Abstract
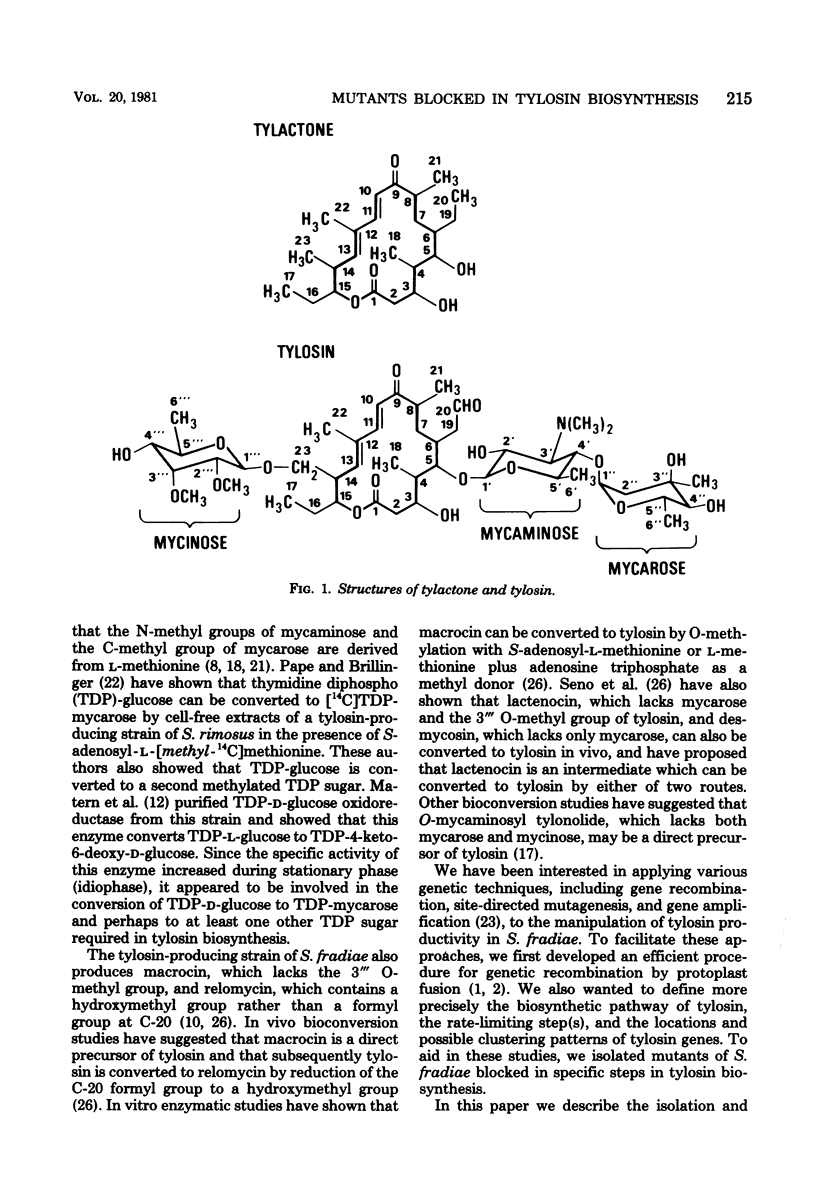
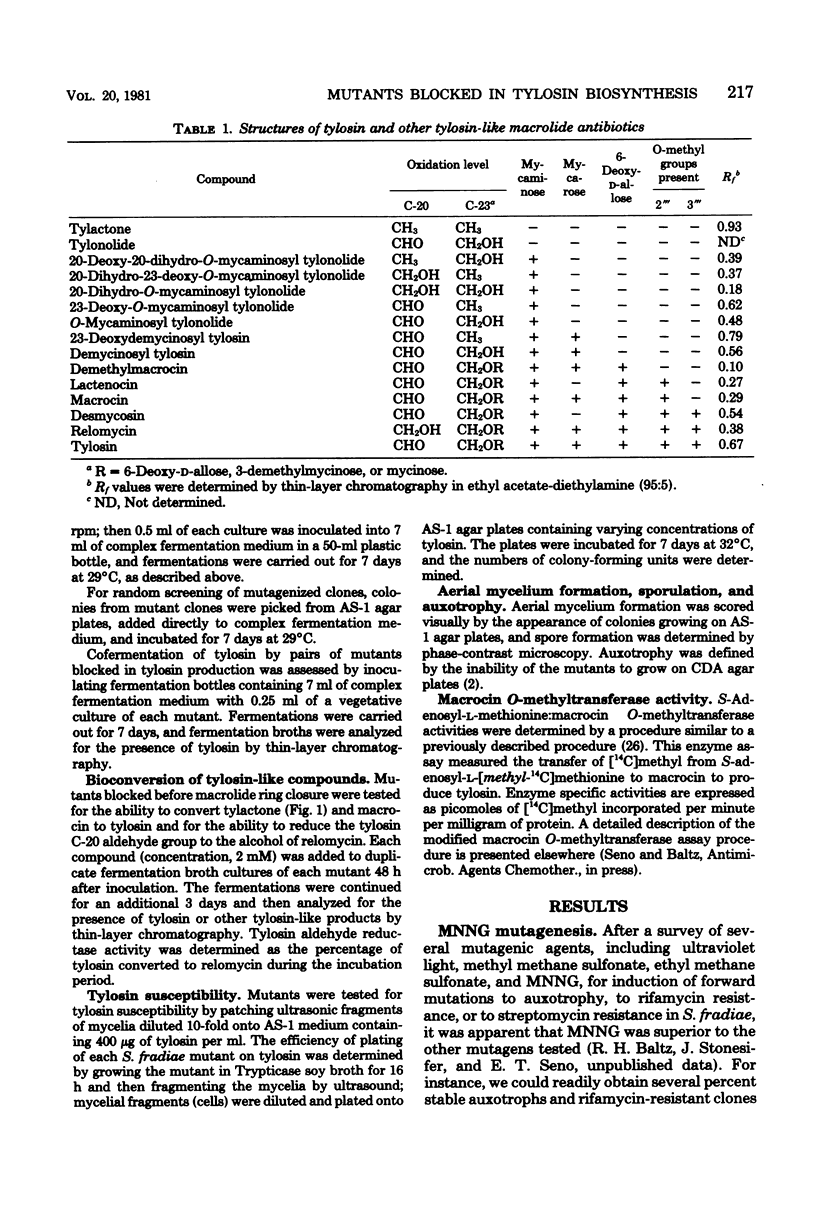
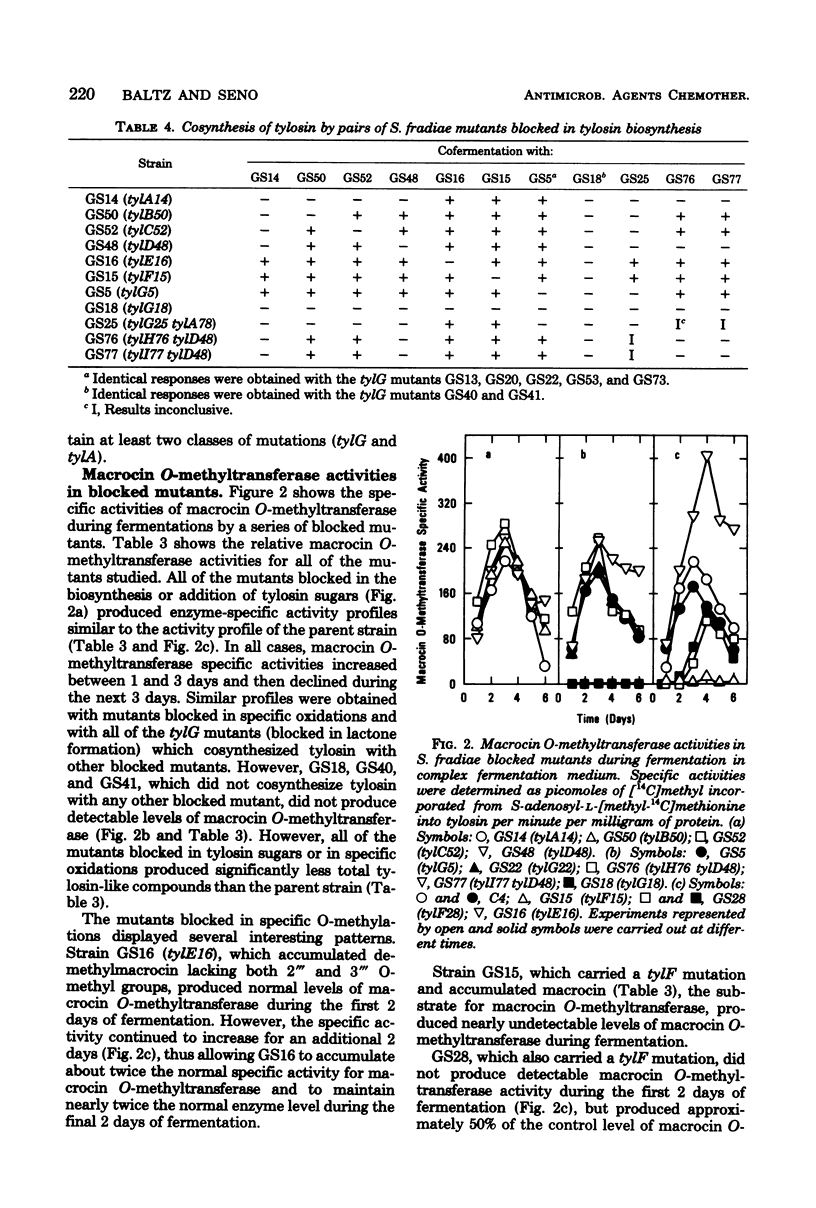
We isolated numerous mutants of Streptomyces fradiae blocked in tylosin biosynthesis after N-methyl-N′-nitro-N-nitrosoguanidine mutagenesis. These mutants were classified into nine groups, based upon the tylosin-like compounds produced and upon cofermentation analyses. More than 80% of the mutants isolated produced no tylosin-like compounds, and the majority of these were blocked only in the formation of tylactone. Four classes of mutants blocked in the biosynthesis or addition of tylosin sugars were isolated; tylA mutants were blocked in the formation of all three tylosin sugars, whereas tylB, tylC, and tylD mutants were blocked specifically in the biosynthesis or the addition of mycaminose, mycarose, and 6-deoxy-d-allose, respectively. Two classes of mutants (tylH and tylI) blocked in specific oxidations of tylactone and two classes (tylE and tylF) blocked in specific O-methylations of demethylmacrocin and macrocin were also characterized. Cofermentation and bioconversion studies with these mutants suggested the following relationships: (i) the tylosin sugars are derived from a common intermediate; (ii) tylactone is the first intermediate which can be excreted in appreciable quantities; (iii) the addition of mycaminose to the C-5 hydroxyl group of tylactone must precede oxidations at C-20 and C-23; (iv) oxidation at C-20 normally precedes the attachment of mycarose to the 4′ hydroxyl position of mycaminose; and (v) 6-deoxy-d-allose is added to the C-23 hydroxyl position of the lactone and subsequently O-methylated at 2‴ and 3‴ positions. The O-methylations appear to be the final two steps in tylosin biosynthesis, and the 2‴ O-methylation must occur before the 3‴ O-methylation can take place. All of the tyl mutants except the tylG mutants produced relatively high levels of tylosin-like intermediates or shunt products. Mutants blocked in specific steps other than 3‴ O-methylation, including a mutant blocked in 2‴ O-methylation of demethylmacrocin, produced normal levels of macrocin O-methyltransferase. Mutants apparently containing specific tylosin structural gene mutations produced normal levels of aerial mycelia and spores, produced low levels of tylosin aldehyde reductase, and were resistant to high levels of tylosin. However, three atypical tylG mutants produced no tylosin-like compounds, could not cosynthesize tylosin with any other tyl mutant, could not bioconvert tylactone or macrocin to tylosin, and produced no macrocin O-methyltransferase. These three mutants produced elevated levels of tylosin aldehyde reductase. In addition, one was very succeptible to tylosin and did not produce aerial mycelia or spores.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Delić V., Hopwood D. A., Friend E. J. Mutangenesis by N-methyl-N'-nitro-N-nitrosoguanidine (NTG) in Streptomyces coelicolor. Mutat Res. 1970 Feb;9(2):167–182. doi: 10.1016/0027-5107(70)90055-2. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Furumai T., Takeda K., Suzuki M. Studies on the biosynthesis of basic 16-membered macrolide antibiotics, platenomycins. IV. Biosynthesis of platenomycins. J Antibiot (Tokyo) 1975 Oct;28(10):789–797. doi: 10.7164/antibiotics.28.789. [DOI] [PubMed] [Google Scholar]
- Gersch D., Bocker H., Thrum H. Biosynthetic studies on the macrolide antibiotic turimycin using 14C-labeled precursors. J Antibiot (Tokyo) 1977 Jun;30(6):488–493. doi: 10.7164/antibiotics.30.488. [DOI] [PubMed] [Google Scholar]
- HAMILL R. L., STARK W. M. MACROCIN, A NEW ANTIBIOTIC, AND LACTENOCIN, AN ACTIVE DEGRADATION PRODUCT. J Antibiot (Tokyo) 1964 Jul;17:133–139. [PubMed] [Google Scholar]
- Matern H., Brillinger G. U., Pape H. Stoffwechselprodukte von Mikroorganismen. 114. Thymidin-diphospho-D-glucose-oxidoreduktase aus Streptomyces rimosus. Arch Mikrobiol. 1973;88(1):37–48. [PubMed] [Google Scholar]
- Morin R. B., Gorman M., Hamill R. L., Demarco P. V. The structure of tylosin. Tetrahedron Lett. 1970 Nov;(54):4737–4740. doi: 10.1016/s0040-4039(00)89382-x. [DOI] [PubMed] [Google Scholar]
- Nakahama K., Igarasi S. Microbial conversion of antibiotics. IV. Reduction of maridomycin. J Antibiot (Tokyo) 1974 Aug;27(8):605–609. doi: 10.7164/antibiotics.27.605. [DOI] [PubMed] [Google Scholar]
- Omura S., Kitao C., Miyazawa J., Imai H., Takeshima H. Bioconversion and biosynthesis of 16-membered macrolide antibiotic, tylosin, using enzyme inhibitor: cerulenin. J Antibiot (Tokyo) 1978 Mar;31(3):254–256. doi: 10.7164/antibiotics.31.254. [DOI] [PubMed] [Google Scholar]
- Omura S., Nakagawa A. Chemical and biological studies on 16-membered macrolide antibiotics. J Antibiot (Tokyo) 1975 Jun;28(6):401–433. doi: 10.7164/antibiotics.28.401. [DOI] [PubMed] [Google Scholar]
- Omura S., Takeshima H., Nakagawa A., Miyazawa J., Piriou F., Lukacs G. Studies on the biosynthesis of 16-membered macrolide antibiotics using carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Jun 28;16(13):2860–2866. doi: 10.1021/bi00632a009. [DOI] [PubMed] [Google Scholar]
- Ono H., Harada S., Kishi T. Maridomycin, a new macrolide antibiotic. VII. Incorporation of labeled precursors into maridomycin and preparation of 14C-labeled 9-propionylmaridomycin. J Antibiot (Tokyo) 1974 Jun;27(6):442–448. [PubMed] [Google Scholar]
- Pape H., Brillinger G. U. Stoffwechselfprodukte von Mikroorganismen. 113. Biosynthese von Thymidin-diphospho-mycarose durch ein zellfreies System aus Streptomyces rimosus. Arch Mikrobiol. 1973;88(1):25–35. [PubMed] [Google Scholar]
- Randazzo R., Sciandrello G., Carere A., Bignami M., Velcich A., Sermonti G. Localized mutagenesis in Streptomyces coelicolor A3 (2). Mutat Res. 1976 Sep;36(3):291–302. doi: 10.1016/0027-5107(76)90239-6. [DOI] [PubMed] [Google Scholar]
- Seno E. T., Pieper R. L., Huber F. M. Terminal stages in the biosynthesis of tylosin. Antimicrob Agents Chemother. 1977 Mar;11(3):455–461. doi: 10.1128/aac.11.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]


