Abstract
Intestinal trefoil factor (ITF), a small, compact protease-resistant peptide, is abundantly expressed in goblet cells of large and small intestine. Although several biological activities of ITF have been identified, including promotion of wound healing, stimulation of epithelial cell migration, and protection of intestinal epithelial barrier, little is known about signaling events through which ITF mediates its physiological function. In this study, the effects of exogenous ITF on mitogen-activated protein kinase (MAPK) signaling cascades were examined in IEC-6 cells, a nontransformed intestinal epithelial cell line that does not express endogenous trefoil peptides. Stimulation with ITF resulted in rapid decrease in extracellular signal-related protein kinase (ERK) activity and concomitant reduced ERK tyrosine phosphorylation. ITF also decreased activation of ERK activity induced by either transforming growth factor-α, which links extracellular stimuli to the Ras/Raf/MEK/ERK pathway via the epidermal growth factor receptor, or phorbol 12-myristate 13-acetate, which activates Raf through protein kinase C. ITF-induced inhibition of ERK activity was blocked by an inhibitor of tyrosine and dual-specific phosphatases, sodium orthovanadate. In summary, ITF leads to inhibition of ERK and the MAPK pathway through activation of tyrosine or dual-specific phosphatase.
Keywords: IEC-6 cell, mitogen-activated protein kinase, TGFα, PMA, phosphatase
The mammalian trefoil peptide family is composed of three small protease-resistant proteins characterized by three interchain disulfide bonds forming the trefoil motif or “P” domain (1, 2). Additional proteins including the trefoil motif have been isolated from amphibian skin and stomach (3, 4). In the normal mammalian gastrointestinal tract, expression of the three known trefoil peptides is generally found in a regional selective distribution, although some interspecies variation in the distribution of one of the trefoil peptide (SP) has been observed (5, 6). Intestinal trefoil factor (ITF) is the most widely expressed member of the family and is present in goblet cells throughout small and large intestine (7, 8).
Increased expression of trefoil peptides has been observed in proximity to sites of injury in the gastrointestinal tract, including peptic ulcers and ulcers resulting from inflammatory bowel disease (9–12). In vitro studies have demonstrated that ITF and other trefoil peptides promote re-establishment of mucosal integrity after injury (13). Trefoil peptides have also been shown to protect intestinal epithelial cell monolayers against a variety of injurious agents in cooperation with mucin glycoproteins (14). Consistent with these in vitro observations, oral administration of ITF or SP has been shown to protect against ethanol- and indomethacin-induced gastric injury in the rat (15), and genetic disruption of the ITF gene results in a marked susceptibility to mucosal injury in mice (16). Despite accumulating knowledge about functions of the trefoil peptides, intracellular signaling events through which they mediate their physiological function remain unknown.
The mitogen-activated protein kinases family, including the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase/stress-activated protein kinases (JNK/SAPK), and p38/HOG1, are important components of an intracellular regulatory network that transduces extracellular stimuli to effect intracellular responses such as proliferation, differentiation, and adaptation to external stress (for reviews see refs. 17 and 18). Because of the pleiotropic function of these kinases, their activation and inactivation need to be tightly regulated. A variety of extracellular stimuli such as growth factors, cytokines, and stress stimuli have been demonstrated to initiate mitogen-activated protein kinase (MAPK) activation. However, little is known about factors that down-regulate the MAPK pathway, although one report has demonstrated inhibition of p38MAPK by insulin in cultured fetal neurons (19). Recent reports have highlighted the potential importance of protein phosphatases that may themselves be activated in response to a variety of stimuli (20–24).
In the present study, we examined the effect of exogenous ITF on MAPK activity in IEC-6 cells, a nontransformed intestinal epithelial cell line that lacks any endogenous trefoil peptide expression. These studies demonstrate that ITF inhibits steady-state levels as well as activated levels of ERK1 and ERK2 in these cells in association with activation of tyrosine or dual-specific phosphatases.
MATERIALS AND METHODS
Reagent.
[γ-32P]ATP was obtained from New England Nuclear. Polyclonal antibodies against JNK1, p38, ERK1, and ERK2 were purchased from Santa Cruz Biotechnology. Monoclonal for phosphotyrosine (4G10) was from Upstate Biotechnology (Lake Placid, NY). Monoclonal antibody for phosphotyrosine (PY20) was from Transduction Laboratories (Lexington, KY). Glutathione S-transferase-c-Jun (amino acid 1–79) and glutathione S-transferase-AFT-2 (amino acid 1–96) were from Santa Cruz. Recombinant human transforming growth factor α (TGFα) was from R & D Systems. Phorbol 12-myristate 13-acetate (PMA) and okadaic acid (tissue culture grade) were from Sigma.
Preparation of Human Recombinant ITF in Yeast.
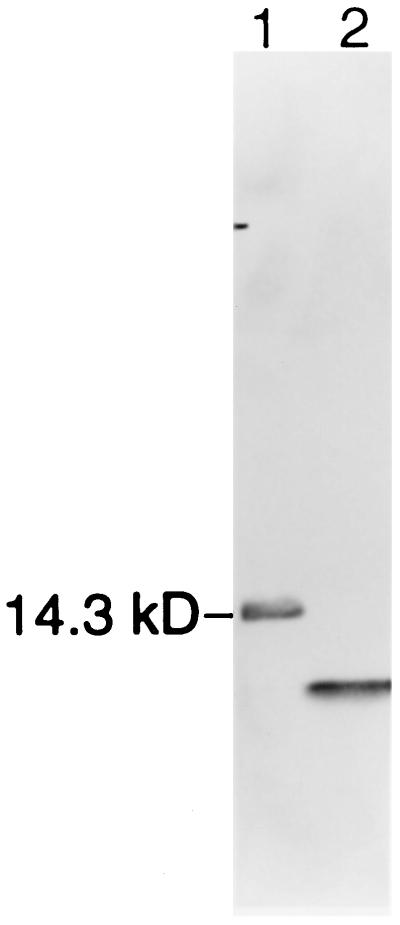
Human intestinal trefoil factor (hITF) was expressed from Pichia pastoris using derivatives of the pPIC9 expression vector (Invitrogen) according to the manufacturer’s instructions. The hITF coding sequences in PCR 1000 vector (Invitrogen) (8) were amplified by PCR using primers 5′-TGCAGTCTCGAGAAAAGAGAGGCTGAGGAGTACGTGGGCCTGTCTGCA-3′ (which adds a XhoI site and junction sequences between the alpha mating factor secretion sequences and hITF) and 5′-GTACGAATTCCTATCAGAAGGTGCATTCTGCTTGCAG-3′ (which adds a stop codon and an EcoRI site downstream of the gene). The PCR product was digested with XhoI and EcoRI and cloned into plasmid pCCM48, which contains a kanamycin-resistance gene inserted into pPIC9 between the HIS4 and AOX 3′ regions, producing pCCM56. Plasmid pCCM56 containing the hITF gene was digested with StuI, electroporated into GS115, and plated on MD agar selecting HIS4. Strains expressing hITF were identified by a colony immunoblot with antiserum raised against recombinant rat ITF (7). SDS/PAGE analysis of culture supernatants from methanol-induced cultures confirmed hITF expression in these strains. One isolate, CCM280, was used for hITF production, and purification was accomplished essentially as previously described (25) (see Fig. 1). SDS/PAGE was carried out in 15% polyacrylamide slab gels by standard techniques after suspending samples in 25 μl of sample buffer, with or without reducing agent (1 mM DTT). Gels were electrophoresed at 25 mA for 1 h followed by 50 mA for approximately 1.5 h. Protein was visualized by staining with silver staining technique.
Figure 1.
SDS/PAGE of recombinant human ITF. hITF was expressed by using a P. pastoris derivative of the pPIL9 expression vector after subcloning of PCR product encoding full-length hITF, as described in the text. Peptide was purified by sequential chromatography as previously described (25). SDS/PAGE of 5 μg of purified ITF in nonreducing (lane 1) or reducing (lane 2, 1 mm DTT) conditions (proteins are localized by the silver stain method) is shown.
Cell Culture.
IEC-6 cells (passage 15–20) derived from rat small intestinal epithelium (26) were maintained in DMEM (Cellgro, Mediatech, Herndon, VA) with 5 μg/ml insulin, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5% heat-inactivated fetal calf serum (FCS) (Sigma).
Immunoprecipitation and Immunoblot Analysis.
After washing with PBS, cells were incubated for 12 h in serum-free media. After stimulation with various factors or reagents, cells were washed with PBS and immediately frozen in liquid nitrogen. The cells were then lysed on ice in buffer (0.5% Nonidet P-40/10 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM EDTA/10 μg/ml aprotinin/100 μM phenylmethylsulfonyl fluoride/10 μg/ml leupeptin) containing phosphatase inhibitors (500 μM sodium orthovanadate, 100 mM NaF, and 10 mM sodium pyrophosphate). The lysates were centrifuged at 10,000 × g for 20 min at 4°C, and the protein concentration in resulting supernatants was quantified by the Bradford method (Bio-Rad). Various antibodies were added to the clarified cell lysates and then incubated for 2 h and precipitated with protein A-Sepharose (Pharmacia). The resulting immune complexes were washed three times with lysis buffer. SDS-sample buffer was added, and immune complexes were boiled for 5 min. Gel electrophoresis and immunoblot analysis with antiphosphotyrosine antibodies or other antibodies using Renaissance chemiluminescent reagents (DuPont) were performed as described before (27, 28). Some immunoblots were stripped of antibodies with 62.5 mM Tris (pH 6.8), 2% SDS containing 100 mM 2-mercaptoethanol at 50°C for 30 min.
Immune Complex Kinase Assay.
Proteins immunoprecipitated by antibodies absorbed on protein A-Sepharose were washed two times with lysis buffer and once with kinase reaction buffer (40 mM Hepes, pH 7.5/10 mM MgCl2/3 mM MnCl2). The immune complexes were resuspended in 50 μl of kinase buffer supplemented with 10 μCi of [γ-32P]ATP and 7.5 μg of myelin basic protein (MBP) (Sigma) for ERK1 and ERK2, 0.3 μg of c-Jun for JNK1, or 0.3 μg of ATF-2 for p38, respectively. Kinase reactions were performed for 30 min at 25°C and terminated by boiling in SDS-sample buffer. Samples were analyzed by SDS-PAGE and autoradiography.
Statistical Analysis.
Student’s t test was used for comparative analysis in studies.
RESULTS
ITF Decreases the Steady-State Level of ERK but Not of JNK1 or p38 MAPK Activity in IEC-6 Cells.
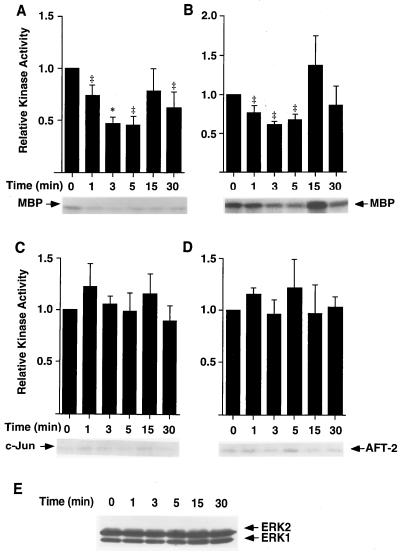
Initial studies were carried out to assess the effects of ITF on MAPK pathways in IEC-6 cells. This cell line has been proven to be responsive to trefoil peptides in past studies but lacks any endogenous ITF production (7). The effect of ITF on the kinase activity of ERK1/2 was evaluated by in vitro phosphorylation of the substrate MBP. As shown in Fig. 2A, stimulation with 0.1 μM ITF resulted in a 50% decrease in ERK1 activity within 3 min. Maximal decrease was achieved at 5 min and persisted at 30 min after stimulation. Decreased ERK1 activity was evident following treatment with concentrations of ITF as low as 10 nM, a concentration substantially below that present on the mucosal surface. ITF did not affect the level of ERK1 and ERK2 expression during the observed time periods (Fig. 2E). ITF stimulation also resulted in a comparable decrease in ERK2 activity (Fig. 2B). In contrast to ERK1 and ERK2, inhibition of MAP kinase activity by ITF was specific for ERKs, as neither JNK1 nor p38 MAP kinase was significantly affected by ITF (Fig. 2 C and D, respectively).
Figure 2.
ITF decreases the steady-state level of ERK activity in IEC-6 cells. Cell or lysates (200 μg) from subconfluent IEC-6 cells stimulated for indicated times with 0.1 μM ITF were immunoprecipitated by anti-ERK1 antibody (A) or anti-ERK2 antibody (B). Resulting immunocomplexes were subjected to kinase assay in the presence of 10 μCi of [γ-32P]ATP and 7.5 μg of MBP. Kinase reactions were performed for 30 min at 25°C, and samples were analyzed by SDS/PAGE and autoradiography. Relative ERK activity was quantified by densitometry. Results are the mean ± SEM of five independent experiments. JNK-1 (C) was assessed by using 0.3 μg of c-Jun as substrate instead of MBP and p38 MAP kinase (D) using 0.3 μg of AFT2. 20 μg of cell lysates prepared in A and B were analyzed by Western blotting using ERK1/2 antibodies. ∗, Significantly different from time 0 at P < 0.05; ‡, P < 0.01.
Stimulation of ITF Results in Decreased Tyrosine Phosphorylation of ERK.
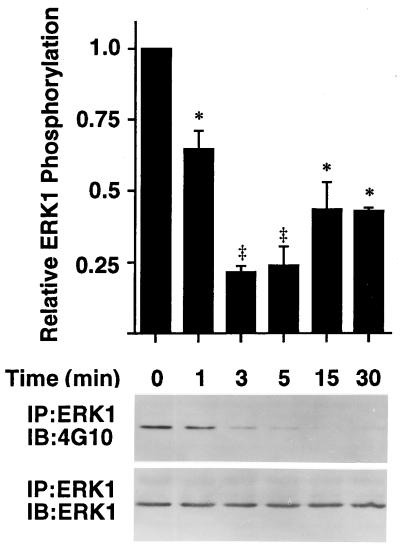
Full activation of MAP kinases requires dual phosphorylation on threonine and tyrosine residues within the kinase domain. To determine if the inhibition of ERK activity by ITF is due to changes in the tyrosine phosphorylation state of the enzyme, ERK1 was immunoprecipitated from IEC-6 cells either unstimulated or stimulated for the indicated time with 0.1 μM ITF, and resulting immunocomplexes were subjected to Western blotting using antiphosphotyrosine antibodies. Consistent with the decreased ERK activity by ITF demonstrated in Fig. 2A, the level of tyrosine phosphorylation of ERK in IEC-6 cells was markedly decreased by ITF stimulation (Fig. 3). The effect of ITF was evident within 1 min, maximal by 3 min, and sustained for 30 min after stimulation.
Figure 3.
Stimulation by ITF results in decreased tyrosine phosphorylation of ERK. Cell lysates (500 μg) from subconfluent IEC-6 cells either unstimulated or stimulated for indicated times with 0.1 μM ITF were immunoprecipitated by anti-ERK1 antibody. Resulting immunocomplexes were subjected to Western blotting by using antiphosphotyrosine antibodies (Upper). The membranes were stripped and immunoblotted with anti-ERK1 antibody (Lower). ERK1 phosphorylation (Upper) at each time period was normalized to that of immunoprecipitated ERK1 (Lower) by using laser densitometry, and normalized content was compared with that at time 0. Results are the mean ± SEM of three independent experiments. ∗, Significantly different from time 0 at P < 0.05; ‡, P < 0.01.
ITF Decreased the Levels of EGF and PMA-Induced ERK Activation.
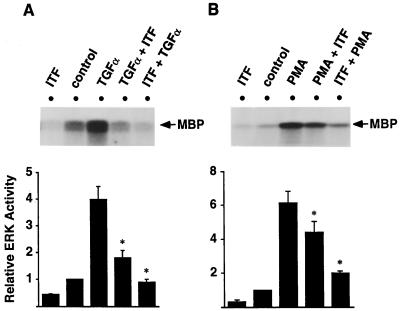
After demonstration that ITF decreased the steady-state level of ERK activity, we examined whether ITF could decrease or inhibit activation of ERK by other factors. Thus, the effect of ITF on ERK1 was assessed in TGFα- and PMA-stimulated cells, agents that activate ERK1 via EGFR (Ras/Raf/MEK/ERK) and protein kinase C (Raf/MEK/ERK), respectively. As shown in Fig. 4A, TGFα-induced activation of ERK1 was inhibited by subsequent treatment with ITF. Moreover, TGFα was not able to activate ERK1 in IEC-6 cells pretreated with ITF. Similar results were obtained in PMA-stimulated cells. This pretreatment with ITF reduced PMA stimulation of ERK. PMA was also unable to stimulate ERK activation in ITF-pretreated cells (Fig. 4B).
Figure 4.
ITF decreased the levels of ERK activated by either TGFα or PMA in IEC-6 cells. Subconfluent IEC-6 cells were treated with several reagents as indicated above. Cell lysates were prepared (200 μg of protein in a 200-μl volume) and immunoprecipitated with anti-ERK1 antibody. Immunoprecipitants were subjected to kinase assay by using MBP as a substrate. (A) Lane 1, ITF (0.1 μM, 3 min); lane 2, control; lane 3, TGFα (10 ng/ml, 5 min) followed by PBS for 3 min; lane 4, TGFα (5 min) followed by ITF for 3 min; lane 5, ITF (3 min) followed by TGFα (8 min). (B) Lane 1, ITF (0.1 μM, 3 min); lane 2, control; lane 3, PMA (100 nM, 10 min) followed by PBS for 3 min; lane 4, PMA (10 min) followed by ITF for 3 min; lane 5 ITF (3 min) followed by PMA (13 min). Before adding another reagent, cells were briefly washed by prewarmed PBS. Relative ERK1 activity was quantified by densitometry ( A and B, Lower). Results are the mean ± SEM of three independent experiments. ∗, Significantly different from TGFα (A) or PMA (B) stimulation at P < 0.05.
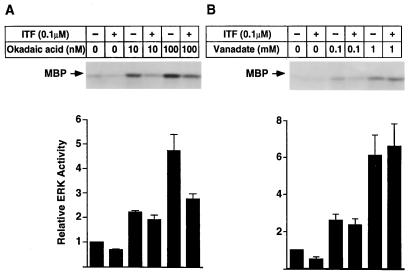
Sodium Orthovanadate but Not Okadaic Acid Blocked the Inhibitory Effect of ITF on ERK1 Activity.
After demonstration of reduced ERK activation and corresponding decreased tyrosine phosphorylation of ERK itself, studies were carried out to determine whether activation of a protein phosphatase might be involved in the inhibition of ERK by ITF. Okadaic acid, a specific inhibitor of serine/threonine phosphatases 1 and 2A (29), has been shown to activate ERK in different cells (30–32). As shown in Fig. 5, okadaic acid also activates ERK1 in IEC-6 cells in a dose-dependent fashion as expected. The level of ERK1 activated by either 10 nM or 100 nM okadaic acid was diminished by subsequent treatment with ITF (Fig. 5A), indicating that the inhibitory effect of ITF on ERK1 activity is insensitive to this serine/threonine phosphatase inhibitor.
Figure 5.
Sodium orthovanadate blocks the inhibitory effect of ITF on ERK1 activity. Subconfluent IEC-6 cells were pretreated with sodium orthovanadate for 15 min (B) or okadaic acid for 45 min (A) before addition of ITF (0.1 μM) for 3 min. Cell lysate were prepared (200 μg of protein in a 200-μl volume) and immunoprecipitated with anti-ERK1 antibody. Immunoprecipitants were subjected to kinase assay using MBP as a substrate. Relative ERK1 activity was quantified by densitometry. Results are the mean ± SEM of three independent experiments.
Sodium orthovanadate, an inhibitor of protein tyrosine and dual-specific phosphatases (29), was added to IEC-6 cell culture 10 min before ITF stimulation. Stimulation with sodium orthovanadate for 15 min markedly activated ERK1 in IEC-6 cells in a dose-dependent manner. In contrast to its inhibition of okadaic acid-induced ERK activation, orthovanadate-activated level of ERK1 was not affected by subsequent treatment with ITF (0.1 μM, 3 min) (Fig. 5B). In aggregate, these findings suggest that inactivation of ERK by ITF is dependent on the activity of vanadate-sensitive phosphatases in IEC-6 cells.
DISCUSSION
The epithelial cell population of the intestinal mucosa is composed of a dynamic continuum ranging from proliferating stem cells in the crypt to terminally differentiated enterocytes and colonocytes. To sustain the integrity of the mucosal surface, exquisite balance of cellular proliferation, differentiation, and senescence must be achieved through regulatory mechanisms. It is increasingly clear that control of these processes is achieved through a complex network of regulatory peptides including growth factors and cytokines. Intestinal epithelial cell populations have been found to be responsive to members of a wide variety of growth factor families including the EGF, IGF, TGFα, FGF, and HGF families (33). In addition, several cytokines have been demonstrated to modulate both proliferative and/or phenotypic features of the intestinal epithelium including interleukin-1, -2, -4, -7, -9, -11, and -15 as well as tumor necrosis factor α and interferon γ (33). Although these regulatory peptides are structurally diverse, all modulate intestinal epithelium through basolateral surface receptors.
In addition to these growth factors and cytokines, intestinal trefoil factor as well as other members of the trefoil family have been shown to modulate key functional responses of the intestinal epithelium. These include most importantly cell migration following injury, which accomplishes rapid repair after disruption of mucosal continuity (12, 13, 16). In contrast to both growth factors and cytokines, the trefoil factors seem to act at the apical surface. Recent studies using an in vitro model of intestinal epithelial wounding suggests that trefoil factors act through mechanisms that are complementary to the wide range of regulatory peptides present at the basolateral pole of the epithelium (13, 14). However, the mechanism of these responses has not been previously defined.
The present studies demonstrate substantial down-regulation of key pathways of intracellular signaling following exposure to the representative trefoil peptide ITF. Most importantly, these studies demonstrate inhibition of the key kinases ERK1 and ERK2. These kinase activities serve as the focal point of cellular responses to many regulatory peptides as well as other external stimuli affecting intestinal epithelial cells in a manner similar to other cell populations.
It is notable that the intestinal trefoil factor seems to have opposite effects to many of the pro-proliferative regulatory peptides in leading to down-regulation of MAPK activity. Furthermore, trefoil peptides seem to have the capability of overriding activation of the MAPK pathway by these pro-proliferative factors. Thus, inactivation of MAPK activity by ITF was observed when intestinal epithelial cells were exposed to either TGFα or PMA stimuli, which activate the MAPK pathway through two distinct but proximal signaling events (i.e., through EGFR formation of the GRB2/SOS complex that leads to Ras-mediated activation of MAPK through Raf-1 and conversely activation through protein kinase C). It seems that the trefoil peptide ITF abrogates MAPK pathway through activation of a tyrosine or a dual-specific phosphatase activity. Activation of protein phosphatase activity as a potent mechanism regulating MAPK signaling pathways has been reported recently in response to other extracellular stimuli (20–24, 34, 35). However, it should be noted that compensatory effects on non-ITF-dependent pathways by vanadate cannot be excluded. We also note that these studies have focused on relatively short-term response to the trefoil peptide. Whether these effects are sustained, modulated, or down-regulated in the context of long term exposure, which might occur in vivo, remains to be determined.
It remains unclear whether these specific effects on key signaling pathways are achieved through cell surface receptors for trefoil peptides. Although preliminary evidence of a trefoil binding peptide has been described (36), a specific signaling trefoil receptor has not been identified. Whereas the presence of these specific effects on key signaling peptides may well suggest the effects of a receptor-induced response, it should be noted that activation of another signaling pathway through p38 and JNK1 in response to physical distortion of myocytes has been described previously (37). In this context, it should be noted trefoil factors effect a pro-migratory response on intestinal epithelial cell populations. It is therefore possible that the modulation of MAP kinase signaling pathways is achieved through induced migration and changes of cytoskeletal structure rather than through a specific cell surface receptor. The modulation of the p38 pathway is presumed to reflect cellular responses secondary to changes in cell shape causing alterations of the cell cytoskeleton (38–40). Further efforts will be directed toward identification of the specific protein phosphatase regulated by the trefoil peptides and further the mechanisms of phosphatase activation. The latter will depend on definitive determination of the presence of cell surface trefoil binding proteins with signaling capability.
Acknowledgments
We thank Ms. Kathy Devaney for expert assistance. These studies were supported by the Donaldson Trust and by National Institutes of Health Grants DK41557 and DK43351.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ERK, extracellular signal-regulated kinase; FCS, fetal calf serum; ITF, intestinal trefoil factor; JNK1, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; MEK, MAP kinase kinase; TGFα, transforming growth factor-α; PMA, phorbol 12-myristate 13-acetate; EGF, epidermal growth factor.
References
- 1.Sands B E, Podolsky D K. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 2.Poulsom R. Baillieres Clin Gastroenterol. 1996;10:113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann W. J Biol Chem. 1988;263:7686–7690. [PubMed] [Google Scholar]
- 4.Hauser F, Hoffmann W. J Biol Chem. 1991;266:21306–21309. [PubMed] [Google Scholar]
- 5.Tomasetto C, Rio M C, Gautier C, Wolf C, Hareuveni M, Chambon P, Lathe R. EMBO J. 1990;9:407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rio M C, Bellocq J P, Daniel J Y, Tomasetto C, Lathe R, Chenard M P, Batzenschlager A, Chambon P. Science. 1988;241:705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- 7.Suemori S, Lynch-Devaney K, Podolsky D K. Proc Natl Acad Sci USA. 1991;88:11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podolsky D K, Lynch-Devaney K, Stow J L, Oates P, Murgue B, DeBeaumont M, Sands B E, Mahida Y R. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 9.Wright N A, Poulsom R, Stamp G W, Hall P A, Jeffery R E, Longcroft J M, Rio M C, Tomasetto C, Chambon P. J Pathol. 1990;162:279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- 10.Hanby A M, Poulsom R, Elia G, Singh S, Longcroft J M, Wright N A. J Pathol. 1993;169:355–360. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- 11.Wright N A, Poulsom R, Stamp G, Van Noorden S, Sarraf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C, Rio M-C, Chambon P. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 12.Alison M R, Chinery R, Poulsom R, Ashwood P, Longcroft J M, Wright N A. J Pathol. 1995;175:405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- 13.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky D K. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky D K. Gastroenterology. 1995;109:516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 15.Babyatsky M W, deBeaumont M, Thim L, Podolsky D K. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 16.Mashimo H, Wu D C, Podolsky D K, Fishman M C. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G L, Vaillancourt R R. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 18.Cano E, Mahadevan L C. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich K A, Kummer J L. J Biol Chem. 1996;271:9891–9894. doi: 10.1074/jbc.271.17.9891. [DOI] [PubMed] [Google Scholar]
- 20.Zheng C F, Guan K L. J Biol Chem. 1993;268:16116–16119. [PubMed] [Google Scholar]
- 21.Guan K L, Butch E. J Biol Chem. 1995;270:7197–7203. doi: 10.1074/jbc.270.13.7197. [DOI] [PubMed] [Google Scholar]
- 22.Bokemeyer D, Sorokin A, Yan M, Ahn N G, Templeton D J, Dunn M J. J Biol Chem. 1996;271:639–642. doi: 10.1074/jbc.271.2.639. [DOI] [PubMed] [Google Scholar]
- 23.Chu Y, Solski P A, Khosravi-Far R, Der C J, Kelly K. J Biol Chem. 1996;271:6497–6591. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 24.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 25.Thim L, Woldike H F, Nielsen P F, Christensen M, Lynch-Devaney K, Podolsky D K. Biochemistry. 1995;34:4757–4764. doi: 10.1021/bi00014a033. [DOI] [PubMed] [Google Scholar]
- 26.Quaroni A, Wands J, Trelstad R L, Isselbacher K J. J Cell Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai M, Goke M, Tsunekawa S, Podolsky D K. J Biol Chem. 1997;272:6621–6628. doi: 10.1074/jbc.272.10.6621. [DOI] [PubMed] [Google Scholar]
- 28.Kanai M, Rosenberg I, Podolsky D K. Am J Physiol. 1997;272:G885–G893. doi: 10.1152/ajpgi.1997.272.4.G885. [DOI] [PubMed] [Google Scholar]
- 29.Hunter T. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 30.Amaral M C, Casillas A M, Nel A E. Immunology. 1993;79:24–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Casillas A M, Amaral K, Chegini-Farahani S, Nel A E. Biochem J. 1993;290:545–550. doi: 10.1042/bj2900545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whisler R L, Newhouse Y G, Bagenstose S E. Cell Immunol. 1996;168:201–210. doi: 10.1006/cimm.1996.0067. [DOI] [PubMed] [Google Scholar]
- 33.Fiocchi C, editor. Cytokines in Inflammatory Bowel Disease. Heidelberg: Springer; 1996. [Google Scholar]
- 34.Cook S J, Beltman J, Cadwallader K A, McMahon M, McCormick F. Proc Natl Acad Sci USA. 1997;272:13309–13319. doi: 10.1074/jbc.272.20.13309. [DOI] [PubMed] [Google Scholar]
- 35.Rich H, J-K, Lebrin F, Rabilloud T, Leory D, Chambaz E M, Goldberg Y. Science. 1997;276:952–955. doi: 10.1126/science.276.5314.952. [DOI] [PubMed] [Google Scholar]
- 36.Chinery R, Cox H M. Peptides. 1995;16:749–755. doi: 10.1016/0196-9781(95)00045-l. [DOI] [PubMed] [Google Scholar]
- 37.Bogoyevitch M A, Gillespie-Brown J, Ketterman A J, Fuller S J, Ben-Levy R, Ashworth A, Marshall C J, Sugden P H. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 38.Landry J, Huot J. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 39.Huot J, Houle F, Marceau F, Landry J. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 40.Guay J, Lambert H, Gingras-Breton G, Lavoie J N, Huot J, Landry J. J Cell Sci. 1997;110:357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]