Abstract
Multiple myeloma (MM) is a B cell malignancy characterized by the expansion of monoclonal Ig-secreting plasma cells with low proliferative activity. It is postulated that inhibition of physiologic cell death is an underlying factor in the pathophysiology of MM. The development of chemoresistance is a common feature in patients with MM. In the present studies, hexamethylene bisacetamide (HMBA), a hybrid polar compound that is a potent inducer of terminal differentiation of various transformed cells, is shown to inhibit the growth of several human myeloma cell lines (ARP-1, U266, and RPMI 8226), including doxorubicin-resistant RPMI 8226 variants that overexpress the multidrug-resistance gene, MDR-1, and its product, p-glycoprotein. In addition to growth arrest and suppression of clonogenicity, HMBA induces apoptosis both in freshly isolated human myeloma cells and in cell lines, as determined by morphologic alterations, cell cycle distribution and endonucleosomal DNA fragmentation. Further, HMBA decreases BCL-2 protein expression in myeloma cells within 12–48 hr. Overexpression of BCL-2 protein in ARP-1 cells confers resistance to HMBA-induced apoptosis. Taken together, these data suggest that HMBA is a potent inducer of apoptosis in human myeloma cells, which may act through suppressing the anti-apoptotic function of the bcl-2 gene. HMBA, and related hybrid polar compounds, may prove useful in the management of this presently incurable disease.
Induced differentiation of transformed cells by hybrid polar compounds, such as hexamethylene bisacetamide (HMBA), is regularly associated with a loss of proliferative capacity (1). This is generally associated with cell cycle arrest in G1 (2). In some cell lineages, inducer-mediated loss of proliferation reflects programmed cell death (3–5).
Multiple myeloma (MM) is an incurable B cell malignancy characterized by the accumulation of large numbers of clonal plasma cells. In general, these are morphologically well differentiated cells that do not grow rapidly (6). While initially sensitive to chemotherapy, in virtually all patients the disease becomes chemoresistant. Overexpression of the multidrug resistant gene and its product, P-glycoprotein, is frequently found in myeloma cells that have become chemoresistant (7). In addition, in many MM patients, serum interleukin 6 (IL-6) levels are elevated and plasma cells express high levels of BCL-2 protein. Both IL-6 and BCL-2 proteins are known inhibitors of apoptosis, and it has been suggested that their overexpression contributes to resistance to therapy (6, 9–13). A major defect in MM may, indeed, be related to an inhibition of the malignant cells’ physiologic rate of apoptosis (6).
In the present study we report that HMBA induces apoptosis in several human myeloma cell lines as well as in freshly isolated human myeloma cells. This effect of HMBA is associated with a decrease in BCL-2 protein levels. Overproduction of BCL-2 protein, by transfection of an exogenous bcl-2 gene, renders myeloma cells resistant to HMBA-induced apoptosis.
MATERIALS AND METHODS
Cell Lines and Culture Conditions.
U266 and RPMI 8226 human MM-derived cell lines were obtained from the American-Type Culture Collection. The ARP-1 MM cell line and the Dox-6 and Dox-40 variants of the RPMI 8266 cell line were provided by J. Hardin (Arkansas Cancer Research Center, Little Rock) and W. Dalton (Arizona Cancer Center, Tucson), respectively. Both Dox-6 and Dox-40 overexpress multidrug resistant and p-glycoprotein (7). Cells were maintained in RPMI 1640, supplemented with 10% fetal bovine serum and 2 mM glutamine, and refreshed every 3–4 days.
Growth Conditions and Proliferation Assay.
Cells were cultured at 2 × 105 cells/ml in the presence or absence of 5 mM HMBA (Sigma) for varying times. Cell density was determined by a colorimetric assay utilizing 3-(4,5-dimethylthiazol-2yl)-2, 5-diphenyl tetrasodium bromide (Chemicon).
Myeloma Colony Assay.
Bone marrow mononuclear cells (2 × 105) were obtained by density gradient centrifugation over Ficoll-Hypaque (Pharmacia) and suspended in 1 ml of RPMI 1640 medium containing 20% fetal calf serum, 2 mM glutamine, 50 μM 2-mercaptoethanol and 0.8% methylcellulose (Stem Cell Technologies, Paisley, Scotland). Irradiated human fetal fibroblasts were added at a concentration of 105 cells per ml. The mixtures were plated onto 35-mm Petri dishes and examined immediately and 24 hr later to document that all cells were plated as a single cell suspension. In some experiments, irradiated bone marrow mononuclear cells were used to prevent aggregate formation. The cultures were incubated for 28 days in a humidified chamber with 5% CO2 in air at 37°C. The medium was replaced every 2 weeks with an equal amount of fresh medium. This assay allows the growth of clonal plasma cells from 70% of the patients. There is a linear correlation between the number of cells seeded and number of colonies generated (8). Colonies (containing ≥50 cells) were directly scored using an inverted microscope.
Morphological Assessment of Apoptosis by Light Microscopy.
Following exposure to HMBA, cells were collected, washed with PBS (pH 7.4), and cytospun onto slides. Cytospins were sequentially fixed in methanol overnight, air-dried, and stained with DiffQuick stain.
DNA Strand Break Labeling.
Apoptotic cells were assayed by determining DNA strand breaks labeled by the terminal transferase assay employing BrdUTP and a fluorescein isothiocyanate-conjugated anti-BrdUrd mAb, as described (14). At each time point, indicated below, 5 × 106 cells were fixed and assayed. Control samples included: (i) untreated cells; (ii) cells incubated with the reaction mixture without the enzyme. Cellular fluorescence was measured using a FACScan flow cytometer (Becton Dickinson) and at least 20,000 cells were analyzed by the cellquest software (Becton Dickinson).
DNA Fragmentation.
Cells (5 × 105) were washed with PBS and pelleted by centrifugation. The cell pellets were then treated for 10 sec with 50 μl lysis buffer (1% Nonidet P-40 in 20 mM EDTA, 50 mM Tris⋅HCl, pH 7.5). Following 5 min centrifugation at 1,600 × g the supernatants were collected and extracted with 50 μl of the same lysis buffer. The supernatants were brought to 1% SDS and treated for 2 hr with 5 μg/μl RNase A (at 56°C) followed by digestion with 2.5 μg/μl proteinase K for 2 hr at 37°C (15). After addition of 1/2 vol of 10 M ammonium acetate, the DNA was precipitated with 2.5 vol of ethanol and dissolved in gel-loading buffer, and the DNA fragments were separated by electrophoresis in 1% agarose gel (15).
Immunohistochemistry.
To evaluate BCL-2 protein expression by immunohistochemical assay, cytospin slides fixed in acetone/methanol were incubated with the primary antibody (anti-BCL-2, Dako) and with isotype-matched mouse IgG control, followed by sequential 15 min incubations with biotinylated anti-mouse Ig and alkaline phosphatase-labeled streptavidin. The phosphatase activity was visualized with new fuschin chromogen and then counterstained with hematoxylin (12).
Western Blot Analysis.
To analyze the BCL-2 protein level in myeloma cells, 107 cells were washed in PBS and resuspended in lysis buffer, and sonicated. Protein determinations were done using the Bradford reaction (16). Protein (50 μg, or as otherwise indicated) were electrophoresed in 12% PAGE gels. Protein was electrotransferred to Immobilin P filters and blocked with 5% BSA. The membrane was incubated with anti-human BCL-2 antibody (Dako) and then horseradish perioxidase-conjugated goat anti-mouse antibody was added. Filters were washed again and developed using enhanced chemiluminscence imaging system (Amersham).
bcl-2 DNA Transfections and in Situ End Labeling.
ARP-1 cells (107 cells per ml) were transfected with 10 μg of either human bcl-2 pSFFV-neo or pSFFV-neo empty plasmid DNA (kindly provided by S. J. Korsmeyer, Washington University School of Medicine, St. Louis, MO.) by electroporation at 275 V and 1,180 μF, using a Cell-Porator (GIBCO/BRL). After 48 hr, bulk transfectants were selected in 800 μg/ml G418 (Life Technologies, Grand Island, NY). Individual clones were then isolated by cell sorting using flow cytometry and characterized for BCL-2 expression. Expression of BCL-2 was determined by Western blot analysis using the mouse anti-human BCL-2 mAb as described above. Following treatment with HMBA, apoptosis was assessed using the in situ end labeling method as described (17). Briefly, cytospin slides were fixed in ethanol for more than 4 hr at 4°C, rinsed, and air-dried. The slides were then incubated for 90 min at 15°C with buffer A (50 mM Tris·HCl, 5 mM MgCl2, 10 mM 2 mercaptoethanol/0.005% BSA, pH 7.5; Chemicon). To this buffer, 0.01 mM deoxyadenosine triphosphate, deoxycytidine triphosphate, deoxyuridine triphosphate, and biotin-11-UTP were added together with 4–10 units/ml of Klenow fragment of DNA polymerase. Nucleotide incorporation was visualized using avidin-conjugated horseradish peroxidase (17) Results are expressed as percent of labeled cells among 200 cells scored per slide.
RESULTS
The Effect of HMBA on MM Cells in Vitro.
To examine whether HMBA affects the growth of human myeloma cell lines, U266 and ARP-1, cells of each type were cultured in the presence or absence of the compound. HMBA, in a dose- and time-dependent manner, causes cell growth inhibition of both these cell lines (Fig. 1); by 4–5 days of culture with 5 m HMBA there was 90% or more inhibition of cell growth.
Figure 1.
Effect of HMBA on myeloma cell line growth. ARP-1 or U266 cells were incubated at 105 cells per ml in RPMI 1640 with 10% FBS. (A) cells were incubated in the presence of the indicated concentration of HMBA. Viable cell numbers were determined at 72 hr by trypan blue exclusion. Inhibition of cell growth is expressed relative to controls. (B) Cells were cultured in the presence of 5 mM HMBA for the indicated periods of time. Inhibition of cell growth is expressed relative to controls.
To determine whether the acquisition of the multidrug resistant phenotype by myeloma cells alters their response to HMBA, two cell lines, RPMI-DOX-6 and RPMI-DOX-40, exhibiting low and high levels of resistance to Doxorubicin, respectively, were cultured in the presence of increasing concentrations of HMBA. Dox-6 and Dox-40 cells retain their sensitivity to growth inhibition by HMBA (data not shown).
HMBA Induces Apoptosis in Human Myeloma Cells.
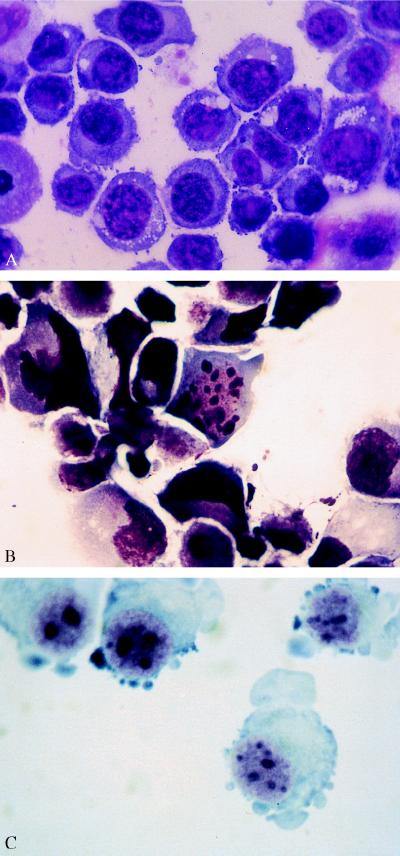
To test whether HMBA-mediated growth inhibition of myeloma cells is associated with the induction of apoptosis, U266 cells cultured in the presence of 5 mM HMBA for 24 hr, were Giemsa-stained and assessed morphologically. HMBA-cultured cells display the characteristic features of apoptotic cell death, namely nuclear chromatin condensation and the appearance of typical apoptotic bodies (Fig. 2 A and B). Similar morphologic alterations were noted when ARP-1, RPM1, RPM1-DOX-6, and RPMI-DOX-40 cells were cultured in the presence of HMBA (data not shown).
Figure 2.
Morphological alterations in cells cultured with HMBA. (A) U266 cells cultured without HMBA for 24 hr. (B) U266 cells, cultured with HMBA for 24 hr, display nuclear condensation and characteristic apoptotic bodies. (C) Myeloma cells freshly isolated from a patient and grown in the presence of 5 mM HMBA for 24 hr, exhibit apoptotic bodies.
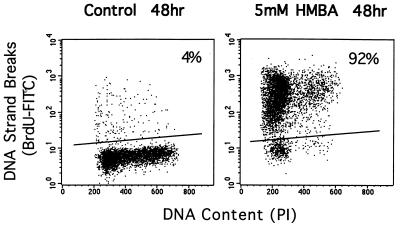
Another characteristic feature of apoptotic cells is the presence of DNA strand breaks. To assay for DNA fragmentation, ARP-1 cells were labeled with BrdUTP, as described in Materials and Methods. After 48 hr in culture without inducer, 4% of cells are labeled with BrdUTP, whereas 92% of the cells cultured with 5 mM HMBA were labeled, indicative of accumulating DNA strand breaks consistent with apoptosis (Fig. 3) (14). While most apoptotic cell death occurred in cells accumulating in G1, apoptosis was not restricted to cells in G1. Cells in S and G2M also exhibited DNA breaks (Fig. 3). Another characteristic of cells undergoing apoptosis is progressive accumulation of cells in the so-called sub-G1 (<2C DNA content) fraction of the cell cycle as measured by flow microfluorometric analysis of DNA content (18). While in control cells, 4–6% of the cells were found in sub-G1, 16% and 35% of HMBA-treated cells were found in sub-G1 after 48 and 72 hr of culture, respectively (data not shown).
Figure 3.
DNA strand breaks induced by HMBA in ARP-1 Cells: Dotblot plots of DNA content (x axis) and DNA strand break labeling by BrdUTP (y axis). Cells in G1 fall between 100 and 250 on the x axis, S-phase cells and G2/M-phase cells lie between 250 and 600 on the x axis.
HMBA-induced apoptosis of myeloma cells was further evaluated by analyzing the DNA fragmentation that is a characteristic of apoptotic cells (15, 19). Using DNA-gel electrophoresis, we have found that ARP-1 cells cultured in the presence of 5 mM HMBA display the characteristic pattern of DNA fragmentation, as early as 24 hr (data not shown).
Effect of HMBA on Freshly Isolated Human Myeloma Cells.
Mononuclear cells from the bone marrow of three patients with advanced MM were cultured in the presence of 5 mM HMBA. After 48 to 72 hr of culture with HMBA, cells exhibited the morphologic features of apoptosis (Fig. 2C). Bone marrow aspirates from three different patients were seeded at 5 × 105 cells per ml in a semisolid medium, employing the myeloma clonogenic assay (see Materials and Methods). While culture of cells without HMBA for 4 weeks generated 20–110 colonies per dish, no myeloma cell colonies were detected if 5 mM HMBA was present in the culture medium. These results indicate that HMBA suppresses the in vitro clonogenic capacity of freshly isolated human myeloma cells.
HMBA Down-Regulates BCL-2 Expression in Myeloma Cell Lines and in Freshly Isolated Myeloma Cells.
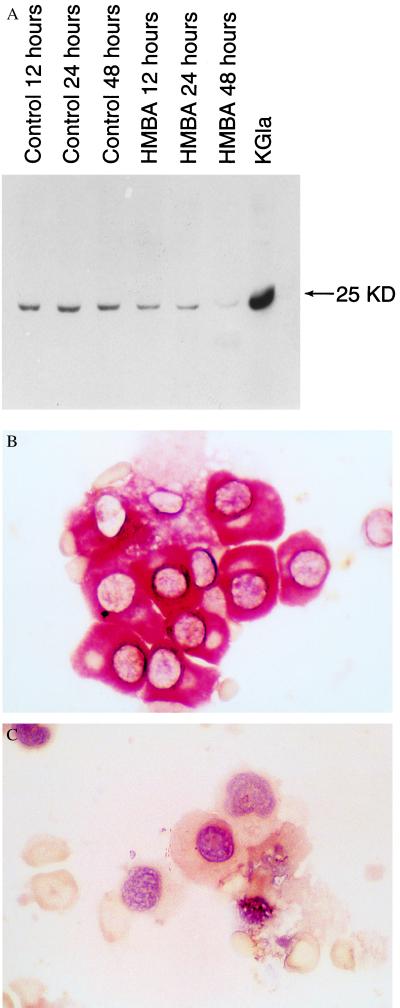
We next asked whether HMBA-mediated apoptosis in myeloma cells is associated with modulation of BCL-2 protein expression. Using Western blot analysis, we found that BCL-2 protein levels decrease as early as 12 hr after onset of culture with HMBA and then decline further by 48 hr of culture (Fig. 4A). We also found a reduction of BCL-2 expression, by immunohistochemistry, in myeloma cells freshly isolated from three patients and cultured for 24 hr in the presence of 5 mM HMBA (Fig. 4 B and C).
Figure 4.
BCL-2 expression in ARP-1 cells. (A) Western blot analysis of BCL-2 protein level in ARP1 cells cultured without and with HMBA for 12, 24, and 48 hr. KG1a is a leukemia cell line overexpressing BCL-2, used as a positive control. (B) Myeloma colony from a patient positively stained with anti-BCL-2 mAb. (C) 24 hr after exposure to 5 mM HMBA, the cells no longer stain for BCL-2 protein and display apoptotic morphological features.
Overexpression of BCL-2 Protein in Myeloma Cells Inhibits HMBA-Induced Apoptosis.
We next asked whether cells expressing higher levels of BCL-2 protein were resistant to HMBA-induced apoptosis. ARP-1 cells were transfected with plasmid DNA; bcl-2 PSFFV neo, by electroporation (20). Transfected cells expressed ≈10-fold higher basal level of BCL-2 protein compared with parental cells (Western blot). Culture of these transfectants with 5 mM HMBA resulted in G1-arrest, as in parental cells, however these cells were resistant to HMBA-induced apoptosis, as assessed by cell viability, morphology and by in situ end labeling of DNA. After 48 hr in culture, parental cells without HMBA showed 9% apoptosis whereas 98% of the cells cultured with HMBA were apoptotic. By comparison, bcl-2 transfectants had 5% and 12% apoptotic cells without and with HMBA, respectively. Both the bulk transfectants as well as the subclones displayed resistance to HMBA-induced apoptosis.
DISCUSSION
In these studies we demonstrate that the hybrid polar compound, HMBA, which is a potent inducer of differentiation of various transformed cells (1, 2), inhibits the growth of human myeloma cells and induces morphological and biochemical changes, consistent with apoptotic cell death. These include DNA fragmentation, accumulation of cells in sub G1 fraction of the cell cycle and morphological evidence of apoptotic nuclear bodies. These effects of HMBA were observed with myeloma cell lines and freshly derived myeloma cells from patients. Apoptotic cells induced by HMBA accumulated predominantly but not exclusively in G1. We find evidence that apoptosis is preceded by down-regulation of BCL-2 protein both in human myeloma cell lines and in freshly isolated human myeloma cells. Overexpression of BCL-2 protein, by transfection of the bcl-2 gene into the human myeloma cell line, ARP-1, renders these cells resistant to HMBA-induced apoptosis. These findings suggest that down-regulation of BCL-2 protein is one of the factors playing an important role in the pathway of HMBA-induced apoptosis of MM cells. Experiments exploring the possible involvement of other pro- and anti-apoptotic genes (12) are underway. Our present data suggest that HMBA does not affect the expression of Bax or BCLXL proteins (personal observations).
Several lines of evidence suggest that failure to undergo apoptosis may contribute to the pathophysiology of MM. Clinically, MM resembles follicular lymphoma, a low grade B cell malignancy also characterized by an inhibited apoptotic response to chemotherapy (13). In both diseases the anti-apoptotic gene, bcl-2, is strongly expressed. While in follicular lymphoma this expression of bcl-2 is attributed to a translocation (14, 18) present in 80% of patients, this translocation is rare in MM (21). Furthermore, no major structural DNA abnormalities involving the bcl-2 gene have been found in MM (21). In addition to BCL-2 protein expression, many patients with MM exhibit increased serum IL-6 levels, a cytokine capable of blocking apoptosis of malignant plasma cells (17). Furthermore, corticosteroids, such as dexamethasone that are frequently effective, at least initially, in the treatment of MM, appear to exert their effect, in part, through induction of apoptosis (17, 22).
It is currently unknown whether the processes of induced differentiation and induced apoptosis are regularly coupled and whether the bcl-2 gene plays a role in modulating both cellular responses. Redirection of bcl-2 expression to cortical thymocytes, in transgenic mice, results in protection of immature CD4+8+ thymocytes from apoptosis as well as in an increased proportion of more mature CD4−8+ and CD3 thymocytes (23). Retinoid acid induced differentiation of HL-60 myeloid leukemia cells into granulocytes, and their subsequent apoptosis, is associated with decreased BCL-2 protein (24–25). Overexpression of BCL-2 protein in HL60 cells blocks apoptosis but not differentiation (25). Transfection of the human bcl-2 gene into an IL-3-dependent multipotent hematopoietic cell line allows these cells to survive in the absence of IL-3, and to undergo multilineage differentiation (26).
The responses to HMBA, namely differentiation or apoptosis, appear to be cell-type dependent. Specific differences in gene expression may be responsible for directing cells toward differentiation or toward apoptosis. Recently we found that HMBA causes a human bladder carcinoma cell line, overexpressing the human papilloma virus-16 E7 gene (T24E7), to become apoptotic, whereas the parental T24 cells lacking E7 expression, arrest in G1 phase and undergo morphological maturation, in response to HMBA (5). HMBA consistently induces murine erythroleukemia cells to terminal erythroid differentiation and cell cycle arrest with minimal evidence of apoptosis (2, 27). The present evidence, taken together with previous observations (1–2, 5), suggests that induction of cell growth arrest, differentiation and/or apoptosis, in response to HMBA, may be determined, in part, by the level of various regulatory proteins such as BCL-2, and cell cycle regulatory proteins such as E2F, RB, and CDK4 (2, 5).
The clinical use of HMBA has been studied in patients with myeloid leukemia and the myelodysplastic syndrome (28). Continuous intravenous infusion of HMBA for 10 days resulted in an ≈30% rate of complete or partial remission and in some of these cases, direct evidence for in vivo induction of cellular differentiation was obtained. The findings that HMBA is a potent inducer of apoptosis in human myeloma cell lines, as well as, in freshly isolated myeloma cells, and that multidrug resistant cells retain sensitivity to HMBA, suggest that this and related hybrid polar compounds might prove useful in the management of MM.
Acknowledgments
We thank Drs. J. Hardin and W. Dalton for providing us with the ARP-1 MM cell line and the Dox-6 and Dox-40 variants of the RPM1 8266 cell line, respectively.
ABBREVIATIONS
- MM
multiple myeloma
- HMBA
hexamethylene bisacetamide
- IL
interleukin
References
- 1.Michaeli J, Rifkind R A, Marks P A. Cancer Chemother Biol Response Modifiers. 1992;13:287–307. [PubMed] [Google Scholar]
- 2.Marks P A, Richon V M, Kiyokawa H, Rifkind R A. Proc Natl Acad Sci USA. 1994;91:10251–10254. doi: 10.1073/pnas.91.22.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams G T. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- 4.Williams G T, Smith C A. Cell. 1993;74:777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- 5.Richon V M, Russo P, Venta-Perez G, Cordon-Cardo C, Rifkind R A, Marks P A. Cancer Res. 1997;57:2789–2798. [PubMed] [Google Scholar]
- 6.Niesvizky R, Siegel D, Michaeli J. Blood Rev. 1993;7:24–33. doi: 10.1016/0268-960x(93)90021-u. [DOI] [PubMed] [Google Scholar]
- 7.Dalton W S, Salmon S E. Hematol Oncol Clin North Am. 1992;6:383–393. [PubMed] [Google Scholar]
- 8.Zhang X, Childs B, Siegel D, Rameche Y, Zelenetz A, Michaeli J. Blood. 1994;84:175a. [Google Scholar]
- 9.Lichtenstein A, Tu Y, Pady C, Vescio R, Berenson J. Cell Immunol. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 10.Pettersson M, Jernberg-Wiklund H, Larsson L-G, Sundstrom C, Givol I, Tsujimoto Y, Nilsson K. Blood. 1992;79:495–502. [PubMed] [Google Scholar]
- 11.Tu Y, Xu F-H, Liu J, Vescio R, Berenson J, Fady C, Lichtenstein A. Blood. 1996;88:1805–1812. [PubMed] [Google Scholar]
- 12.Miyashita T, Reed J C. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 13.Hannum Y A. Blood. 1997;89:1845–1853. [PubMed] [Google Scholar]
- 14.Li X, Darzynkiewicz Z. Cell Prolif. 1995;28:571–579. doi: 10.1111/j.1365-2184.1995.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann M, Lorenz H-M, Voll R, Grunke M, Woith W, Kalden J R. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford M M. Ann Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Ziao H, Epstein J. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 18.Gorezyca W, Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. Cancer Res. 1993;53:3186–3192. [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben-Sasson S A. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarze M M K, Hawley R G. Cancer Res. 1995;55:2262–2265. [PubMed] [Google Scholar]
- 21.Landanyi M, Wang S, Niesvizky R, Feiner H, Michaeli J. Am J Pathol. 1992;141:949–953. [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia U, Traganos F, Darzynkiewicz Z. Cell Growth Differ. 1995;6:937–944. [PubMed] [Google Scholar]
- 23.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 24.Naumovski L, Cleary M L. Blood. 1994;83:2261–2267. [PubMed] [Google Scholar]
- 25.Park J R, Robertson K, Hickstein D D, Tsai S, Hockenbery D M, Collins S J. Blood. 1994;84:440–445. [PubMed] [Google Scholar]
- 26.Fairbairn L J, Cowling G J, Reipert B M, Dexter T M. Cell. 1993;74:823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- 27.Hueng Y, Waxman S. Mol Cell Differ. 1994;2:83–100. [Google Scholar]
- 28.Andreeff M, Stone R, Michaeli J, Young C E, Tong W F, Sogoloff H, Ervin T, Kufe D, Rifkind R A, Marks P A. Blood. 1992;80:2604–2609. [PubMed] [Google Scholar]