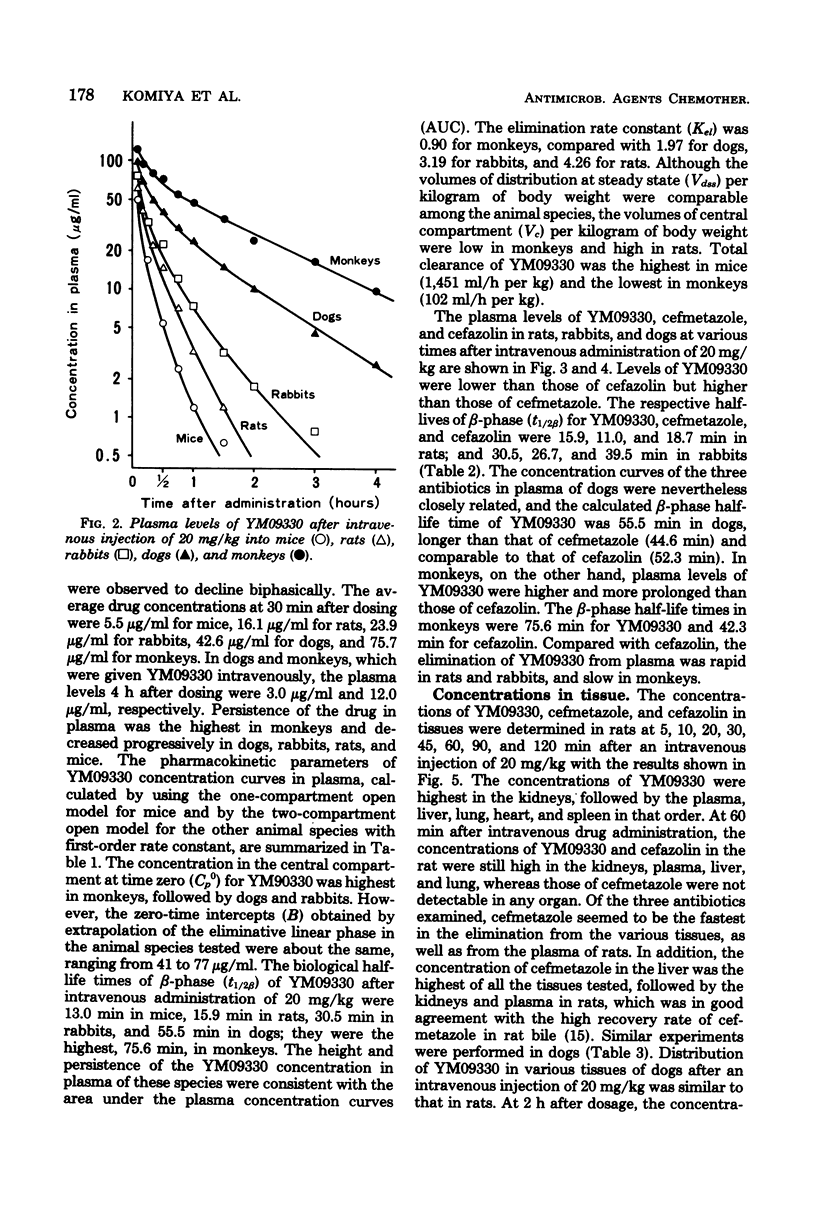
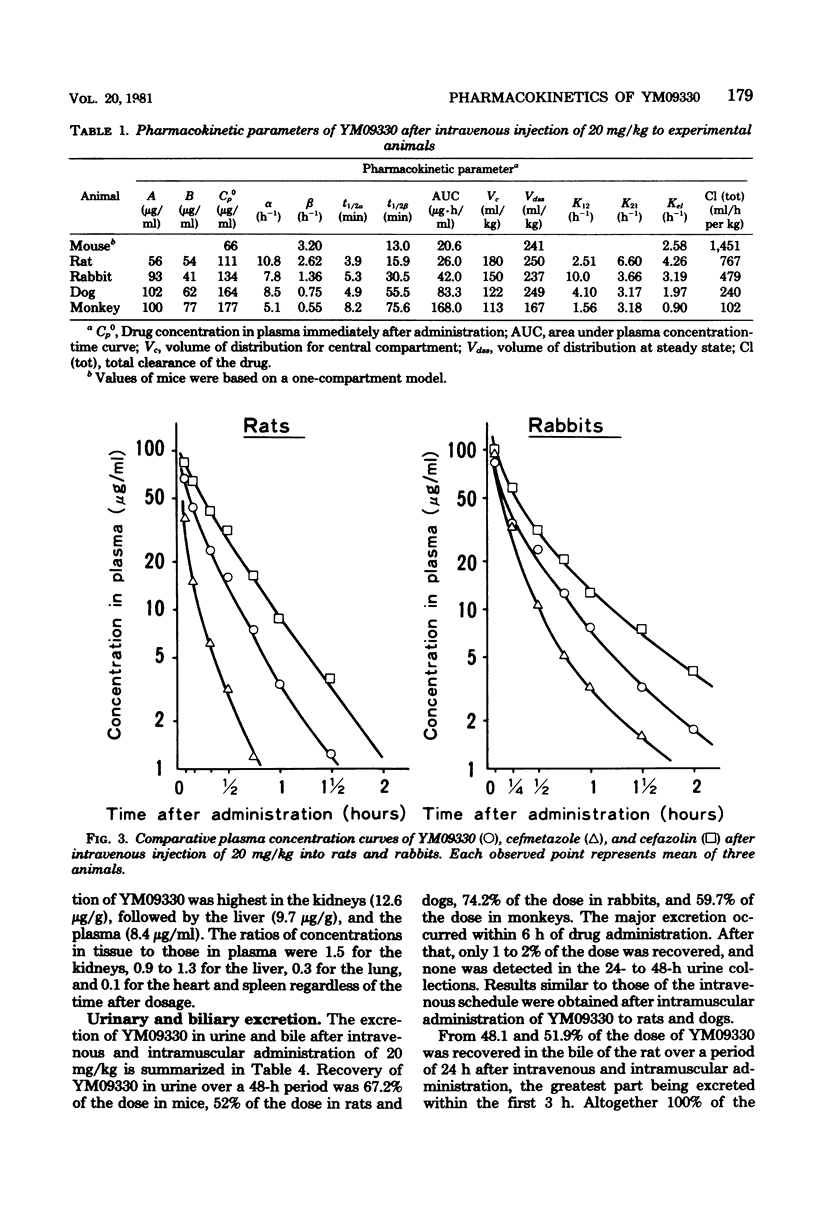
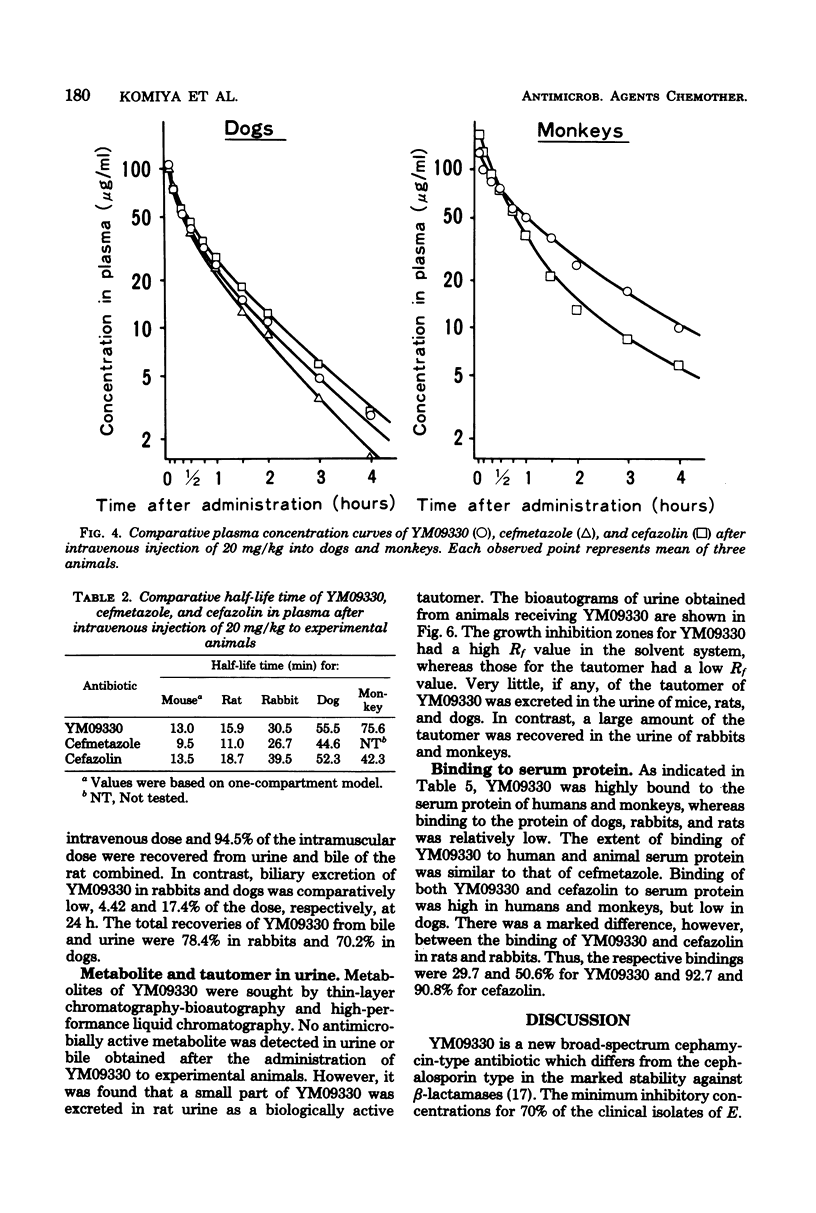
Abstract
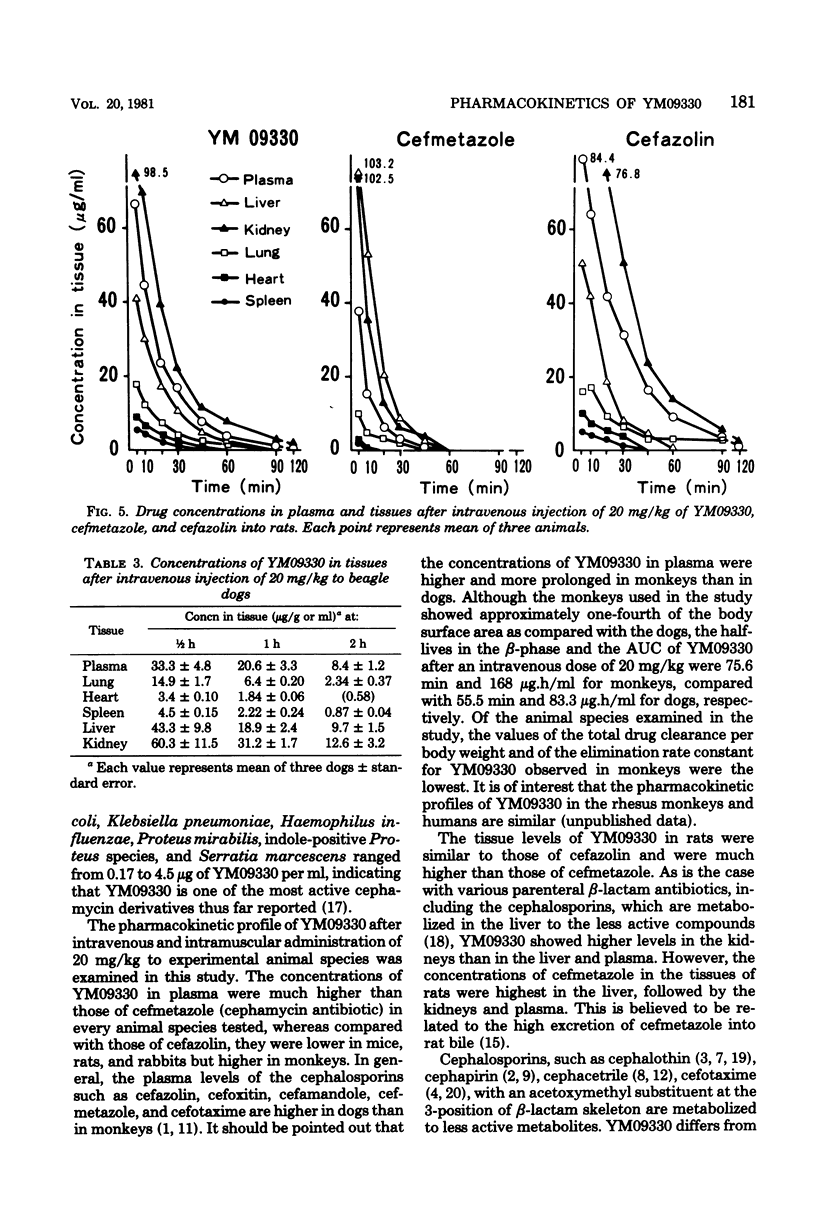
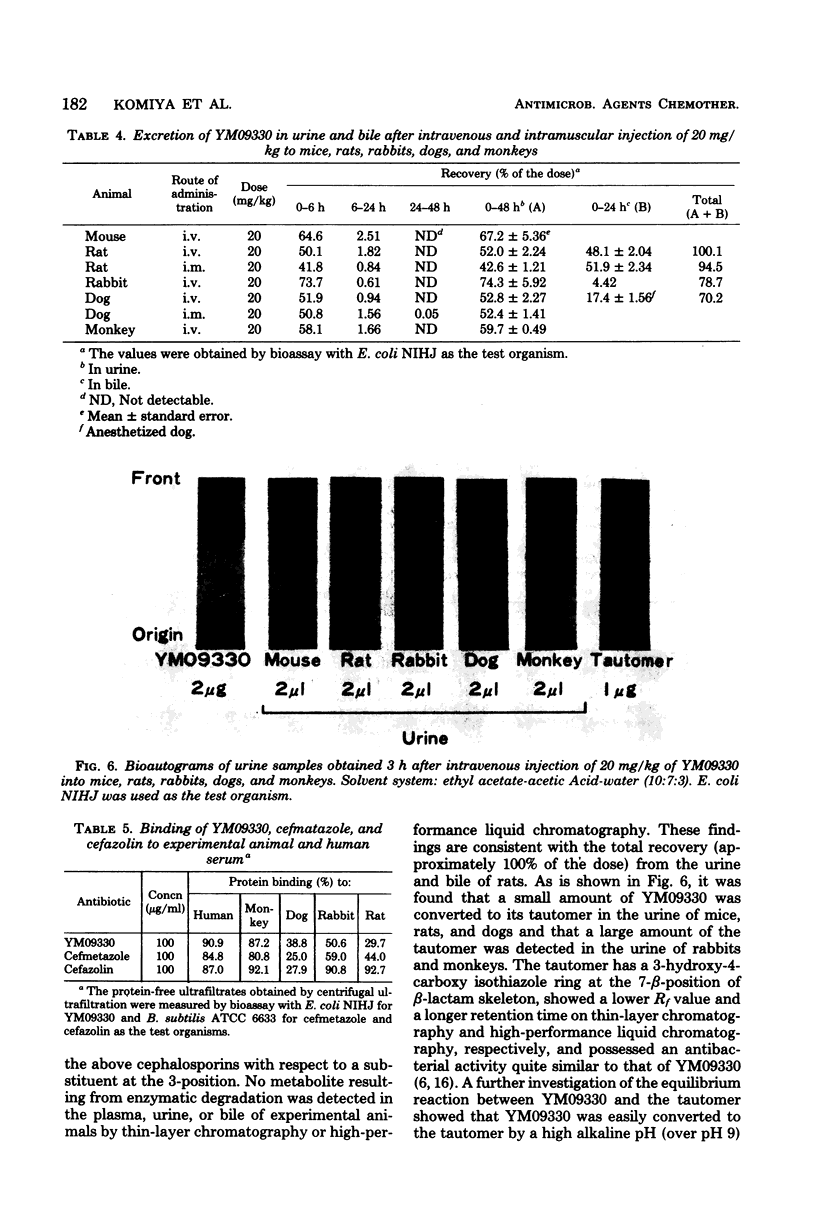
The pharmacokinetics of YM09330, a new semisynthetic cephamycin, were determined after intravenous and intramuscular administration to experimental animals. Mean plasma levels of YM09330 at 30 min after intravenous administration of 20 mg/kg were 5.5 micrograms/ml for mice, 17 micrograms/ml for rats, 24 micrograms/ml for rabbits, 42 micrograms/ml for dogs, and 76 micrograms/ml for monkeys; plasma half-lives were 13.0, 15.9, 30.5, 55.5, and 75.6 min, respectively. The half-lives of YM09330 were longer than those of cefmetazole in all species tested. In monkeys, plasma levels of YM09330 were higher and more prolonged than those of cefazolin. In rats and dogs, the concentrations of YM09330 were highest in the kidneys, followed by the liver, plasma, lung, spleen, and heart in that order; they were similar to those of cefazolin in rats. Urinary excretion of YM09330 within 48 h of intravenous administrations was 67% of the dose in mice, 52% in rats, 74% in rabbits, 53% in dogs, and 60% in monkeys. In rats, 48% of the dose of YM09330 was detected in the plasma, urine, or bile. However, small amounts of an antibacterially active tautomer of YM09330 were recovered in the urine of mice, rats, and dogs, whereas large amounts of the tautomer were recovered in the urine of rabbits and monkeys. Serum protein binding of YM09330 was 30% of rats, 51% for rabbits, 39% for dogs, 87% for monkeys, and 91% for humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actor P., Uri J. V., Zajac I., Guarini J. R., Phillips L., Pitkin D. H., Berges D. A., Dunn G. L., Hoover J. R., Weisbach J. A. SK&F 75073, new parenteral broad-spectrum cephalosporin with high and prolonged serum levels. Antimicrob Agents Chemother. 1978 May;13(5):784–790. doi: 10.1128/aac.13.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana B. E., van Harken D. R., Hottendorf G. H. Comparative pharmacokinetics and metabolism of cephapirin in laboratory animals and humans. Antimicrob Agents Chemother. 1976 Aug;10(2):307–317. doi: 10.1128/aac.10.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. J., Anders M. W., Mirkin B. L. Ion-pair extraction and high-speed liquid chromatography of cephalothin and deacetylcephalothin in human serum and urine. Drug Metab Dispos. 1973 Sep-Oct;1(5):659–662. [PubMed] [Google Scholar]
- Fu K. P., Aswapokee P., Ho I., Matthijssen C., Neu H. C. Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother. 1979 Nov;16(5):592–597. doi: 10.1128/aac.16.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami M., Maeda T., Fujimoto M., Nagano Y., Nagano N., Yamazaki A., Shibanuma T., Tamazawa K., Yano K. Synthesis of new cephamycin derivatives and a novel rearrangement between isothiazolethioacetamides and 1,3-dithietanecarboxamides. Chem Pharm Bull (Tokyo) 1980 Sep;28(9):2629–2636. doi: 10.1248/cpb.28.2629. [DOI] [PubMed] [Google Scholar]
- LEE C. C., HERR E. B., Jr, ANDERSON R. C. Pharmacological and toxicological studies on cephalotin. Clin Med (Northfield) 1963 Jun;70:1123–1138. [PubMed] [Google Scholar]
- McCloskey R. V., Terry E. E., McCracken A. W., Sweeney M. J., Forland M. F. Effect of hemodialysis and renal failure on serum and urine concentrations of cephapirin sodium. Antimicrob Agents Chemother. 1972 Feb;1(2):90–93. doi: 10.1128/aac.1.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa T., Sakamoto H., Fukada S., Nakamoto S., Hirose T., Itoh N., Nishida M. Pharmacokinetics of ceftizoxime in animals after parenteral dosing. Antimicrob Agents Chemother. 1980 Feb;17(2):157–164. doi: 10.1128/aac.17.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y., Kanai Y., Fugono T., Tanayama S. Metabolic fate of cephacetrile after parenteral administration in rats and rabbits. J Antibiot (Tokyo) 1976 Jan;29(1):81–90. doi: 10.7164/antibiotics.29.81. [DOI] [PubMed] [Google Scholar]
- Nightingale C. H., Greene D. S., Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975 Dec;64(12):1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y. Cefazolin, a new semisynthetic cephalosporin antibiotic. II. In vitro and in vivo antimicrobial activity. J Antibiot (Tokyo) 1970 Mar;23(3):137–148. doi: 10.7164/antibiotics.23.137. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Kita Y., Yamazaki I., Kondo M., Noji Y., Fugono T. Absorption, distribution and excretion of cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin, in mice, rats, rabbits and dogs. J Antibiot (Tokyo) 1980 Dec;33(12):1532–1544. doi: 10.7164/antibiotics.33.1532. [DOI] [PubMed] [Google Scholar]
- Wise R., Wills P. J., Andrews J. M., Bedford K. A. Activity of the cefotaxime (HR756) desacetyl metabolite compared with those of cefotaxime and other cephalosporins. Antimicrob Agents Chemother. 1980 Jan;17(1):84–86. doi: 10.1128/aac.17.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]



