Abstract
Mucoid Pseudomonas aeruginosa strains isolated from cystic fibrosis patients are very heterogeneous and include a class which is hypersusceptible to carbenicillin (minimum inhibitory concentration, less than or equal to 1 microgram/ml). Hypersusceptible mucoid P. aeruginosa isolates were found in 12 of 22 cystic fibrosis patients examined. In cystic fibrosis patients having both resistant and hypersusceptible mucoid strains, 24 of 54 mucoid colonies obtained from a sputum sample were found to belong to the hypersusceptible class. In most instances, hypersusceptible and resistant strains isolated from the same sputum sample were indistinguishable, aside from their antibiotic susceptibilities, by classical methods. A particular pair of mucoid isolates (one hypersusceptible and one resistant) was chosen for further study. The hypersusceptibility was not limited to carbenicillin but was found to extend to other penicillins, tetracycline, and trimethoprim but not to the aminoglycosides gentamicin and tobramycin. The hypersusceptibility of the mucoid strain was found to be unrelated to amount or ability to synthesize alginate. The hypersusceptible strain was found to have two additional outer membrane proteins (32,000 and 25,000 daltons) as compared with the resistant strain. The 32,000-dalton protein, termed protein N1, was found to be correlated to the hypersusceptibility phenotype, as all spontaneous mutants of the hypersusceptible mucoid strain which were capable of growing in the presence of 50 microgram of carbenicillin per ml had lost the 32,000-dalton outer membrane protein. The possible origins of the hypersusceptibility phenotype and the implications of the heterogeneity of mucoid P. aeruginosa in the pathogenesis of P. aeruginosa are discussed.
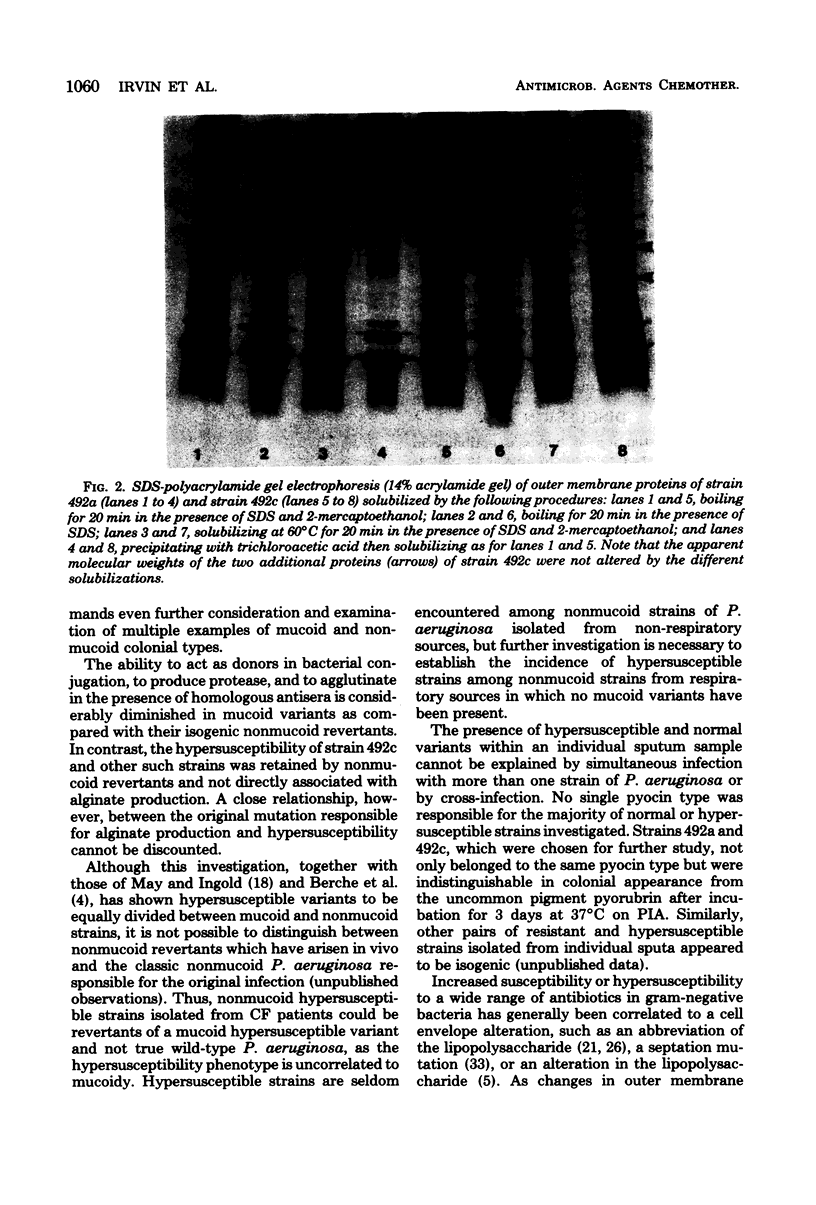
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R., Haas D., Yagil E. Mutants of Escherichia coli "cryptic" for certain periplasmic enzymes: evidence for an alteration of the outer membrane. J Bacteriol. 1977 Feb;129(2):1034–1044. doi: 10.1128/jb.129.2.1034-1044.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Oppici M., Shapiro J., Fennewald M. Regulation of membrane peptides by the Pseudomonas plasmid alk regulon. J Bacteriol. 1979 Dec;140(3):754–762. doi: 10.1128/jb.140.3.754-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Descamps P., Avril J. L., Daoulas-Lebourdelles F., Véron M. Effect of antibiotics on mucoid strains of Pseudomonas aeruginosa. Ann Microbiol (Paris) 1979 Apr;130A(3):315–330. [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969 Nov;18(5):936–937. doi: 10.1128/am.18.5.936-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Rehn K., Hoehn B. Cell envelope and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2033–2036. doi: 10.1073/pnas.70.7.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E. T., Rolinson G. N., Sutherland R. Carbenicillin: a new semisynthetic penicillin active against Pseudomonas pyocyanea. Br Med J. 1967 Jul 8;3(5557):75–78. doi: 10.1136/bmj.3.5557.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. R., Ingold A. Sensitivity of respiratory strains of Pseudomonas aeruginosa to carbenicillin. J Med Microbiol. 1973 Feb;6(1):77–82. doi: 10.1099/00222615-6-1-77. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I. Identification of Pseudomonas aeruginosa in the clinical laboratory. J Med Microbiol. 1969 Feb;2(1):9–16. doi: 10.1099/00222615-2-1-9. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale T. W., Thirkill H., Tarpay M., Flux M., Rennert O. M. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J Clin Microbiol. 1979 Jan;9(1):72–78. doi: 10.1128/jcm.9.1.72-78.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Boxerbaum B., Stern R. C., Kuchenbrod P. J. Multiple of isolates of Pseudomonas aeruginosa with differing antimicrobial susceptibility patterns from patients with cystic fibrosis. J Infect Dis. 1979 Dec;140(6):873–880. doi: 10.1093/infdis/140.6.873. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weigand R. A., Rothfield L. I. Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol. 1976 Jan;125(1):340–345. doi: 10.1128/jb.125.1.340-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Govan J. R. Pyocine typing of mucoid strains of Pseudomonas aeruginosa isolated from children with cystic fibrosis. J Med Microbiol. 1973 Aug;6(3):409–412. doi: 10.1099/00222615-6-3-409. [DOI] [PubMed] [Google Scholar]