Abstract
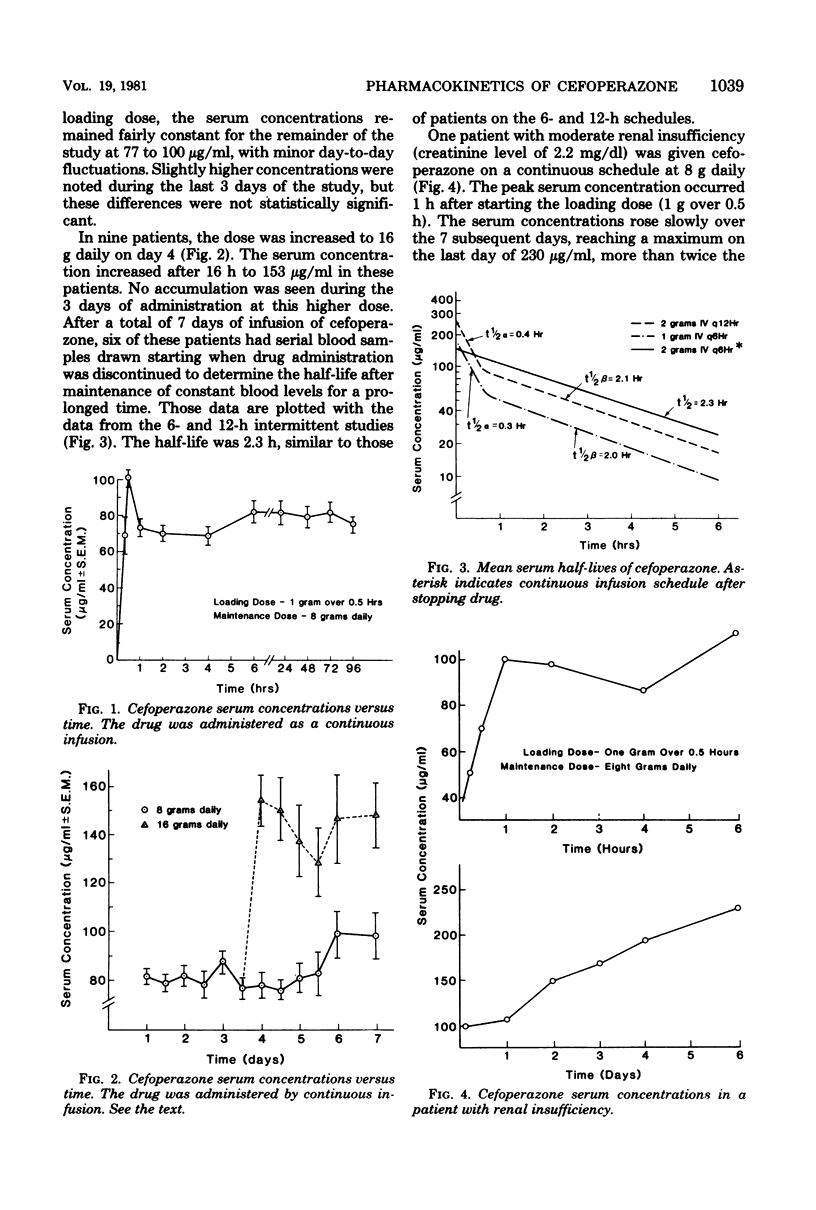
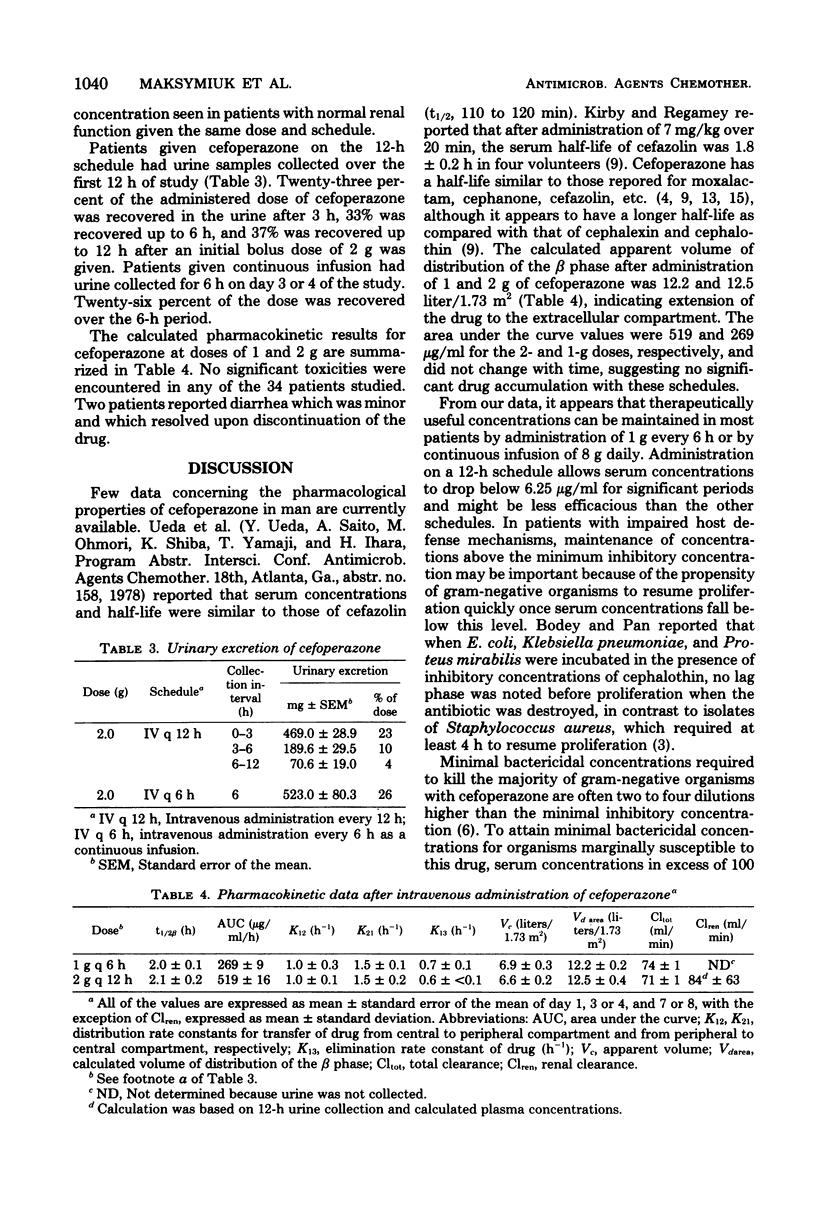
The pharmacokinetics of cefoperazone, a new semisynthetic cephalosporin, were studied in 34 patients with neoplastic disease. This compound was administered in a variety of doses and schedules without observable toxicity in any patient. The mean peak serum concentration after a 15-min intravenous infusion of 2 g was 264 microgram/ml after the first dose; the serum half-life was 2.1 h. There was no significant change in half-life or serum concentrations after 4 or 7 days of therapy. The mean peak serum concentration after infusion of 1 g over 15 min was 133 microgram/ml, with a mean of 10.7 microgram/ml at 6 h. The serum half-life was 2 h. The mean peak serum concentration after infusion of 1 g over 0.5 h was 101 microgram/ml. When 8 g was subsequently administered daily by a continuous infusion schedule, levels were maintained at 80 microgram/ml. When the dose was increased to 16 g daily, serum concentrations were maintained at an average of 153 microgram/ml. Only 37% of cefoperazone was recovered in the urine in a 12-h period after the initial dose, suggesting the importance of other mechanisms of excretion; however, serum concentrations in one patient with renal insufficiency were significantly higher than serum concentrations in patients with normal renal function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriole V. T. Pharmacokinetics of cephalosporins in patients with normal or reduced renal function. J Infect Dis. 1978 May;137 (Suppl):S88–S99. doi: 10.1093/infdis/137.supplement.s88. [DOI] [PubMed] [Google Scholar]
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Pan T. Effect of cephalothin on growth patterns of micro-organisms. J Antibiot (Tokyo) 1976 Oct;29(10):1092–1095. doi: 10.7164/antibiotics.29.1092. [DOI] [PubMed] [Google Scholar]
- Estey E. H., Weaver S. S., Ho D. H., Bodey G. P. Clinical pharmacology of moxalactam in patients with malignant disease. Antimicrob Agents Chemother. 1981 Apr;19(4):639–644. doi: 10.1128/aac.19.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle A. M., LeBlanc B. M., Bodey G. P. In vitro evaluation of cefoperazone. Antimicrob Agents Chemother. 1980 Mar;17(3):423–427. doi: 10.1128/aac.17.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki J., Rodriguez V., Bodey G. P. Proceedings: Causes of death in cancer patients. Cancer. 1974 Feb;33(2):568–573. doi: 10.1002/1097-0142(197402)33:2<568::aid-cncr2820330236>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kaye D., Kobasa W., Kaye K. Susceptibilities of anaerobic bacteria to cefoperazone and other antibiotics. Antimicrob Agents Chemother. 1980 Jun;17(6):957–960. doi: 10.1128/aac.17.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M., Regamey C. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis. 1973 Oct;128(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara N., Minami S., Muraoka T., Saikawa I., Mitsuhashi S. In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1979 Dec;16(6):731–735. doi: 10.1128/aac.16.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Comparison of the pharmacokinetics of cefamandole and other cephalosporin compounds. J Infect Dis. 1978 May;137 (Suppl):S80–S87. doi: 10.1093/infdis/137.supplement.s80. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P., Aswapokee N., Aswapokee P., Kung K. Comparative activity and beta-lactamase stability of cefoperazone, a piperazine cephalosporin. Antimicrob Agents Chemother. 1979 Aug;16(2):150–157. doi: 10.1128/aac.16.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H. Description and classification of the newer cephalosporins and their relationships with the established compounds. J Antimicrob Chemother. 1979 Nov;5(6):635–671. doi: 10.1093/jac/5.6.635. [DOI] [PubMed] [Google Scholar]


