Abstract
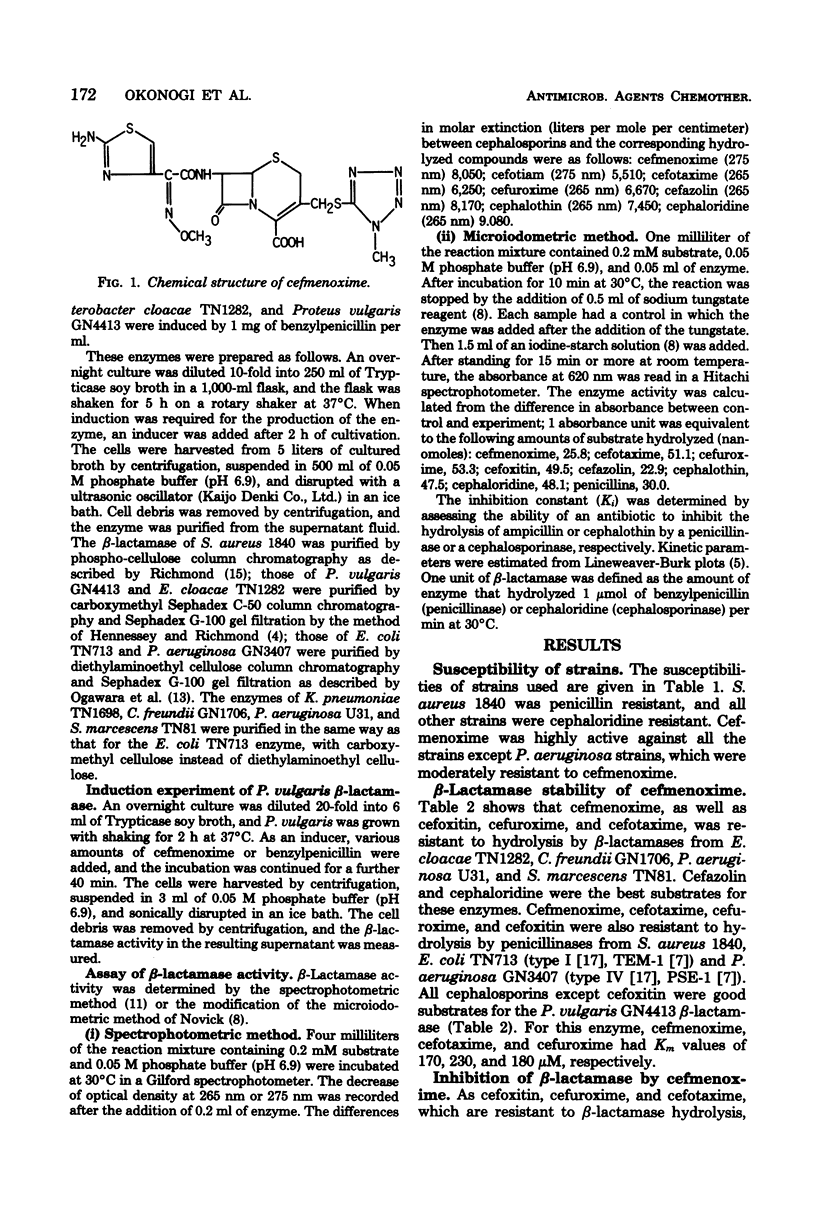
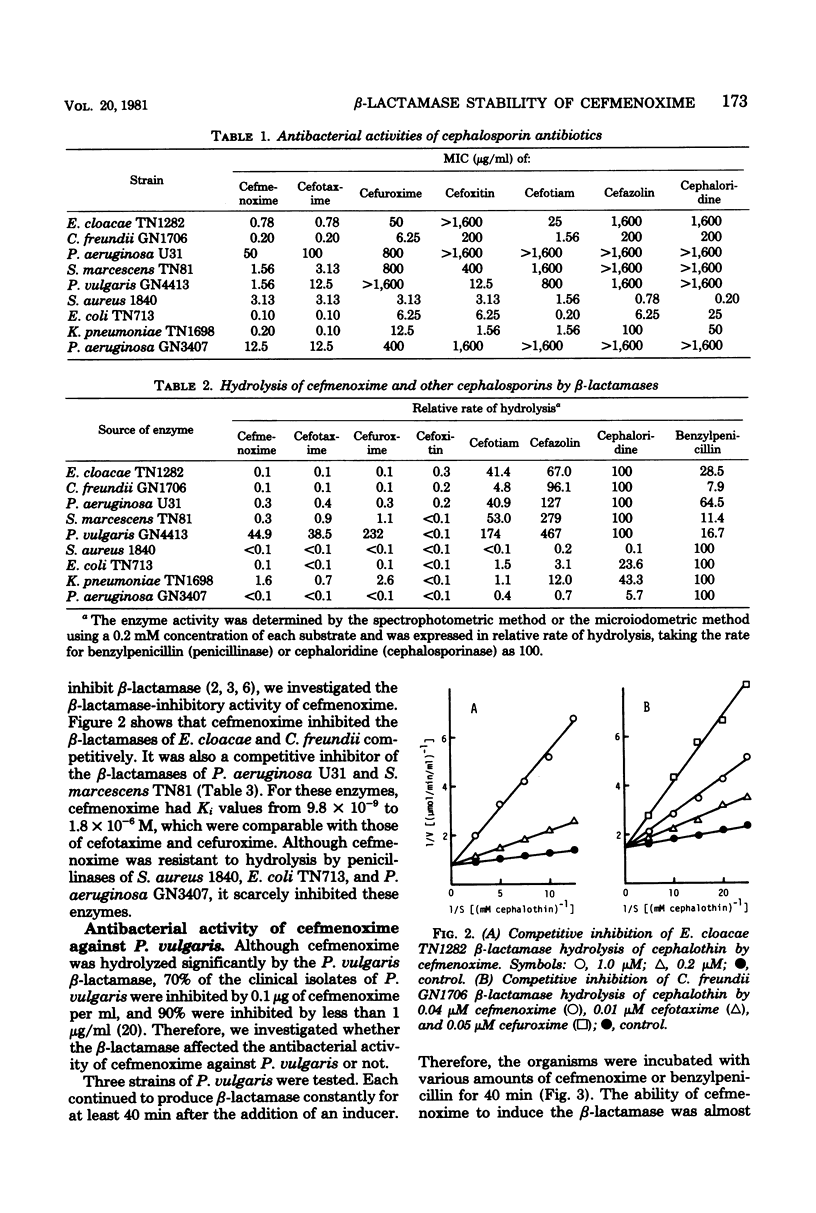
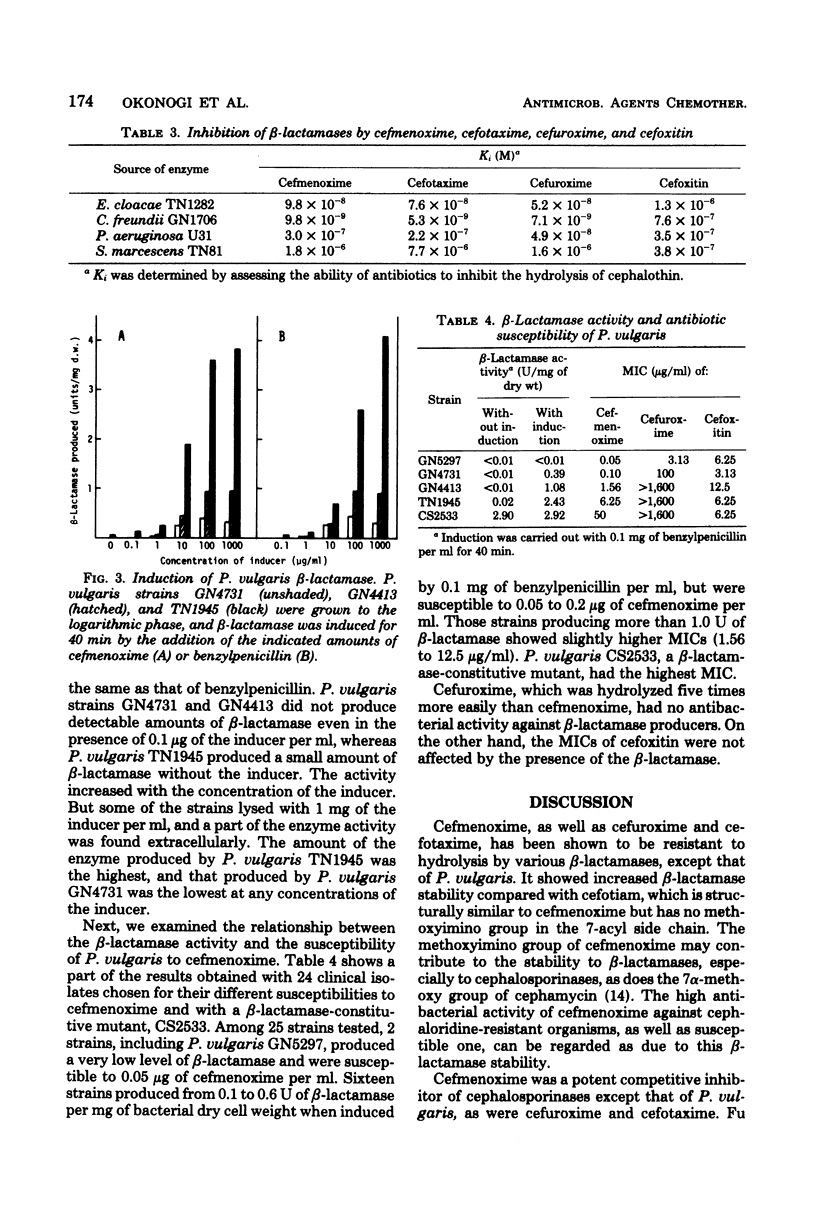
Cefmenoxime, a new cephalosporin antibiotic, has been shown to be stable to a Staphylococcus aureus penicillinase and R plasmid-mediated type I and type IV penicillinases. It was also resistant to hydrolysis by most cephalosporinases, but was susceptible to hydrolysis by a Proteus vulgaris beta-lactamase. Cefmenoxime was active against cephaloridine-resistant species, except Pseudomonas aeruginosa, which was moderately resistant to cefmenoxime. Cefmenoxime was an inducer of P. vulgaris beta-lactamase biosynthesis, but 1 microgram or more of the drug per ml, which inhibits most of the clinical isolates of P. vulgaris, was required for the production of detectable amounts of the enzyme. Cefmenoxime was a strong competitive inhibitor of beta-lactamases of Enterobacter cloacae, Citrobacter freundii, P. aeruginosa, and Serratia marcescens, but it did not inhibit penicillinases in spite of its resistance to hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland G., Birnbaum J. Cefoxitin resistance to beta-lactamase: a major factor for susceptibility of bacteroides fragilis to the antibiotic. Antimicrob Agents Chemother. 1977 Apr;11(4):725–734. doi: 10.1128/aac.11.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. beta-lactamase stability of HR 756, a novel cephalosporin, compared to that of cefuroxime and cefoxitin. Antimicrob Agents Chemother. 1978 Sep;14(3):322–326. doi: 10.1128/aac.14.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney D. F., Koppel G. A., Turner J. R. Substrate inhibition of beta-lactamases, a method for predicting enzymatic stability of cephalosporins. Antimicrob Agents Chemother. 1976 Sep;10(3):470–475. doi: 10.1128/aac.10.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M. Plasmid-mediated beta-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979 Jul;5(4):349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Imada A., Yoneda M. SCE-963, a new potent cephalosporin with high affinity for penicillin-binding proteins 1 and 3 of Escherichia coli. Antimicrob Agents Chemother. 1979 Jan;15(1):20–27. doi: 10.1128/aac.15.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata M., Minamida I., Yamaoka M., Shiraishi M., Miyawaki T., Akimoto H., Naito K., Kida M. A new cephalosporin. SCE-963: 7-[2-(2-aminothiazol-4-yl)-acetamido]-3-[[[1-(2-dimethylaminoethyl)-1h-tetrazol-5-yl]-thio]methyl]ceph-3-em-4-carboxylic acid. Chemistry and structure-activity relationships. J Antibiot (Tokyo) 1978 Dec;31(12):1262–1271. doi: 10.7164/antibiotics.31.1262. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Ryan D. M., Foord R. D., Muggleton P. W. Cefuroxime - a new cephalosporin antibiotic. J Antibiot (Tokyo) 1976 Jan;29(1):29–37. doi: 10.7164/antibiotics.29.29. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Maeda K., Umezawa H. A -lactamase of Escherichia coli. Biochim Biophys Acta. 1972 Nov 10;289(1):203–211. doi: 10.1016/0005-2744(72)90123-4. [DOI] [PubMed] [Google Scholar]
- Onishi H. R., Daoust D. R., Zimmerman S. B., Hendlin D., Stapley E. O. Cefoxitin, a semisynthetic cephamycin antibiotic: resistance to beta-lactamase inactivation. Antimicrob Agents Chemother. 1974 Jan;5(1):38–48. doi: 10.1128/aac.5.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Yaginuma S., Tai M., Iyobe S., Mitsuhashi S. Plasmid-mediated penicillin beta-lactamases in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jan;9(1):55–60. doi: 10.1128/aac.9.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Warren C., Phillips I. In vitro antibacterial activity and susceptibility of the cephalosporin Ro 13-9904 to beta-lactamases. Antimicrob Agents Chemother. 1980 Aug;18(2):292–298. doi: 10.1128/aac.18.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kida M., Kondo M., Ono H., Takeuchi M., Nishi T. SCE-963, a new broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Oct;14(4):557–568. doi: 10.1128/aac.14.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


