Abstract
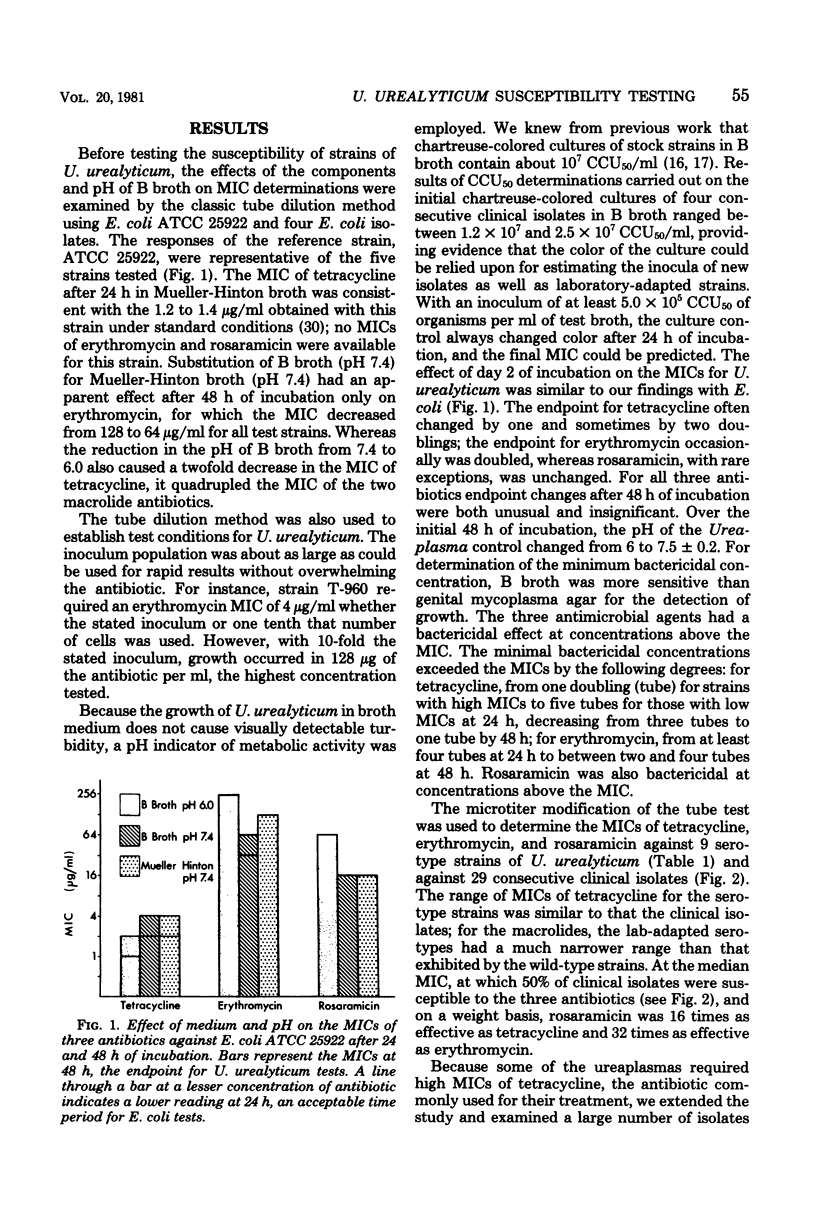
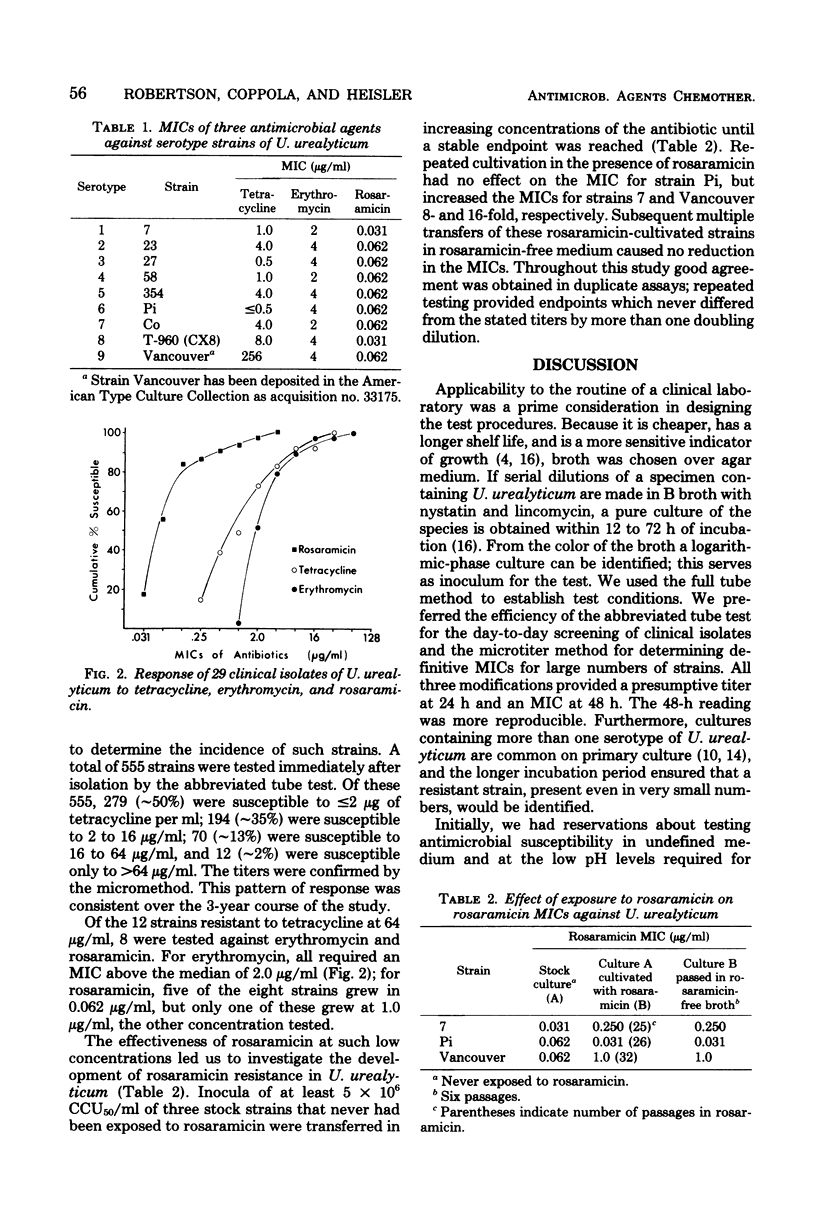
We describe a method for determining the minimal inhibitory concentrations (MICs) of antibiotics for Ureaplasma urealyticum which is compatible with current standard of susceptibility testing. A presumptive MIC is available after 24 h of incubation, and the definitive MIC is available at 48 h. The MICs for 9 serotype strains and 27 clinical isolates ranged from less than or equal to 0.5 to 256 microgram of tetracycline per ml, greater than or equal to 1 to 64 microgram of erythromycin per ml, and 0.031 to 4.0 microgram of rosaramicin per ml. Of an additional 555 isolates screened for their response to tetracycline, 2% required MICs of greater than 64 microgram/ml, which we believe is near the concentration at which in vivo resistance to this agent is expressed. After prolonged exposure to rosaramicin, the resistance of two of three serotype strains of U. urealyticum was increased 8- and 16-fold, but the MICs still did not exceed 1.0 microgram/ml.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddle J. W., Thornsberry C. In vitro activity of rosamicin, josamycin, erythromycin, and clindamycin against beta-lactamase-nagative and beta-lactamase-positive strains of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1979 Feb;15(2):243–245. doi: 10.1128/aac.15.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonissol C., Daoulas-Lebourdelles F. Valeur de l'antibiogramme pour le traitement des infections urogénitales à Ureaplasma. Pathol Biol (Paris) 1978 Dec;26(9-10):539–546. [PubMed] [Google Scholar]
- Braun P., Klein J. O., Kass E. H. Susceptibility of genital mycoplasmas to antimicrobial agents. Appl Microbiol. 1970 Jan;19(1):62–70. doi: 10.1128/am.19.1.62-70.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P., Klein J. O., Lee Y. H., Kass E. H. Methodologic investigations and prevalence of genital mycoplasmas in pregnancy. J Infect Dis. 1970 Apr;121(4):391–400. doi: 10.1093/infdis/121.4.391. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. The incidence of tetracycline-resistant strains of Ureaplasma urealyticum. J Antimicrob Chemother. 1978 Jan;4(1):57–63. doi: 10.1093/jac/4.1.57. [DOI] [PubMed] [Google Scholar]
- Ford D. K. CULTURE OF HUMAN GENITAL "T-STRAIN" PLEUROPNEUMONIA-LIKE ORGANISMS. J Bacteriol. 1962 Nov;84(5):1028–1034. doi: 10.1128/jb.84.5.1028-1034.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K., Smith J. R. Non-specific urethritis associated with a tetracycline-resistant T-mycoplasma. Br J Vener Dis. 1974 Oct;50(5):373–374. doi: 10.1136/sti.50.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber C. D., Creticos P., Valicenti J., Williamson H. O. T mycoplasma in human reproductive failure. Obstet Gynecol. 1979 Nov;54(5):558–561. [PubMed] [Google Scholar]
- Hill A. C., Sutton G. Rosamicin, a macrolide with in vitro activity against Ureaplasma urealyticum. J Antibiot (Tokyo) 1979 Sep;32(9):915–919. doi: 10.7164/antibiotics.32.915. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Antimicrobial activity of several antibiotics and a sulfonamide against Chlamydia trachomatis organisms in cell culture. Antimicrob Agents Chemother. 1977 Jul;12(1):80–83. doi: 10.1128/aac.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S., Kass E. H. Serotypic heterogeneity in isolates of human genital T-mycoplasmas. Infect Immun. 1973 Mar;7(3):499–500. doi: 10.1128/iai.7.3.499-500.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P. Comparison of growth inhibition and immunofluorescence tests in serotyping clinical isolates of Ureaplasma urealyticum. Br J Vener Dis. 1977 Jun;53(3):186–189. doi: 10.1136/sti.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice M. J., Taylor-Robinson D., Csonka G. W. Non-specific urethritis. A placebo-controlled trial of minocycline in conjunction with laboratory investigations. Br J Vener Dis. 1976 Aug;52(4):269–275. doi: 10.1136/sti.52.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A. Bromothymol blue broth: improved medium for detection of Ureaplasma urealyticum (T-strain mycoplasma). J Clin Microbiol. 1978 Feb;7(2):127–132. doi: 10.1128/jcm.7.2.127-132.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Stemke G. W. Modified metabolic inhibition test for serotyping strains of Ureaplasma urealyticum (T-strain Mycoplasma). J Clin Microbiol. 1979 Jun;9(6):673–676. doi: 10.1128/jcm.9.6.673-676.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root T. E., Edwards L. D., Spengler P. J. Nongonococcal urethritis: a survey of clinical and laboratory features. Sex Transm Dis. 1980 Apr-Jun;7(2):59–65. [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr In vitro activity of rosamicin against Neisseria and Haemophilus, including penicillinase-producing strains. Antimicrob Agents Chemother. 1977 Aug;12(2):293–294. doi: 10.1128/aac.12.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Howard D. R. Identification of "T" mycoplasmas in primary agar cultures by means of a direct test for urease. Ann N Y Acad Sci. 1970 Oct 30;174(2):809–819. doi: 10.1111/j.1749-6632.1970.tb45598.x. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D., Baker R. L. T-strain Mycoplasma. Selective inhibition by erythromycin in vitro. Br J Vener Dis. 1966 Mar;42(1):21–24. doi: 10.1136/sti.42.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F. In vitro susceptibility of Ureaplasma urealyticum to rosaramicin. Antimicrob Agents Chemother. 1979 Jul;16(1):106–108. doi: 10.1128/aac.16.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Washton H. E. In vitro susceptibility of 30 strains of Chlamydia trachomatis to rosamicin. Antimicrob Agents Chemother. 1978 Sep;14(3):493–494. doi: 10.1128/aac.14.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaepen M. S., Kundsin R. B., Horne H. W. Tetracycline-resistant T-mycoplasmas (Ureaplasma urealyticum) from patients with a history of reproductive failure. Antimicrob Agents Chemother. 1976 Jun;9(6):1012–1018. doi: 10.1128/aac.9.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaepen M. S., Kundsin R. B. Simple, direct broth-disk method for antibiotic susceptibility testing of Ureaplasma urealyticum. Antimicrob Agents Chemother. 1977 Feb;11(2):267–270. doi: 10.1128/aac.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D. Mycoplasmas of various hosts and their antibiotic sensitivities. Postgrad Med J. 1967 Mar;43(Suppl):100–104. [PubMed] [Google Scholar]
- Taylor-Robinson D. Pathogenicity of ureaplasmas for animals and man. Zentralbl Bakteriol Orig A. 1979 Oct;245(1-2):150–163. [PubMed] [Google Scholar]


