Abstract
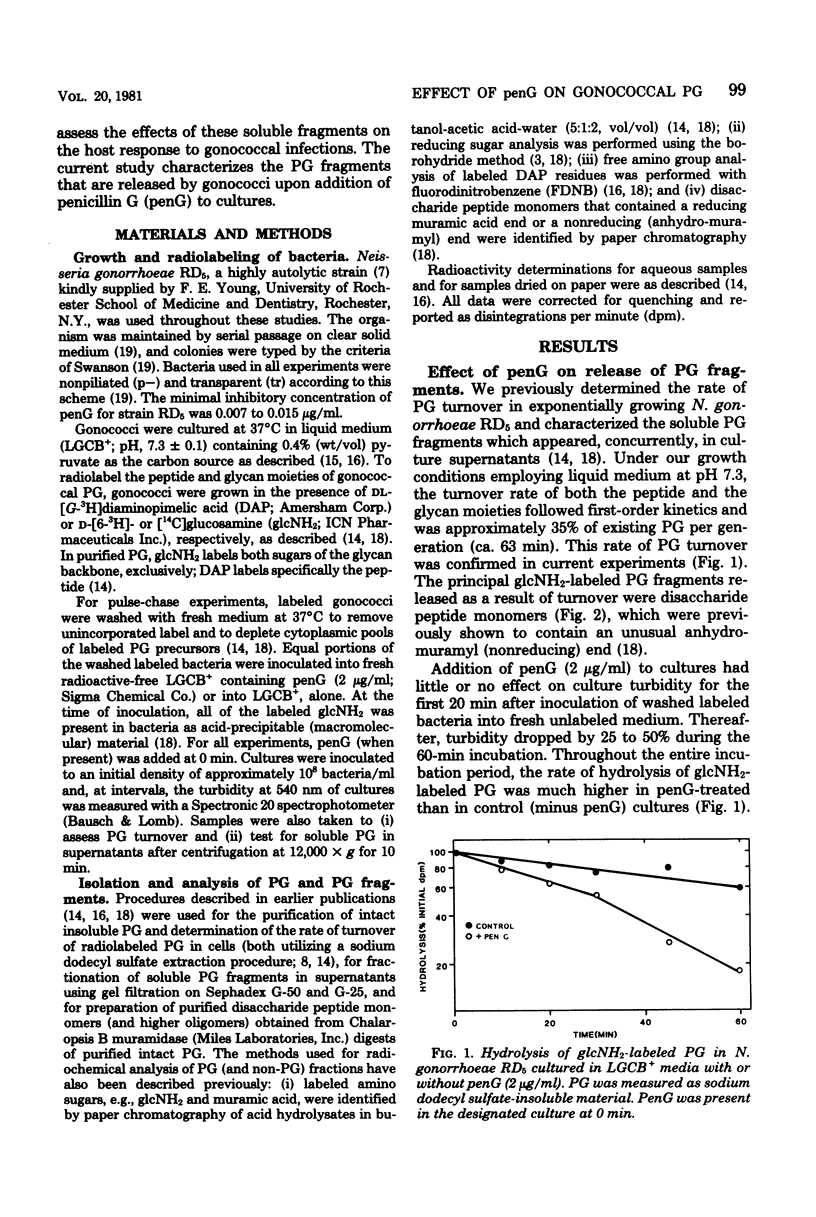
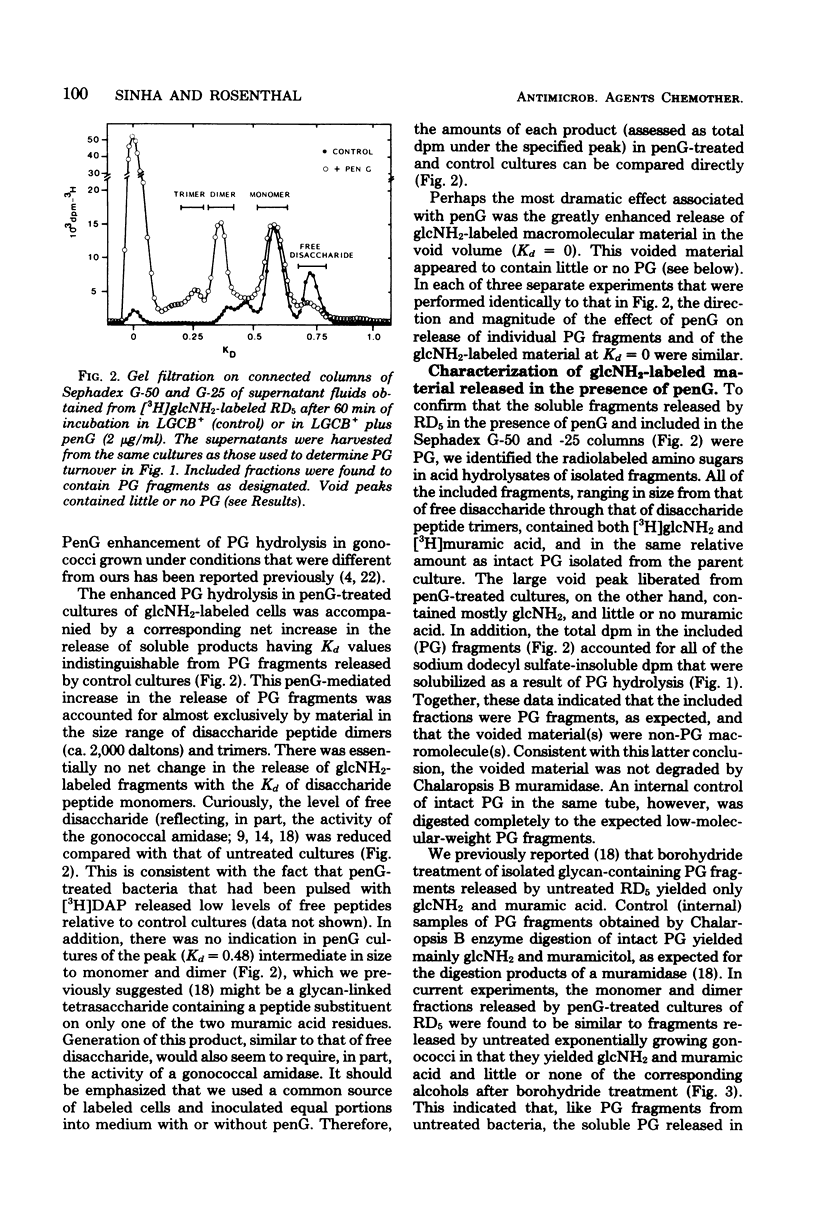
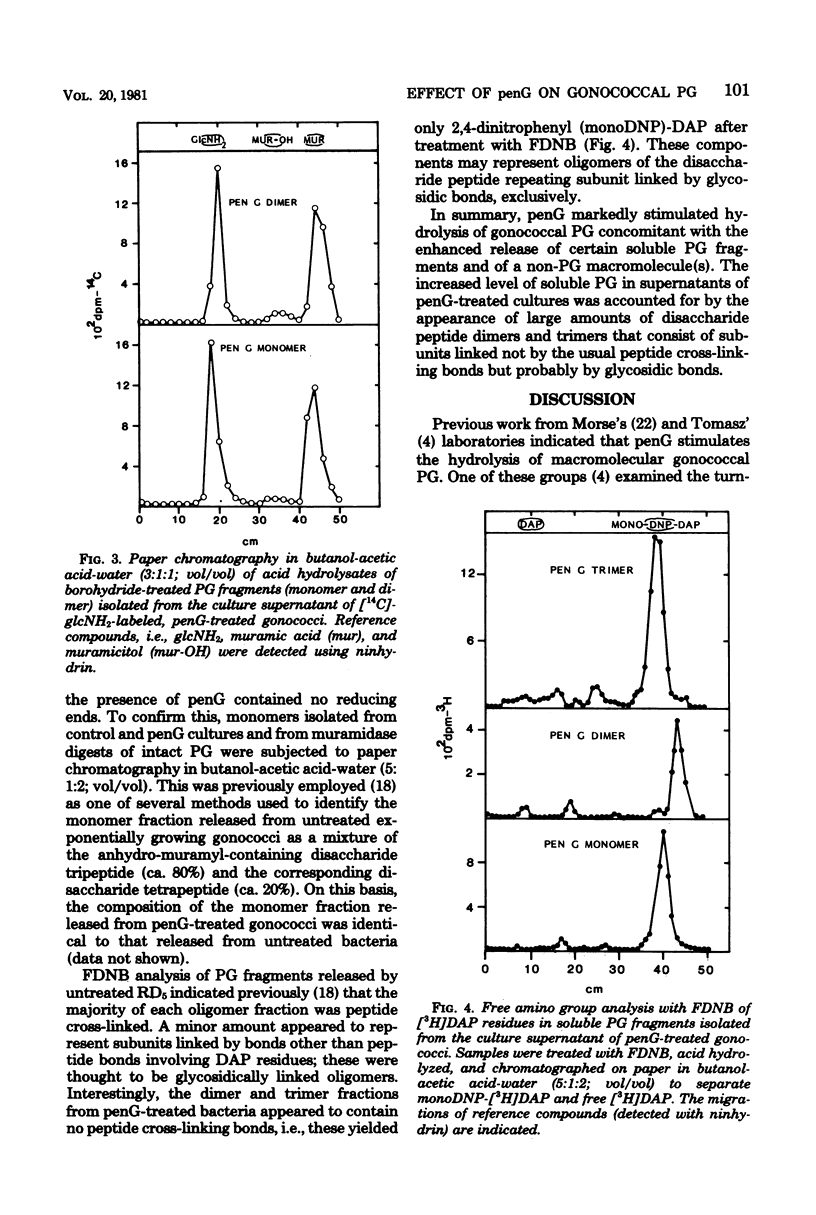
The effect of penicillin G (penG) on the turnover and release of peptidoglycan (PG) by Neisseria gonorrhoeae RD5 was investigated. We previously showed that exponentially growing gonococci (labeled in the glycan moiety with glucosamine and muramic acid and in the peptide with diaminopimelic acid) turn over ca. 35% of their PG per generation. In current studies, addition of penG accelerated the rate of PG hydrolysis by more than twofold and resulted in a corresponding increase in the amount of soluble PG fragments found in the medium. This increase of soluble PG was accounted for mainly by the release of nonreducing (anhydro-muramyl-containing) disaccharide peptide dimers (molecular weight, about 2,000) and trimers that were composed of subunits, linked not by peptide cross-linking bonds, but probably only by glycosidic bonds. The enhanced release of these products suggested that penG, directly or indirectly, stimulates the activity of a glycan-splitting, gonococcal PG:PG-6 muramyl transferase (transglycosylase). PG monomers that were released in the presence of penG were identical, both qualitatively and quantitatively, to the monomers released as a result of turnover in the absence of penG and consisted of anhydro-muramyl-containing disaccharide tripeptides and tetrapeptides. PenG-treated bacteria consistently released slightly less free disaccharide and free peptides than did control cultures, implying a penG-associated depression in the activity of the gonococcal N-acetylmuramyl-L-alanine amidase. In addition to stimulating the release of PG fragments, penG was also associated with greatly enhanced release from cells of glucosamine-containing non-PG macromolecule(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodell E. W., Fazio M., Tomasz A. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Mar;13(3):514–526. doi: 10.1128/aac.13.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman R. K., Lawson J. W. Isolation of a stable cell wall-defective form of Neisseria gonorrhoeae from a case of untreated gonococcal urethritis. J Clin Microbiol. 1980 Oct;12(4):603–605. doi: 10.1128/jcm.12.4.603-605.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Gutman L. T., Belding M. E., Turck M. Recovery of Neisseria gonorrhoeae from "Sterile" synovial fluid in gonococcal arthritis. N Engl J Med. 1971 Feb 11;284(6):318–320. doi: 10.1056/NEJM197102112840609. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S. Preparation of arthritogenic hydrosoluble peptidoglycans from both arthritogenic and non-arthritogenic bacterial cell walls. Infect Immun. 1977 Jun;16(3):861–866. doi: 10.1128/iai.16.3.861-866.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis. 1979 May-Jun;1(3):434–467. doi: 10.1093/clinids/1.3.434. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: penicillin enhancement of peptidoglycan hydrolysis. Infect Immun. 1977 Dec;18(3):717–725. doi: 10.1128/iai.18.3.717-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


