Abstract
The maturation of dendritic cells is accompanied by the redistribution of major histocompatibility complex (MHC) class II molecules from the lysosomal MHC class II compartment to the plasma membrane to mediate presentation of peptide antigens. Besides MHC molecules, dendritic cells also express CD1 molecules that mediate presentation of lipid antigens. Herein, we show that in human monocyte-derived dendritic cells, unlike MHC class II, the steady-state distribution of lysosomal CD1b and CD1c isoforms was unperturbed in response to lipopolysaccharide-induced maturation. However, the lysosomes in these cells underwent a dramatic reorganization into electron dense tubules with altered lysosomal protein composition. These structures matured into novel and morphologically unique compartments, here termed mature dendritic cell lysosomes (MDL). Furthermore, we show that upon activation mature dendritic cells do not lose their ability of efficient clathrin-mediated endocytosis as demonstrated for CD1b and transferrin receptor molecules. Thus, the constitutive endocytosis of CD1b molecules and the differential sorting of MHC class II from lysosomes separate peptide- and lipid antigen-presenting molecules during dendritic cell maturation.
INTRODUCTION
Dendritic cells (DCs) are professional antigen presenting cells that distribute from blood to various tissues to sample antigens from invading pathogens. To optimize the uptake of pathogens, immature DCs (IMDCs) exhibit a high rate of pinocytosis and phagocytosis. After endocytosis of microbes or activation by inflammatory stimuli such as lipopolysaccharide (LPS), DCs undergo maturation, while migrating to the T cell-rich lymph nodes where they optimize presentation of pathogen-derived peptide antigens via major histocompatibility complex (MHC) class II molecules.
The DC maturation-associated changes in MHC class II intracellular distribution and trafficking have been well characterized (Lanzavecchia and Sallusto, 2001; Mellman and Steinman, 2001). In IMDCs, a majority of MHC class II molecules are stored in a late endosomal/lysosomal compartment, called the MHC class II compartment (MIIC) (Peters et al., 1991), where peptide antigen loading onto MHC class II molecules is proposed to occur (Watts, 1997). In mature DCs (MDCs), the MIIC is reorganized and peptide antigen-loaded MHC class II molecules are efficiently transported in a retrograde manner in tubular transport containers. These tubes pull out and polarize toward specific T cells and fuse with the DC plasma membrane (Turley et al., 2000; Kleijmeer et al., 2001). Internalization of MHC class II from the cell surface is down-regulated upon maturation and the class II dimers become more stable on the cell surface with an increased half-life (Cella et al., 1997; Pierre et al., 1997; Turley et al., 2000). These DC maturation-associated cellular changes contribute to efficient activation of peptide antigen-specific, MHC class II-restricted T cells.
Human DC also prominently express CD1a, b, c, and d members of a distinct lineage of MHC-like antigen-presenting, molecules. Unlike MHC class I and class II molecules, which bind short peptides, CD1 molecules bind (glyco-)lipid antigens in their hydrophobic cavity for presentation to the T cell receptor complex of a variety of T cells that function against microbial infection (Porcelli et al., 1992; Beckman et al., 1994; Sieling et al., 1995; Zeng et al., 1997; Jackman et al., 1998; Gumperz and Brenner, 2001; Gadola et al., 2002).
Like MHC class II molecules, CD1b and CD1c molecules can be detected in lysosomal MIICs. By virtue of the tyrosine-based endosomal targeting sequence in the cytoplasmic tail, CD1b and CD1c are internalized in clathrin-coated pits and vesicles from the plasma membrane and distribute to endocytic subcompartments, including the MIIC (Peters et al., 1995; Sugita et al., 1996, 2000). It has been shown that AP3 is essential for proper targeting of CD1b molecules, whereas MHC class II molecules traffic independent of AP3 (Briken et al., 2002; Sugita et al., 2002). When the cytoplasmic tyrosine in the tail is deleted, CD1b molecules are unable to target to endocytic subcompartments and reside primarily at the PM. Cytoplasmic targeting motifs can be classified based on common amino acid sequences and the most common tyrosine-based motif is the YXXΦ motif in which Y is the tyrosine residue, X any amino acid, and Φ a bulky hydrophobic residue. A list of relevant transmembrane molecules containing a tyrosine-based motif is given in Table 1 (Honing et al., 1996; Sugita et al., 1999, 2000). In contrast, CD1a lacks the tyrosine-based motif and is expressed abundantly on the plasma membrane and in early recycling endosomes (Sugita et al., 1999).
Table 1.
The tyrosine-based sorting motifs of relevant transmembrane proteins
| CD1a* | TM-RKRCFC |
| CD1b | TM-RRRSYQNIP |
| CD1c | TM-KKHCSYQDIL |
| CD1d | TM-KRQTSYQGVL |
| LAMP-1 | TM-RKRSHAGYQTI |
| LAMP-2 | TM-KHHHAGYEQF |
| CD63 | TM-KSIRSGYEVM |
| M6PR | TM-24aa-YKYSKV-135aa |
| Transferrin receptor | 17aa-LSYTRF-45aa-TM |
The sequences of the cytoplasmic tails are depicted for CD1a, CD1b, CD1c, and CD1d, lysosomal proteins LAMP1, LAMP2, and CD63, and early endosomal transferrin receptor, late endosomal CI mannose-6-phosphate receptor. TM denotes the transmembrane domain and the YXXØ motif is underlined. The tyrosine (Y) and the bulky hydrophobic residues are bold typed.
Note CD1a lacks the tyrosine motif.
The striking changes in MHC class II intracellular trafficking and the lack of knowledge about CD1 lipid antigen presentation during DC maturation urged us to initiate the current study to address how CD1 trafficking might be altered upon maturation. Surprisingly, we found that CD1b and CD1c molecules segregated from the MHC class II molecules in the MIIC. Whereas MHC class II trafficked out of the MIIC, no such steady-state redistribution of CD1b and c molecules occurred. CD1b and CD1c molecules were primarily detected in late endocytic compartments, herein termed mature dendritic cell lysosome (MDL). We further show that independently of the DC maturation status, CD1b molecules are continuously endocytosed from the plasma membrane via clathrin-coated vesicles. The continued internalization of CD1b molecules probably prevents substantial increases of the surface levels for CD1b, resulting in a striking difference in changes in cell surface expression between the lipid-presenting CD1 molecules and the peptide presenting MHC class II molecules.
MATERIALS AND METHODS
Monocyte Culture
Human monocytes were isolated from peripheral blood from donors (Porcelli et al., 1992). The cells were cultured in RPMI 1640 medium containing 10% fetal calf serum, 10 mM HEPES, 2.8 mM l-glutamine, 0.8 mM nonessential amino acids, 0.4 mM essential amino acids, 100 μg/ml penicillin-streptomycin, 50 μM 2-Mercaptoethanol, 300 U/ml human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (PeproTech, Rocky Hill, NJ), and 200 U/ml human recombinant interleukin-4 (IL-4) (PeproTech) for 6 d to generate CD1-positive immature DCs. Maturation of the DCs was induced by addition of 1 μg/ml LPS (Salmonella abortus equi; Sigma-Aldrich, St. Louis, MO).
Electron Microscopy
Monocytes were cultured in medium containing GM-CSF/IL-4 for 6 d, and the cells were fixed before stimulation and at different time points (1, 2, 4, 8, 24, 40, and 48 h) after stimulation with LPS. As a control, cells remained in GM-CSF and IL-4 for the different time points. Fixation was performed by adding an equal volume of 4% paraformaldehyde, and 0.4% glutaraldehyde in PIPES/HEPES/EGTA/magnesium buffer to the warm culture medium. Fixed cells were collected, embedded, and processed for cryosectioning with a Leica FCS as described previously (Peters and Hunziker, 2001). Samples were trimmed using a diamond Cryotrim 90 knife at –100°C (Diatome, Biel, Switzerland), and ultrathin sections of 50 nm were cut at –120°C by using an ultramicrotome cryo knife. Immunogold labeling was performed using mouse monoclonal antibodies against CD1a (10H3.9.3), CD1b (BCD1b2.1), and CD1c (F10/21A3) and rabbit polyclonal antisera against MHC class II (α-chain; Neefjes et al., 1990), mannose 6-phosphate receptor (M6PR; a gift from Dr. V. Hsu, Harvard Medical School, Boston, MA), early endosome antigen1 (EEA1) (PA1-063; Affinity Bioreagents, Golden, CO), CD63 (M1544; CLB Amsterdam, Amsterdam, The Netherlands), and lysosome-associated membrane protein 1 (LAMP1 931.1; a gift from M. Fukuda, La Jolla Cancer Research, La Jolla, CA). Detection of bound antibodies was performed directly using protein-A conjugated to 10-nm gold or via rabbit anti-mouse bridging serum (DAKO, Glostrup, Denmark) and protein-A conjugated to 15-nm gold (Electron Microscopy Laboratory, Utrecht University, Utrecht, The Netherlands). Sections were studied using a CM10 and CM12 transmission electron microscope (Fei, Endhoven, The Netherlands).
Flow Cytometry
Flow cytometric analysis was performed as described previously (Porcelli et al., 1992). The antibodies (see “Electron Microscopy”) were detected using fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG/IgM (Jackson Immunoresearch Laboratories, West Grove, PA).
Endocytosis Assays
Measurement of the pinocytic capacity was performed by incubating LPS-stimulated DC and unstimulated DC with FITC-conjugated dextran. First, it was determined whether the uptake of dextran is saturable by incubating the cells with increasing concentrations of dextran-FITC. Incubations were performed for 1 h in 0.5 mg/ml dextran–FITC (Molecular Probes, Eugene, OR) at 4°C to determine the portion of dextran that would bind to the plasma membrane without being endocytosed. Incubation at 37°C for 1 h allows the cells to pinocytose the dextran, whereas control cells were kept on ice.
The endocytic capacity via clathrin-coated pits was measured by using Alexa 488-conjugated transferrin (Molecular Probes) as a tracer. First, the optimal transferrin concentration was determined by titration to find the receptor saturation point. Immature and mature DCs were incubated in medium containing 10 μg/ml transferrin for 1 h at 4°C. Of these cells, one pool was incubated at 37°C for 1 h, whereas the control cells were kept on ice. The monocyte-derived DCs were then washed and analyzed by flow cytometry or fixed and processed for electron/fluorescence microscopy.
The internalization of CD1b molecules was studied using Fab fragments of CD1b monoclonal antibody BCD1b3.1. Fragments were cleaved using papain-agarose beads (Sigma-Aldrich) at 37°C, and undigested antibodies and Fc parts were purified from the Fab fragment solution by protein G-Sepharose beads. IMDCs and MDCs were collected and resuspended to a concentration of 2 × 107 cells/ml in cold IMDM medium supplemented with 10% fetal bovine serum and 6 μg/ml CD1b-specific Fab fragments. The cells were incubated on ice for 1 h to allow binding of the Fab fragments and subsequently washed three times and incubated at 37°C for various times (30, 60, and 120 min). Control samples were kept on ice for 120 min. Cells were transferred to ice to stop internalization and incubated for 30 min in FITC-conjugated goat anti-mouse IgG F(ab′)2 (Pierce Chemical, Rockford, IL) to detect the Fab fragments on the cell surface by flow cytometry.
Chimeric Constructs
Construction of plasmids encoding chimeric CD1b molecules and transfection in T2 cells is described previously (Porcelli et al., 1992). In all chimeric constructs, the luminal and transmembrane portion remained of CD1b origin. Generation of the DNA construct has been fully described (Sugita et al., 2002), and its identity was confirmed by DNA sequencing. The CD1b:CD63 tail-encoding plasmid was constructed in the pCEP4 expression vector (Invitrogen, Carlsbad, CA) and transfected into human lymphoblastoid T2 cells by electroporation. Selection was performed in the presence of 0.2 mg/ml hygromycin B (Invitrogen), and positive cells were sorted by two cycles of flow cytometric procedure.
RESULTS
Differential Expression of CD1b and MHC Class II in Lysosomal Subdomains of Immature DCs
MHC class II and CD1b molecules are both prominently expressed in a specialized lysosomal MIIC and mediate presentation of endocytosed antigens. However, given the distinct nature of antigens they bind, we hypothesized that some parts of the intracellular pathways for MHC class II- and CD1b-mediated antigen presentation might differ. Therefore, we carefully analyzed and quantified the MIIC in ultrathin cryosectioned human DCs by immunogold-labeled transmission electron microscopy for MHC class II and CD1b expression. When human peripheral blood monocytes were stimulated with GM-CSF and IL-4, the cells differentiated into IMDCs, and a large quantity of MHC class II molecules was detected intracellularly in the MIIC. Unlike the multivesicular structure often seen in B cells and murine DC cell lines, the MIIC developed in freshly isolated human DCs typically contained numerous membrane lamellae closely packed in concentric arrangement, and MHC class II molecules were mainly detected on internal membranes of the MIIC rather than on the limiting membrane. Besides MHC class II, these human monocyte-derived DCs also expressed CD1b molecules in the same MIIC, but unlike MHC class II, CD1b molecules seemed to be differentially localized to the limiting membrane (Figure 1A). To appreciate the differential expression of MHC class II and CD1b molecules on the inner and limiting membranes in a quantitative way, lysosomes of monocyte-derived DCs obtained from different donors were randomly analyzed and gold particles present on the internal and limiting membranes were counted for MHC class II and CD1b. As summarized in the Table 2, 85% of CD1b molecules expressed in the MIIC were found on the limiting membrane, whereas 15% were detected on the internal membranes. In sharp contrast, only 5% of MHC class II molecules expressed in the MIIC were found on the limiting membrane, and the majority of the molecules was distributed to the internal membranes. For comparison, other proteins that resided in the MIIC were also analyzed for their differential expression in the internal and the limiting membranes. As shown in Table 2, LAMP1 was localized preferentially to the limiting membrane, albeit less prominently as CD1b, whereas CD63 was expressed more abundantly in the inner membranes than in the limiting membrane.
Figure 1.
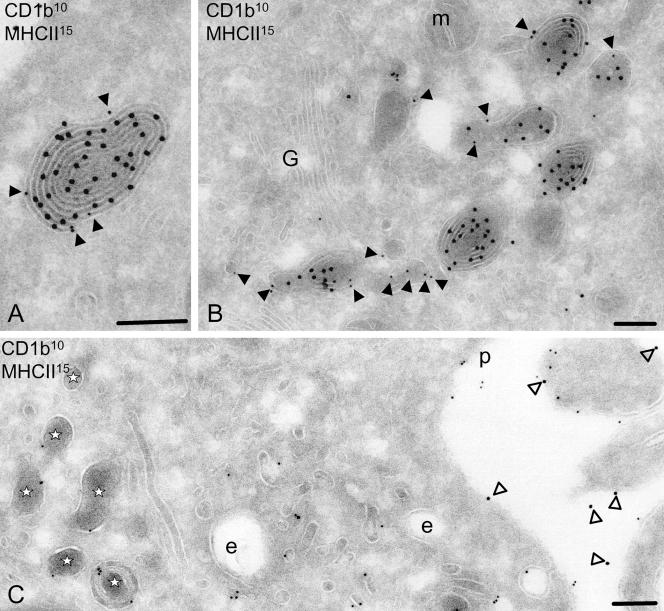
After maturation, CD1b localization remains lysosomal and separates from MHC class II. Immunogold labeling of CD1b (closed arrowheads) and MHC class II (open arrowheads) on ultrathin sections of IMDC (A), 8-h LPS-stimulated DCs (B), and 48-h LPS-stimulated MDC (C). In MIICs of IMDCs (A), CD1b molecules (10-nm gold) are located at the limiting membrane (see Table 2) and MHC class II molecules (15-nm gold) are present at the internal membranes. After 8 h of maturation (B), the multilamellar structures are altering and often CD1b (10-nm gold) is no longer colocalizing with MHC class II (15-nm gold). At 48 h after maturation (C), most MHC class II is present on the plasma membrane (15-nm gold; open arrowheads), and CD1b (10-nm gold) is detected on the plasma membrane, early endosomes, and electron-dense single membrane MDLs (indicated by asterisks). Scale, 200 nm. G, Golgi complex; m, mitochondria; p, plasma membrane; and e, endosomes.
Table 2.
Distribution of transmembrane proteins on the limiting membranes relative to the internal membranes of MIICs from IMDC
| % Internal membrane | % Limiting membrane | N | |
|---|---|---|---|
| MHC class II | 95 | 5 | 282 |
| CD1b | 15 | 85 | 117 |
| CD1c | 55 | 45 | 146 |
| LAMP1 | 22 | 78 | 144 |
| CD63 | 68 | 32 | 203 |
The immunogold labeling on multilaminar lysosomal MIICs from immature monocyte-derived dendritic cells was determined and the percentage of labeling on the limiting membranes and the internal membranes was quantified (N is the number of gold particles counted). MHC class II and CD63 molecules were detected mostly on the internal membranes and CD1b and LAMP1 on the limiting membranes. CD1c is nearly equally distributed amongst both membrane categories.
Segregation of CD1b and MHC Class II Molecules in Maturing DCs
DCs undergo dynamic cellular changes upon exposure to bacterial products, such as LPS. These changes, collectively called DC maturation, include efficient transport of peptide antigen-loaded MHC class II molecules from the lysosomal MIIC to the plasma membrane. DC maturation-associated changes in lysosomal morphology has been studied extensively at the ultrastructural level, by using a LPS-stimulated murine DC cell line, in which tubulization of the multivesicular MIIC extends toward the plasma membrane (Kleijmeer et al., 2001). However, these murine cells lack the expression of group 1 CD1 molecules and thus, it remained to be determined how CD1b and MHC class II molecules might be differentially transported from lysosomes in maturing DCs.
To investigate morphological changes in the multilamellar MIIC of maturing human DCs, monocyte-derived immature DCs were stimulated with LPS and harvested after 8 h for transmission electron microscopy. At this time point, the membrane lamellae tightly packed in concentric arrangement were appreciably loosened, and some membranes spread out and bud off to form electron-dense tubulo/vesicular structures that contained CD1b molecules, but often lacked the expression of MHC class II molecules (Figure 1B). MHC class II molecules tended to be excluded from the newly formed CD1b-containing vesicles, resulting in partial segregation of CD1b and MHC class II in these lysosomal compartments.
Distinct Steady-State Localization of CD1b and MHC Class II in Fully Matured DCs
To evaluate late morphological changes in the MIIC in fully MDCs, IMDCs were stimulated with LPS and harvested after 24 h for transmission electron microscopy. At this time point, the internal membranes of the lysosomes disappeared and the structure now was filled with electron-dense proteinaceous content. The limiting membrane still contains CD1b, whereas virtually no MHC class II molecules were detected (Figures 1C and 2). Considering the major ultrastructural changes and the changes in the presence of resident molecules, we herein propose to denote the altered mature DC lysosome MDL. The limiting membrane of the MDL contained lysosomal resident molecules LAMP1 (Figure 4). Less than 2% of the CD1b-containing compartments seemed to be late endosomes as was shown by double labeling with late endosomal marker M6PR (our unpublished data). We therefore concluded that the MDL represents a subclass of lysosomal compartments.
Figure 2.
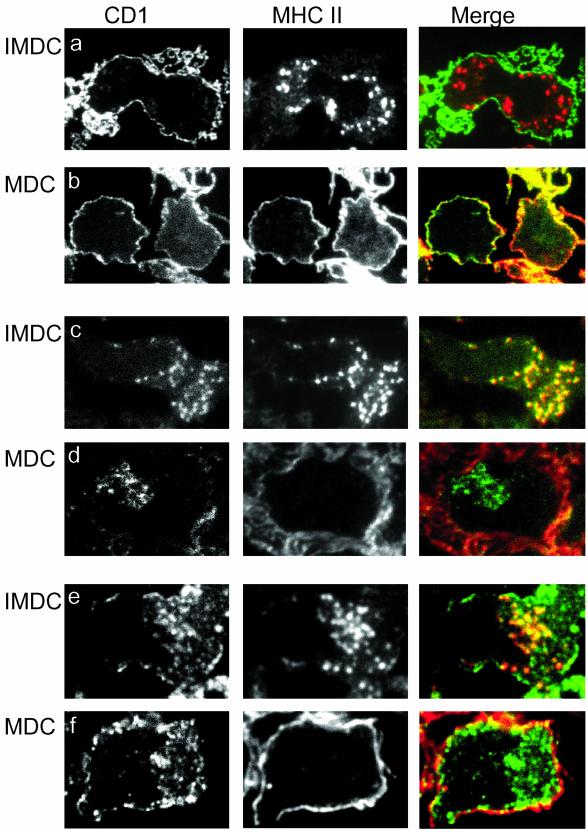
Maturation of DCs causes segregation of CD1b and CD1c from MHC class II molecules. MHC class II was detected together with CD1a (a and b), CD1b (c and d), or CD1c (e and f) by immunofluorescence on semithin cryosections of 280 nm IMDCs cultured in GM-CSF, IL-4 (IMDC; a, c, and e) and after maturation with LPS (MDC; b, d, and f). The CD1 isoforms are presented in the left panel, MHC class II in the middle panel, and merge of the pictures is shown in right panel. After maturation, CD1b and CD1c are located intracellular, and MHC class II molecules are located to the plasma membrane.
Figure 4.
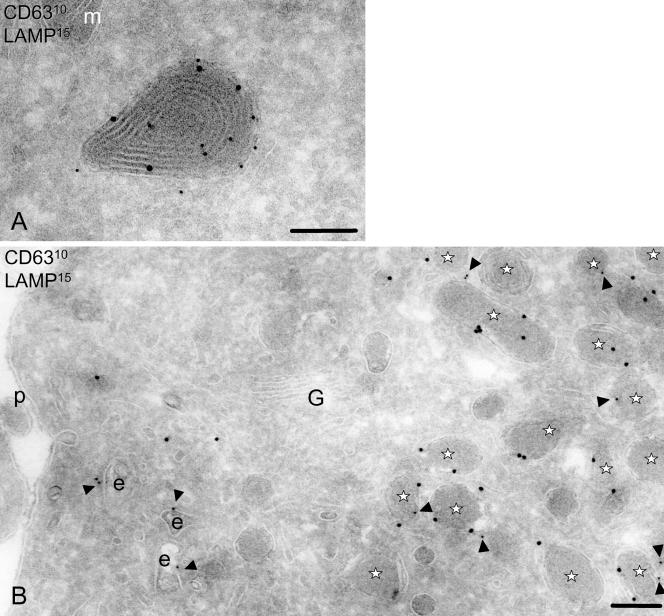
Localization of lysosomal markers CD63 and LAMP1 indicate that the electron-dense MDLs are lysosomal. In IMDCs (A), LAMP1 (15-nm gold) is mostly present on the limiting membrane of the multilamellar lysosomes, whereas CD63 (10-nm gold) can be detected on both internal and limiting membranes. On maturation with LPS (B), both LAMP1 (15 nm) and CD63 (10 nm, arrowheads) can be detected on the membrane of electron-dense structures with altered ultrastructure lacking the internal membrane structures and denoted MDLs and indicated by asterisks. Scale, 200 nm. G, Golgi complex; e, endosomes; m, mitochondria; p, plasma membrane.
Cytoplasmic Tail Determines Internal and Limiting Membrane Lysosomal Localization of CD1b, CD1c, and CD63
In IMDCs, the localization of CD1b is on the limiting membrane of the lysosomal MIIC, whereas the MHC class II is primarily on the internal membranes. This difference in localization within the lysosome might determine the localization after maturation in the MDL. Because the internal membranes are lost in MDL, it is possible that only the molecules that form the limiting membrane of the lysosomes in IMDC are present in the MDL. Other lysosomal residents such as CD63 and LAMP1 seem to be localized preferentially to the internal and the limiting membranes of MIICs, respectively (Table 2). In contrast to CD1b, CD63, and LAMP1, CD1c molecules are almost equally distributed over the internal and limiting membrane domains. The specific enrichment of molecules on the limiting membrane could be caused by the targeting information present in their cytoplasmic tails. CD1b, CD1c, LAMP1, and CD63 all have similar tyrosine motifs in their cytoplasmic tails that is believed to target these molecules to lysosomes (Table 1). It is unknown whether the cytoplasmic motif also determines the microanatomic localization within the lysosome. In contrast to the tyrosine motifs, the lysosomal targeting of MHC class II is dependent on several factors, including the dileucine targeting signal on the beta chain and of the invariant chain. Herein, we analyzed the role of the CD1b cytoplasmic tail in sublocalization in lysosomes. First, we generated chimeras of CD1b, in which the cytoplasmic tail is exchanged for the tail of CD63, which preferentially localizes to the internal membranes of the MIIC. Also, tail chimeras of CD1b with CD1a and CD1c were evaluated to determine whether CD1b chimera's remained at the limiting membrane. As a control, we determined the localization of endogenous CD63.
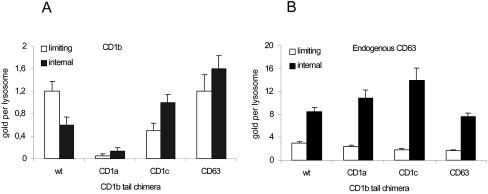
The results showed that the localization of CD1b wild-type and endogenous CD63 is comparable with the distribution determined in IMDC. CD1b molecules with the CD1b tail are targeted to the limiting membrane of the lysosomes. Both the CD63 and the CD1c tail chimeric constructs target CD1b more prominently to the internal membranes (Figure 3). The CD1b/CD1a tail chimera was found in early endosomal compartments and not in lysosomes. These results demonstrate that the cytoplasmic tail of CD1b determines the targeting of CD1b molecules to the limiting membrane of the lysosome.
Figure 3.
CD1b tail targets the CD1b molecule to the limiting membrane of the lysosome. The localization of CD1b wt and CD1b tail chimera within LAMP1-positive compartments was determined in T2 cells transfected with the CD1b constructs (A). As a control, the localization of endogenous CD63 within these transfected cells was determined (B). Immunogold labeling with CD1b- or CD63-specific antibodies was performed on ultrathin cryosections, and the average number (and SE) of gold particles present on the limiting membrane and internal membranes was determined per lysosome.
Localization on the Internal Membranes of Lysosomes Does Not Determine Trafficking during DC Maturation
We next asked whether prior sublocalization in the internal or limiting membranes of lysosomes determines localization after DC maturation. If the internal membranes of the MIIC with MHC class II and its other molecules such as CD63 are all relocated after maturation, one would predict that CD63 would be absent from the MDL. However, immunogold localization of CD63 demonstrated its abundant presence on the electron-dense LAMP1-positive structures in MDC (Figure 4B). Thus, molecules present on the internal membranes of the MIIC can also be detected on the MDL. Also, CD1c molecules could be detected in these compartments but CD1a was not (Figures 2 and 5). Apparently, MHC class II molecules follow a different subcellular pathway in mature dendritic cells than CD1b, CD1c, CD63, or LAMP1. The segregation of the pathway might be at the level of the plasma membrane by constant internalization of CD1b, CD1c, CD63, and LAMP1.
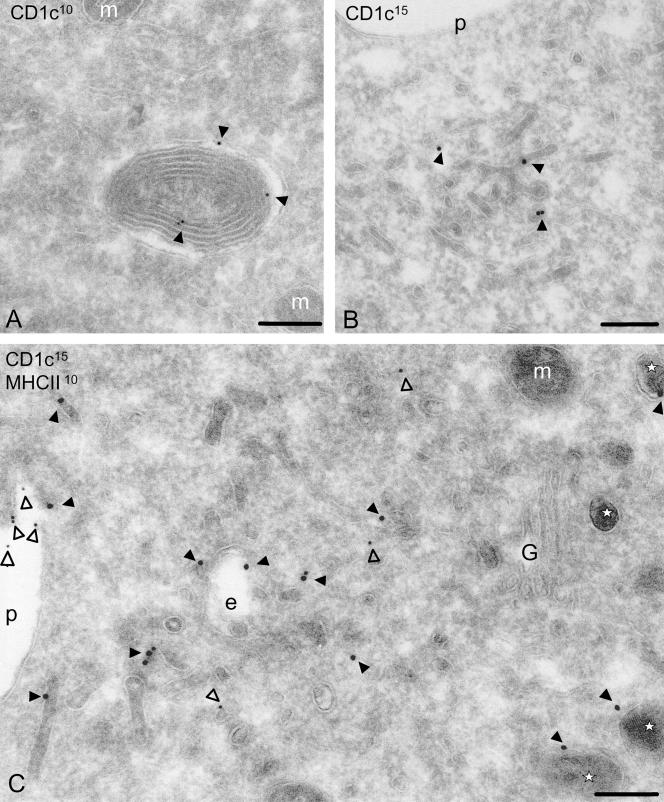
Figure 5.
Localization CD1c in immature and mature DCs. CD1c molecules are present in the multilamellar structures (A; 10-nm gold) and in early endosomes of IMDCs (B; 15-nm gold). Maturation of IMDCs to MDCs (C) shows the presence of CD1c molecules (15-nm gold, closed arrowheads) in MDLs (indicated by asterisks) and in early endosomes, whereas MHC class II molecules (10-nm gold, open arrowheads) are mostly present on the plasma membrane. Scale, 200 nm. p, plasma membrane; G, Golgi complex; e, endosomes; and m, mitochondria.
CD1 Continues to Be Internalized from the Plasma Membrane in Clathrin-Coated Vesicles to a Similar Degree before and after Maturation
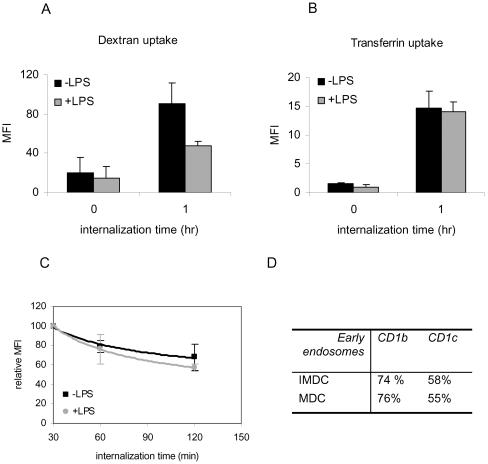
Besides localization to lysosomal MIICs, CD1b and CD1c were detected in early endocytic structures in IMDCs, suggesting internalization from the plasma membrane. However, previous studies on LPS-stimulated DCs have shown a sharp overall reduction of endocytic capacity after DC maturation (Inaba et al., 1993; Sallusto et al., 1995). Thus, we wished to determine whether clathrin-mediated endocytosis was blocked after maturation of DC. Internalization of transferrin conjugated with a fluorescent probe and internalization of CD1b detected with Fab-fragments against CD1b were used as markers for clathrin-mediated endocytosis in both MDC and IMDC. As a control, the fluid phase pinocytic capacity of MDC and IMDC was evaluated using fluorescent-labeled dextran. As expected, the uptake of the pinocytic marker dextran was reduced dramatically after DC maturation (Figure 6A). In contrast, the endocytosis of transferrin was not affected and remained at a constant level (Figure 6B). Also, we determined the localization of the endocytosed transferrin in the cells by using immunofluorescence and as expected, observed that the transferrin almost totally colocalized with EEA1 and transferrin receptor (TfR) (our unpublished data). Importantly, the plasma membrane levels of the Fab-fragments recognizing CD1b decreased in a similar manner during incubation at 37°C, in the absence or presence of LPS, suggesting equal internalization rates in MDC and IMDC (Figure 6C).
Figure 6.
Endocytosis of dextran decreases but transferrin and CD1b internalization
are unaltered after maturation. Receptor-mediated endocytic, pinocytic
capacity and the internalization of CD1b were determined before and after
stimulation with LPS (+LPS). Cells were incubated with fluorescently labeled
dextran (A) or transferrin (B) and analyzed by flow cytometry directly after
addition of the fluorescent probes (0-h incubation on ice) and after 1 h of
uptake (1-h incubation at 37°C). Data represent the average fluorescent
intensities measured in four different experiments, and the error bars
indicate the SE. Maturation with LPS decreased pinocytosis nearly to half the
value it was before maturation. The receptor-mediated endocytosis of
transferrin was not affected by maturation. The uptake of transferrin used was
below the saturation of the receptor as determined by titration experiments
preventing fluid phase uptake. (C) The internalization rate of cell surface
CD1b molecules labeled with Fab′ fragments on immature DCs (▪) and
LPS-maturated DCs ( ). After internalization time of 30, 60, and 120 min,
the average and the SE of relative mean fluorescence intensity are given.
Monocyte-derived DCs of five different donors were used for five independent
experiments. At t = 0, the labeling was not optimal most likely due to low
temperature, and therefore the first measurement at 30 min is used to start
calculating the graphs. The rate of internalization of the Fab-labeled CD1b
molecules is not statistically different before and after maturation. (D)
Percentages of early endosomes positive for CD1b or CD1c in IMDCs and MDCs. At
least 40 different cells were used for detection of early endosomes that were
classified as such using EEA1 labeling.
). After internalization time of 30, 60, and 120 min,
the average and the SE of relative mean fluorescence intensity are given.
Monocyte-derived DCs of five different donors were used for five independent
experiments. At t = 0, the labeling was not optimal most likely due to low
temperature, and therefore the first measurement at 30 min is used to start
calculating the graphs. The rate of internalization of the Fab-labeled CD1b
molecules is not statistically different before and after maturation. (D)
Percentages of early endosomes positive for CD1b or CD1c in IMDCs and MDCs. At
least 40 different cells were used for detection of early endosomes that were
classified as such using EEA1 labeling.
Next, using cryoimmunogold electron microscopy we determined the steady-state localization of CD1a, CD1b, and CD1c molecules to early endocytic compartments in IMDC and MDC. We noted that in contrast to the MIIC, no apparent change in the structure of the early endosomes was observed. CD1b and CD1c but not MHC class II were detected in structures morphologically similar to early endosomes, clathrin-coated pits, and coated vesicles in IMDCs (Sugita et al., 1996) and MDCs (Figure 5B). To ensure that these structures were early endosomes, colocalization studies with the TfR and EEA1 were performed. Both markers were detected in these CD1b-containing compartments. Early endosomes (immunogold labeled with antibodies against EEA1) in both unstimulated and LPS-stimulated DCs was counted, and the percentage in which colocalization with CD1b or CD1c occurred was determined. These percentages demonstrated that the amount of EEA-positive early endosomes in which CD1b or CD1c is present has not changed after maturation. Also, for CD1a localization, no quantifiable difference was noticed (our unpublished data). In IMDC and MDC, CD1a expression remained restricted to the plasma membrane and early endocytic tubulovesicular structures. Thus, we detected no differences in the occurrence of CD1 molecules in the early endocytic structures in mature compared with immature DCs, confirming the internalization data using fluorescent probes. Together, these studies emphasize that in contrast to MHC class II, CD1b (and c) molecules continue to be mainly in lysosomes, despite the drastic changes that occur in these structures. Such persistence in lysosomes, even after DC maturation, may be accounted for in part by the continued internalization from the cell surface.
No Up-Regulation of CD1 Cell Surface Expression after DC Maturation
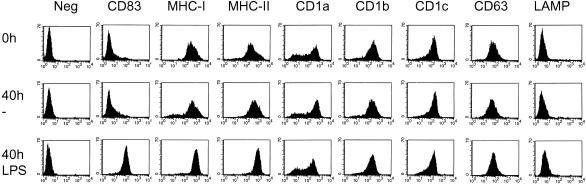
Maturation of DCs has been shown to induce increased levels of MHC class II expression on the cell surface. Because the subcellular trafficking of CD1 molecules after maturation seems to differ dramatically from the MHC class II pathway, involving lysosomes and early endocytic structures, the effect of maturation on cell surface levels of CD1 was determined. IMDC were stimulated with LPS to develop into MDC. After LPS stimulation, virtually all the cells strikingly up-regulated the surface expression of CD83, one of the most reliable markers for DC maturation, confirming that all cells obtained in our experiments were MDCs (Figure 7). These cells also expressed markedly increased levels of MHC class II molecules on the cell surface. In contrast, up-regulation of cell surface expression was not readily detected for CD1 molecules. The surface expression of CD1a was apparently slightly down-regulated, whereas that of CD1b and CD1c was not changed after LPS stimulation. Similarly, the surface expression of LAMP1 and CD63 remained low and was not significantly altered. These data demonstrate that despite the fact that MHC class II, CD1b, CD1c, LAMP1, and CD63 were expressed in the same lysosomal compartments in IMDC, the up-regulation of surface expression after DC maturation was detected only for MHC class II, but not for other lysosomal residents. This finding further underscores how intracellular trafficking pathways of CD1b and CD1c differ from MHC class II.
Figure 7.
LPS stimulation matures DCs but did not raise cell surface levels of CD1 molecules. Monocyte-derived DCs cultured in GM-CSF and IL-4 were analyzed by flow cytometry to determine the expression of cell surface markers. Similar analyses were done on cells stimulated by addition of LPS for 48 h and control cells kept in GM-CSF, IL-4 medium for that period. Maturation marker CD83 is like MHC class II up-regulated in LPS-treated cells. Cell surface levels are not raised for CD1a, CD1b, and CD1c and lysosomal markers CD63 and LAMP1 on the LPS-treated cells. Data shown are representative for six separate experiments in which monocytes from different donors were used.
DISCUSSION
Our study detected a striking difference in CD1 and MHC class II trafficking during DC maturation. CD1b and CD1c molecules as well as MHC class II molecules were detected in MIICs of DCs at the immature stage, but after maturation only CD1b and CD1c molecules but not MHC class II remained in lysosomal compartments. We demonstrated that the cytoplasmic tail of CD1b mediates the localization to the limiting membrane of lysosomes. However, this localization does not predict localization to the limiting membrane of the MDLs. These MDLs also contain CD1b, LAMP1, CD1c, and CD63, the latter deriving from the internal membranes of the MIICs. This study shows the process of lysosomal sorting of different antigen-presenting molecules in mammalian cells and demonstrates that maturation does not exert the same influence on lipid presentation as peptide presentation.
It has already been shown that the internal storage of MHC class II molecules is relocated to the cell surface where the MHC molecules reside with increased stability (Cella et al., 1997; Pierre et al., 1997; Turley et al., 2000). In addition, it was shown that the internalization of MHC class II molecules in mature DCs is largely blocked after LPS stimulation (Kleijmeer et al., 2001). The blockage of MHC class II internalization on matured cells has, in part, been explained by a decreased rate of total endocytosis in LPS-maturated DC (Cella et al., 1997). Indeed, we confirmed that the uptake of FITC-dextran was decreased after maturation. However, receptor-mediated endocytosis detected by the uptake of transferrin by its receptor, revealed no statistical difference between MDCs and IMDCs. Importantly, we found that the internalization rate of CD1b in IMDCs and MDCs also was similar. Consistent with these results, it has previously been shown that maturation of DCs caused no decrease in clathrin-coated pits and vesicles (Garrett et al., 2000). These results indicate that clathrin-mediated endocytosis is unimpaired in MDCs, enabling the cells to internalize along the clathrin-coated pit pathway. The internalization must be selective and discriminate between molecules that stay on the surface (like MHC class II) and molecules that need to be internalized. Because the surface levels of CD1b and CD1c do not change appreciably, we suggest that CD1b and CD1c are internalized at the same rate as their delivery to the plasma membrane. The unchanged presence of CD1b and CD1c in coated vesicles and early endosomes both before and after maturation supports no substantial difference in their rate of internalization.
The present study, however, cannot distinguish whether these lysosomal molecules are retained in the lysosome during the process of maturation or accumulated after internalization from the plasma membrane. The transition of CD63 from the internal membranes of the MIIC to the limiting membrane in the MDL suggests that the molecules are not stably at one location.
In conclusion, our studies demonstrate that CD1b and CD1c segregate from MHC class II after DC stimulation. The different CD1 isoforms and MHC class II molecules are present on the cell surface and will interact with T cells at this site. It has been assumed that professional antigen-presenting cells such as the DCs consume their antigens or pathogens in the peripheral tissues and process them into presentable antigens so that DCs can interact with the T cells in lymphoid tissue. However, the continuous recycling of the CD1 molecules suggests that the lipid antigens may be loaded in CD1 both during mature and immature stages. Therefore, presentation of lipid antigens might occur in immature DCs at the peripheral tissues were T cells reside and precede MHC class II presentation, and continue after the DC matures and travels to the lymph node. Antigen presentation by MHC class II may be focused on the later stages of the adaptive immune response and require DC maturation to function. In contrast, CD1 is likely to function on DCs that are capable of sampling and presenting lipid antigens throughout their maturational life span.
Acknowledgments
We thank Jacques Neefjes and Crislyn D'Souza-Schorey for helpful suggestions, Nico Ong for photographic work, and Erik Bos for technical assistance. This work was supported by the Netherlands Leprosy Relief (International Federation of Anti-Leprosy Associations 702.02.20) and National Institutes of Health grant R31-AI28973.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-11-0744. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0744.
References
- Beckman, E.M., Porcelli, S.A., Morita, C.T., Behar, S.M., Furlong, S.T., and Brenner, M.B. (1994). Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372, 691–694. [DOI] [PubMed] [Google Scholar]
- Briken, V., Jackman, R.M., Dasgupta, S., Hoening, S., and Porcelli, S.A. (2002). Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J. 21, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella, M., Engering, A., Pinet, V., Pieters, J., and Lanzavecchia, A. (1997). Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388, 782–787. [DOI] [PubMed] [Google Scholar]
- Gadola, S.D., Zaccai, N.R., Harlos, K., Shepherd, D., Castro-Palomino, J.C., Ritter, G., Schmidt, R.R., Jones, E.Y., and Cerundolo, V. (2002). Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat. Immunol. 3, 721–726. [DOI] [PubMed] [Google Scholar]
- Garrett, W.S., Chen, L.M., Kroschewski, R., Ebersold, M., Turley, S., Trombetta, S., Galan, J.E., and Mellman, I. (2000). Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334. [DOI] [PubMed] [Google Scholar]
- Gumperz, J.E., and Brenner, M.B. (2001). CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 13, 471–478. [DOI] [PubMed] [Google Scholar]
- Honing, S., Griffith, J., Geuze, H.J., and Hunziker, W. (1996). The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 15, 5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Inaba, K., Inaba, M., Naito, M., and Steinman, R.M. (1993). Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman, R.M., Stenger, S., Lee, A., Moody, D.B., Rogers, R.A., Niazi, K.R., Sugita, M., Modlin, R.L., Peters, P.J., and Porcelli, S.A. (1998). The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351. [DOI] [PubMed] [Google Scholar]
- Kleijmeer, M., et al. (2001). Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J. Cell Biol. 155, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia, A., and Sallusto, F. (2001). Regulation of T cell immunity by dendritic cells. Cell 106, 263–266. [DOI] [PubMed] [Google Scholar]
- Mellman, I., and Steinman, R.M. (2001). Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258. [DOI] [PubMed] [Google Scholar]
- Neefjes, J.J., Stollorz, V., Peters, P.J., Geuze, H.J., and Ploegh, H.L. (1990). The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell 61, 171–183. [DOI] [PubMed] [Google Scholar]
- Peters, P.J., and Hunziker, W. (2001). Subcellular localization of Rab17 by cryo-immunogold electron microscopy in epithelial cells grown on polycarbonate filters. Methods Enzymol. 329, 210–225. [DOI] [PubMed] [Google Scholar]
- Peters, P.J., Neefjes, J.J., Oorschot, V., Ploegh, H.L., and Geuze, H.J. (1991). Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments [see comments]. Nature 349, 669–676. [DOI] [PubMed] [Google Scholar]
- Peters, P.J., Raposo, G., Neefjes, J.J., Oorschot, V., Leijendekker, R.L., Geuze, H.J., and Ploegh, H.L. (1995). Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes. J. Exp. Med. 182, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre, P., Turley, S.J., Gatti, E., Hull, M., Meltzer, J., Mirza, A., Inaba, K., Steinman, R.M., and Mellman, I. (1997). Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388, 787–792. [DOI] [PubMed] [Google Scholar]
- Porcelli, S., Morita, C.T., and Brenner, M.B. (1992). CD1b restricts the response of human CD4–8-T lymphocytes to a microbial antigen. Nature 360, 593–597. [DOI] [PubMed] [Google Scholar]
- Sallusto, F., Cella, M., Danieli, C., and Lanzavecchia, A. (1995). Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling, P.A., Chatterjee, D., Porcelli, S.A., Prigozy, T.I., Mazzaccaro, R.J., Soriano, T., Bloom, B.R., Brenner, M.B., Kronenberg, M., and Brennan, P.J. (1995). CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230. [DOI] [PubMed] [Google Scholar]
- Sugita, M., Cao, X., Watts, G.F., Rogers, R.A., Bonifacino, J.S., and Brenner, M.B. (2002). Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity 16, 697–706. [DOI] [PubMed] [Google Scholar]
- Sugita, M., Grant, E.P., van Donselaar, E., Hsu, V.W., Rogers, R.A., Peters, P.J., and Brenner, M.B. (1999). Separate pathways for antigen presentation by CD1 molecules. Immunity 11, 743–752. [DOI] [PubMed] [Google Scholar]
- Sugita, M., Jackman, R.M., van Donselaar, E., Behar, S.M., Rogers, R.A., Peters, P.J., Brenner, M.B., and Porcelli, S.A. (1996). Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science 273, 349–352. [DOI] [PubMed] [Google Scholar]
- Sugita, M., van Der, W.N., Rogers, R.A., Peters, P.J., and Brenner, M.B. (2000). CD1c molecules broadly survey the endocytic system. Proc. Natl. Acad. Sci. USA 97, 8445–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley, S.J., Inaba, K., Garrett, W.S., Ebersold, M., Unternaehrer, J., Steinman, R.M., and Mellman, I. (2000). Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288, 522–527. [DOI] [PubMed] [Google Scholar]
- Watts, C. (1997). Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 15, 821–850. [DOI] [PubMed] [Google Scholar]
- Zeng, Z., Castano, A.R., Segelke, B.W., Stura, E.A., Peterson, P.A., and Wilson, I.A. (1997). Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277, 339–345. [DOI] [PubMed] [Google Scholar]