection is due to iron overload. Finally, in vitro studies using isolated macrophages also suggest that accessibility to iron is essential for mycobacterial survival and replication in macrophages (Olakanmi et al., 2000). More recent studies by Olakanmi et al. (2002) indicate that the mycobacterial phagosome can access iron both from endocytosed transferrin and through some internal pool of iron.
On the basis of the above, we propose that expression of Rab5(S34N) in macrophages limits the concentration of iron within the M. avium phagosome, resulting in its inability to inhibit the phagosome maturation process and increased mycobacterial killing. As mentioned previously, dead mycobacteria are trafficked to the lysosome, and therefore metabolic activity of the Mycobacterium is important for this inhibitory process. Our data support this hypothesis of iron deprivation because “iron loading,” mediated by the addition of ferric citrate to the M. avium infected Rab5(S34N)-expressing macrophages, resulted in a significant decrease in LAMP1 staining of the M. avium phagosome. In contrast, addition of ferric citrate had no affect on trafficking of heat-killed mycobacteria in Rab5(S34N)-expressing macrophages. Further, in control macrophages, or macrophages expressing wild-type Rab5, where the availability of iron is not as limited, we did not observe any effect of iron on M. avium localization within the macrophage. Future analysis of the genes regulated by iron may provide us with important clues as to proteins required for Mycobacterium's ability to inhibit the phagosome maturation process.
Acknowledgments
We thank Dr. John Collard for the retroviral expression vectors, Felipe Palacios for the Rab constructs, and Dr. Crislyn D'Souza-Schorey and Dr. Guangpu Le for their insightful comments and suggestions. This work was supported, in part, through grants from the American Heart Association and the USDA CSREES. V.A.K. is a National Institutes of Health predoctoral trainee.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-12-0780. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-12-0780.
References
- Atkinson, P.G., Blackwell, J.M., and Barton, C.H. (1997). Nramp1 locus encodes a 65 kDa interferon-gamma-inducible protein in murine macrophages. Biochem. J. 325, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, E., Baker, S.M., and Morgan, E.H. (1998). Characterisation of non-transferrin-bound iron (ferric citrate) uptake by rat hepatocytes in culture. Biochim. Biophys. Acta 1380, 21–30. [DOI] [PubMed] [Google Scholar]
- Barbieri, M.A., Li, G., Colombo, M.I., and Stahl, P.D. (1994). Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269, 18720–18722. [PubMed] [Google Scholar]
- Bellamy, R. (1999). The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1, 23–27. [DOI] [PubMed] [Google Scholar]
- Bohlson, S.S., Strasser, J.A., Bower, J.J., and Schorey, J.S. (2001). Role of complement in Mycobacterium avium pathogenesis: in vivo and in vitro analyses of the host response to infection in the absence of complement component C3. Infect. Immun. 69, 7729–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshans, R.L., Szanto, S., van Aelst, L., and D'Souza-Schorey, C. (2000). ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol. Cell. Biol. 20, 3685–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar, C.R., Matthaeus, W.G., Antholine, W.E., Graff, K., and O'Brien, W.J. (1988). Inhibition of leukemic HL60 cell growth by transferrin-gallium: effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood 72, 1930–1936. [PubMed] [Google Scholar]
- Chitambar, C.R., and Zivkovic, Z. (1987). Uptake of gallium-67 by human leukemic cells: demonstration of transferrin receptor-dependent and transferrin-independent mechanisms. Cancer Res. 47, 3929–3934. [PubMed] [Google Scholar]
- Clemens, D.L. (1996). Characterization of the Mycobacterium tuberculosis phagosome. Trends Microbiol. 4, 113–118. [DOI] [PubMed] [Google Scholar]
- Clemens, D.L., and Horwitz, M.A. (1995). Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voss, J.J., Rutter, K., Schroeder, B.G., and Barry, C.E., 3rd. (1999). Iron acquisition and metabolism by mycobacteria. J. Bacteriol. 181, 4443–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, V., and Fratti, R.A. (1999). Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31, 1603–9. [DOI] [PubMed] [Google Scholar]
- Deretic, V., Via, L.E., Fratti, R.A., and Deretic, D. (1997). Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis 18, 2542–2547. [DOI] [PubMed] [Google Scholar]
- Dhople, A.M., Ibanez, M.A., and Poirier, T.C. (1996). Role of iron in the pathogenesis of Mycobacterium avium infection in mice. Microbios 87, 77–87. [PubMed] [Google Scholar]
- Duclos, S., Diez, R., Garin, J., Papadopoulou, B., Descoteaux, A., Stenmark, H., and Desjardins, M. (2000). Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J. Cell Sci. 113(Pt 19), 3531–3541. [DOI] [PubMed] [Google Scholar]
- Edwards, K.M., Cynamon, M.H., Voladri, R.K., Hager, C.C., DeStefano, M.S., Tham, K.T., Lakey, D.L., Bochan, M.R., and Kernodle, D.S. (2001). Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 164, 2213–9. [DOI] [PubMed] [Google Scholar]
- Feng, Y., Press, B., and Wandinger-Ness, A. (1995). Rab 7, an important regulator of late endocytic membrane traffic. J. Cell Biol. 131, 1435–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, J.R., and Gros, P. (2001). Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9, 397–403. [DOI] [PubMed] [Google Scholar]
- Fratti, R.A., Backer, J.M., Gruenberg, J., Corvera, S., and Deretic, V. (2001). Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti, R.A., Chua, J., and Deretic, V. (2002). Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 277, 17320–17326. [DOI] [PubMed] [Google Scholar]
- Govoni, G., Vidal, S., Cellier, M., Lepage, P., Malo, D., and Gros, P. (1995). Genomic structure, promoter sequence, and induction of expression of the mouse Nramp1 gene in macrophages. Genomics 27, 9–19. [DOI] [PubMed] [Google Scholar]
- Li, G. (1996). Rab5 GTPase and endocytosis. Biocell 20, 325–330. [PubMed] [Google Scholar]
- Li, G., Barbieri, M.A., Colombo, M.I., and Stahl, P.D. (1994). Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J. Biol. Chem. 269, 14631–14635. [PubMed] [Google Scholar]
- Lounis, N., Truffot-Pernot, C., Grosset, J., Gordeuk, V.R., and Boelaert, J.R. (2001). Iron and Mycobacterium tuberculosis infection. J. Clin. Virol. 20, 123–126. [DOI] [PubMed] [Google Scholar]
- Martinez, O., and Goud, B. (1998). Rab proteins. Biochim. Biophys. Acta 1404, 101–112. [DOI] [PubMed] [Google Scholar]
- McCaffrey, M.W., Bielli, A., Cantalupo, G., Mora, S., Roberti, V., Santillo, M., Drummond, F., and Bucci, C. (2001). Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 495, 21–30. [DOI] [PubMed] [Google Scholar]
- Michiels, F., van der Kammen, R.A., Janssen, L., Nolan, G., and Collard, J.G. (2000). Expression of Rho GTPases using retroviral vectors. Methods Enzymol. 325, 295–302. [DOI] [PubMed] [Google Scholar]
- Mohrmann, K., and van der Sluijs, P. (1999). Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol. Membr. Biol. 16, 81–87. [DOI] [PubMed] [Google Scholar]
- Murray, M.J., Murray, A.B., Murray, M.B., and Murray, C.J. (1978). The adverse effect of iron repletion on the course of certain infections. Br. Med. J. 2, 1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olakanmi, O., Schlesinger, L.S., Ahmed, A., and Britigan, B.E. (2002). Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. J. Biol. Chem. 277, 49727–49734. [DOI] [PubMed] [Google Scholar]
- Olakanmi, O., Britigan, B.E., and Schlesinger, L.S. (2000). Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68, 5619–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olakanmi, O., Stokes, J.B., and Britigan, B.E. (1994). Acquisition of iron bound to low molecular weight chelates by human monocyte-derived macrophages. J. Immunol. 153, 2691–2703. [PubMed] [Google Scholar]
- Palacios, F., Price, L., Schweitzer, J., Collard, J.G., and D'Souza-Schorey, C. (2001). An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin, E.L., and Swanson, J.A. (1993). Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 121, 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu, B., Sarma, G.R., and Venkatesan, P. (1993). Effect of iron on the growth and siderophore production of mycobacteria. Biochem. Mol. Biol. Int. 31, 341–348. [PubMed] [Google Scholar]
- Roach, S.K., and Schorey, J.S. (2002). Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect. Immun. 70, 3040–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.G., Dant, J., and Sturgill-Koszycki, S. (1996). Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J. Immunol. 156, 4764–4773. [PubMed] [Google Scholar]
- Russell, D.G., Sturgill-Koszycki, S., Vanheyningen, T., Collins, H., and Schaible, U.E. (1997). Why intracellular parasitism need not be a degrading experience for Mycobacterium. Philos. Trans. R Soc. Lond. B Biol. Sci. 352, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaccabarozzi, A. et al. (2000). Relationship between TNF-alpha and iron metabolism in differentiating human monocytic THP-1 cells. Br. J. Haematol. 110, 978–984. [DOI] [PubMed] [Google Scholar]
- Schaible, U.E., Collins, H.L., Priem, F., and Kaufmann, S. (2002). Correction of the iron overload defect in β-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 196, 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsel Rodman, J., and Wandinger-Ness, A. (2000). Rab GTPases coordinate endocytosis. J. Cell Sci. 113(Pt 2), 183–192. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Parton, R.G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S. et al. (1994). Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678–681. [DOI] [PubMed] [Google Scholar]
- Thilo, L., Stroud, E., and Haylett, T. (1995). Maturation of early endosomes and vesicular traffic to lysosomes in relation to membrane recycling. J. Cell Sci. 108, 1791–1803. [DOI] [PubMed] [Google Scholar]
- Via, L.E., Deretic, D., Ulmer, R.J., Hibler, N.S., Huber, L.A., and Deretic, V. (1997). Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272, 13326–13331. [DOI] [PubMed] [Google Scholar]
- Vidal, S., Gros, P., and Skamene, E. (1995). Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J Leukoc. Biol. 58, 382–390. [DOI] [PubMed] [Google Scholar]
- Vitelli, R., Santillo, M., Lattero, D., Chiariello, M., Bifulco, M., Bruni, C.B., and Bucci, C. (1997). Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272, 4391–4397. [DOI] [PubMed] [Google Scholar]
- Woodman, P.G. (2000a). Biogenesis of the sorting endosome: the role of Rab5. Traffic 1, 695–701. [DOI] [PubMed] [Google Scholar]
- Woodman, P.G. (2000b). Biogenesis of the sorting endosome: the role of Rab5. Traffic 1, 695–701. [DOI] [PubMed] [Google Scholar]
- Xu, S., Cooper, A., Sturgill-Koszycki, S., van Heyningen, T., Chatterjee, D., Orme, I., Allen, P., and Russell, D.G. (1994). Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153, 2568–2578. [PubMed] [Google Scholar]
- Zerial, M. (1993). Regulation of endocytosis by the small GTP-ase rab5. Cytotechnology 11, S47–S49. [DOI] [PubMed] [Google Scholar]