Abstract
Metrosideros subg. Metrosideros (Myrtaceae) comprises ≈26 species distributed widely across the Pacific basin. They occur on the ancient Gondwanan landmasses of New Zealand and New Caledonia, as well as on the volcanic islands of the remote Pacific, from Melanesia to tropical Polynesia and the Bonin Island. Phylogenetic analysis based on nuclear ribosomal DNA spacer sequences from all named species showed Metrosideros umbellata of New Zealand as basal in the subgenus, with the remaining species falling into three monophyletic clades. One includes the seven New Caledonian species together with three daughters in western Oceania that probably dispersed during the mid/late Tertiary. A second contains six taxa located in east Melanesia and Samoa that may also have arisen from a mid/late Tertiary dispersal, in this instance from New Zealand. The third includes three New Zealand endemics along with all of the taxa in remote Polynesia and accounts for much of the total range of the subgenus. These dispersed taxa in Polynesia either are identical to the New Zealand species Metrosideros excelsa or differ by a single nucleotide change. We suggest that they are all derived from a Pleistocene dispersal out of New Zealand. A relatively recent dispersal is surprising, given that this wind-dispersed genus has occupied New Zealand for much of the Tertiary and that some of the islands in remote Polynesia date to at least the Miocene. We attribute this dramatic range expansion to climate change—specifically changes in wind flow patterns—in the southern hemisphere during worldwide glaciation.
Keywords: climate change, dispersal
The remote Pacific supports a terrestrial flora that has many close relationships between far flung archipelagoes, some of quite recent origin (1, 2). Transoceanic dispersal by natural vectors must have contributed to this relative homogeneity. Molecular examinations of plant phylogenetic relationships provide opportunities to assess this long-distance dispersal. An appropriate genus for such work is Metrosideros sens. lat., with about 50 species (3). This genus of woody plants is one of the most widely distributed in the Pacific. In the west, it occurs from the sub-Antarctic islands of New Zealand northward to the Bonin Island (Is) near Japan and in the east from Pitcairn Is northward to Hawaii. Many of the islands where Metrosideros is found are remote and of relatively recent origin, indicating the importance of long-distance dispersal to the distribution of this wind-dispersed genus (4). Only one of the two component subgenera, subg. Metrosideros (≈26 spp.; ref. 5), is widespread. By contrast, subg. Mearnsia (≈24 spp.) is largely confined to landmasses around Australia of Gondwanan affinity: New Zealand, New Caledonia, and New Guinea (6). Metrosideros does not, however, occur in Australia, Indonesia, or Micronesia (6, 7). The genus has diversity centers in temperate New Zealand (12 spp.) and adjacent subtropical New Caledonia (16 spp.). New Zealand has the only recorded fossil pollen (late Paleocene/early Eocene; ref. 8) and macrofossils (early Miocene; ref. 9).
The very small seeds of Metrosideros require wind speeds of only 5–19 km per h to be lofted. The seeds can retain viability in temperatures of −30°C for at least 6 h and after seawater immersion for more than 1 month (10). Carlquist (1) suggested that the more remote taxa had achieved long-distance dispersal to the isolated islands of Oceania on high-altitude jet streams that traverse the tropical Pacific from west to east. The most recent speculation on the dispersal history of subg. Metrosideros (6) points to New Zealand rather than New Caledonia as the landmass of origin and suggests that Samoa is the secondary axis of dispersal into remote Polynesia.
Unlike subg. Mearnsia, subg. Metrosideros is notably homogeneous and without sectional divisions (11). For example, the existing taxonomy has Metrosideros collina and the closely allied Metrosideros polymorpha complex occupying a vast range in Oceania, from Vanuatu in the southwest to French Polynesia in the east and Hawaii in the north (5, 12, 13). Molecular phylogenetics can therefore add a potentially useful dimension to the study of the origins and dispersal of the subgenus. We have examined interspecific and intraspecific relationships within subg. Metrosideros by using parsimony analysis of nuclear ribosomal DNA sequence variation. The ITS region encompasses the 5.8S gene and the two flanking internally transcribed spacers (ITS1 and ITS2). All named species in the Pacific basin have been sequenced, and the results have been interpreted both in terms of the likely origins of subg. Metrosideros and its likely dispersal history.
Materials and Methods
All Metrosideros taxa were sampled from naturally occurring populations in the countries of origin and were transported as live material with subsequent storage at −80°C. Vouchers for these samples are held at the Auckland Museum Herbarium in Auckland, New Zealand; the Victoria University Herbarium in Wellington, New Zealand; and the Solomon Is National Herbarium in Honiara, Solomon Is. Collection localities were mostly identified by the Global Positioning System or by grid reference. The outgroup taxon is Cloezia floribunda; a species endemic to New Caledonia and classified within the Metrosideros alliance of the Myrtaceae (14).
Genomic DNA was prepared from frozen leaf material (0.5–2 g) by using a modified Doyle and Doyle (15) procedure. The ITS region of rDNA was amplified with the primers ITSa and ITSb (16) in the following conditions: 50 pmol primers, 0.2 mM each dNTP, 1–100 ng of genomic DNA, 1.5–2 mM MgCl2, and 1 unit AmpliTaq Gold (Perkin–Elmer) in a reaction volume of 100 μl. PCR products were purified with the High Pure PCR kit (Roche Molecular Biochemicals) and sequenced with an Applied Biosystems 363A or 367 sequencer. Double-stranded sequences (length range of 594–611 bp) were edited visually with the Applied Biosystems auto assembler program and initially aligned with the clustal option of the Applied Biosystems sequence navigator, with final alignment done manually.
Phylogenies were generated with paup 4.0d65 provided by D. L. Swofford of the Smithsonian Institution (Washington, DC). Parsimony analysis for all 36 taxa was applied by using the heuristic search option with tree-bisection-reconnection branch swapping, random addition sequence (10 replicates), and gaps treated as “missing.” Of a total of 24 indels, (size range 1–15 bp), 5 informative indels (shared between two or more taxa) were treated as presence/absence characters in a supplemental matrix. Trees were also constructed by neighbor joining under a variety of substitution models; the derived topologies confirmed the relationships shown in the parsimony consensus.
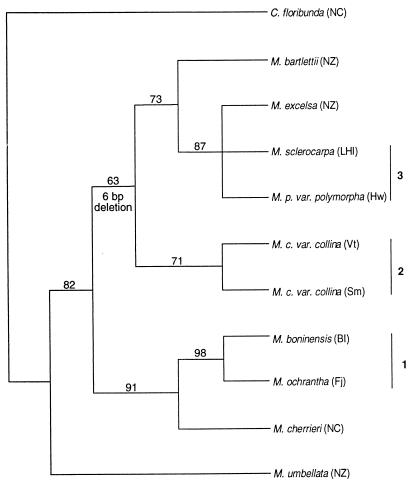
Bootstrapping (17) was applied to a group of taxa arising from data set compression. The bootstrap analysis was performed under parsimony by using the branch and bound option with 1,000 replicates. Taxa included in the compressed data set (see Fig. 2) were (i) the outgroup, C. floribunda, (ii) the basal species, Metrosideros umbellata, (iii) the two Pacific taxa at the geographic poles for each of the three dispersed groups in Oceania (see Fig. 3), and (iv) the phylogenetically most proximate taxon from New Zealand or New Caledonia for each of Groups 1–3. The parsimony-derived consensus (Fig. 1) identified Metrosideros bartlettii as the proximate taxon for Group 2 and Metrosideros excelsa for Group 3. Because the strict consensus topology did not indicate a single New Caledonian taxon, Metrosideros cherrieri was selected as proximate for Group 1 based on the frequency of bipartition association under majority rule.
Figure 2.
Bootstrap analysis of subset of taxa identifies relationships between dispersed taxa in Oceania. Bootstrap values >50% are indicated.
Figure 3.
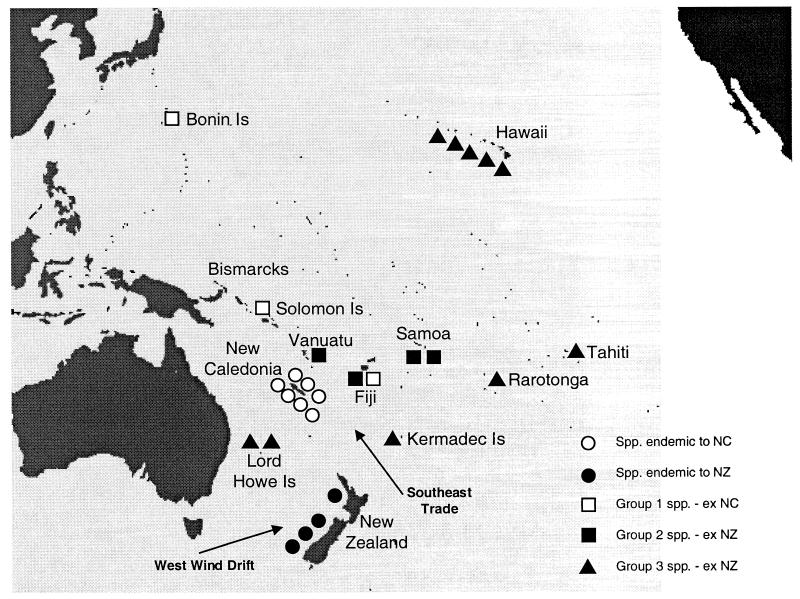
Distribution of subg. Metrosideros taxa sampled in the Pacific. Taxa from Fig. 1 are indicated by symbols, and prevailing winds are indicated by arrows.
Figure 1.
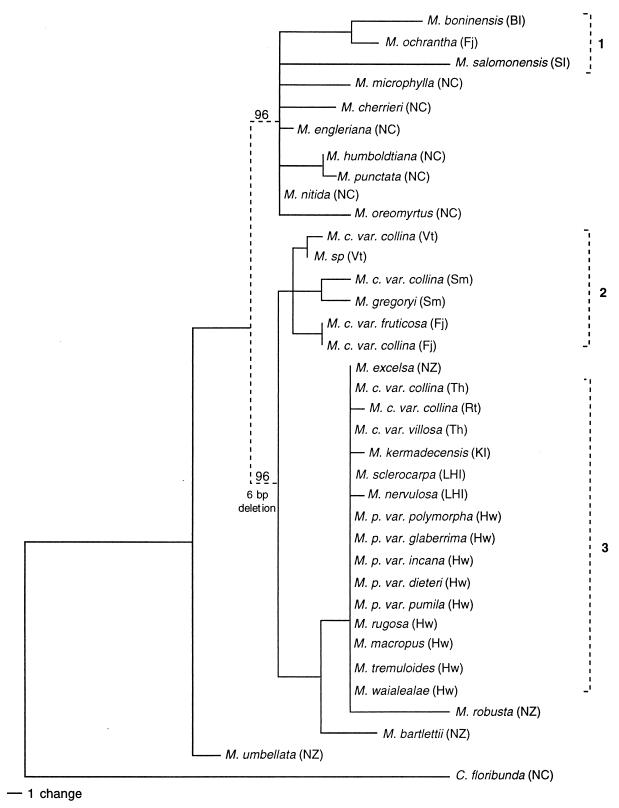
Consensus of parsimony analysis for 36 taxa. Strict consensus is indicated by solid lines; majority rule values >90% are indicated by dashed lines. Numbers refer to dispersed groups (see text). BI, Bonin Is; Fj, Fiji; Hw, Hawaii; KI, Kermadec Is; LHI, Lord Howe Is; NC, New Caledonia; NZ, New Zealand; Rt, Rarotonga; SI, Solomon Is; Sm, Samoa; Th, Tahiti; Vt, Vanuatu.
From parsimony analyses deleting individual taxa from the data set, four species were identified that make large contributions to the homoplasy index. Three of these species, comprising all of Group 1 (Metrosideros salomonensis, Metrosideros boninensis, and Metrosideros ochrantha), are apparent vagrants from New Caledonia occurring as geographic and probably temporal (see Fig. 1) isolates in western Oceania (Solomon Is, Bonin Is, and Mount Kasi, Fiji). The fourth, Metrosideros oreomyrtus, occurs as small scattered populations in New Caledonia itself (18). Removal of these four species from the full parsimony data set substantially reduced the total number of trees retained (from 342 to 12) and also markedly decreased the homoplasy index (from 0.182 to 0.128). Their removal does not, however, lead to a change in topology. Inclusion of these four apparently divergent species in the compressed data set also substantially affects bootstrap values (decreasing them to <50% for the key nodes), again without affecting topology. The relatively robust bootstrap values from the compressed data set shown in Fig. 2 still include two of the four divergent species.
Results and Discussion
Phylogenetic Analysis.
PCR products from the 36 taxa collected throughout the Pacific were sequenced for ITS1–5.8S-ITS2. When more than one individual was sequenced per taxon (six taxa), the sequences were identical except that two samples of M. umbellata, taken from the geographical extremes of its range, differed by 1 bp. Several different species gave identical sequences to each other, including all of the Hawaiian species and M. excelsa from New Zealand.
The 36 sequences were aligned and subjected to parsimony and distance-based analyses (see Materials and Methods). A strict consensus parsimony tree, calculated for the 342 equally most parsimonious trees (121 steps), is shown as Fig. 1 (majority rule values >90% are indicated by dashed lines). Of 641 total characters, 546 were constant; 64 were uninformative; and 31 (including 5 indels) were informative among the 25 divergent sequences obtained. Pairwise sequence divergence ranged from 0 to 0.035 (and to 0.091 for outgroup). Bootstrapping was applied to a subset sample of 11 (Fig. 2) taxa (see Materials and Methods) to examine the important relationships suggested by the consensus topology (Fig. 1). In this case, a single most parsimonious tree was retained with 19 informative characters.
In its lower order structure, the consensus topology shows three distinct clades within subg. Metrosideros, with M. umbellata of New Zealand basal to all three (Fig. 1). Among these groupings, the Gondwanan species have a clear geographic separation, with species endemic to New Caledonia or New Zealand present in separate clades. The remote Pacific island taxa fall into three groups on the consensus tree (numbered 1–3 in Figs. 1 and 2). Group 1 consists of three species lying within the New Caledonian clade with a western Pacific distribution. Group 2, with six taxa sampled, is located as a sister clade to the New Zealand species and is distributed through east Melanesia to Samoa. Group 3 consists of nine species (15 taxa) nested terminally among the New Zealand endemics. This group is dispersed widely across the Pacific from Lord Howe Is to remote Polynesia, including Hawaii.
Thus, the dispersed species of the subgenus in Oceania seem not to be monophyletic and not to have a single landmass of origin (Figs. 1 and 2). Instead, the consensus and bootstrapped topologies suggest that at least three separate groups have dispersed from the two Gondwanan landmasses into the rest of Oceania, as shown in Fig. 3. Bootstrap support for the topology derived from selected dispersed taxa is generally moderate to high (Fig. 2).
Weakest support (63%) is for the node supporting the New Zealand species other than M. umbellata and all probable Pacific relatives (Groups 2 and 3). However, these taxa share a distinctive 6-bp deletion, shown in Fig. 4, which is not present in either M. umbellata or any of the species from the New Caledonian clade. The calculation of Lloyd and Calder (19) was used to obtain a multiresidue gap probability for the 6-bp deletion. To derive a maximum estimate of the probability that this character is shared by Group 2 and 3 taxa in Oceania because of chance rather than through common ancestry, a second hypothetical 6-bp gap was posited. The resultant probability estimate is E6 < 1.2 × 10−3. The actual probability is likely to be considerably less given that a second 6-bp deletion does not exist in the ITS data set (≈600-bp). For example, Lloyd and Calder (19) found only two 6-bp gaps in a search of 9,893 bp of noncoding DNA from humans, chimpanzees, and gorillas (E6 = 1.01 × 10−4). We therefore consider that this deletion is highly unlikely to be polyphyletic. The two Fijian taxa sampled also have a duplication of three nucleotides in the same region (Fig. 4).
Figure 4.
The 6-bp deletion indicated in Fig. 1 and 2 is shown for selected taxa. The Fijian taxon also has an adjacent 3-bp insertion.
Some indication of dispersal history for the three Pacific groups can be taken from nucleotide divergence. The nine species (15 taxa) of Group 3 either are base perfect with or differ by a single base pair change/indel from the New Zealand endemic M. excelsa. By contrast, the three species (six taxa) of Group 2 differ by a minimum of 6-bp changes and two indels (1-bp insertion and 2-bp insertion) from the closest congener in New Zealand, whereas the three species in Group 1 differ by a minimum of 7-bp changes and one indel (1-bp insertion) from the closest congener in New Caledonia. These differences strongly suggest that dispersal of Group 3 into the most distant parts of the subgenus range substantially postdates dispersal for the other two groups.
Relative rate tests with the one-dimensional method of Tajima (20) were done on the full species complements of the three clades. Each clade was separately compared with the other two by using 10 randomly selected pairwise comparisons and with basal M. umbellata as the taxon of reference. All χ2 values (means shown) are substantially less than the critical value (3.841) at the 5% level (Group 1 vs. Group 2 = 0.45; Group 2 vs. Group 3 = 0.381; Group 1 vs. Group 3 = 0.73). We could not therefore reject rate constancy of ITS sequence evolution in subg. Metrosideros.
We conclude that remote and less speciose New Zealand, rather than closer, more speciose New Caledonia, is the likely source of the great majority of dispersed taxa in Oceania. The probable landmass of origin for the subgenus also seems to be New Zealand, because the endemic M. umbellata is located basal to all other species in the various analyses. We propose at least four dispersal events in the history of Metrosideros subg. Metrosideros: an initial mid/late Tertiary dispersal from New Zealand to New Caledonia, a mid/late Tertiary dispersal from New Caledonia into the western Pacific, again a mid/late Tertiary dispersal from New Zealand into the southwestern Pacific, and finally a Pleistocene dispersal from New Zealand to remote Polynesia. We discuss and justify this interpretation in detail below.
Late Arrivals from New Zealand in Remote Polynesia (Group 3).
Nine species (15 taxa) are all very closely related to M. excelsa of New Zealand but are widely scattered across Oceania (from Lord Howe Is to Hawaii; Fig. 3). Three species in this group are endemic to two island groups lying close to New Zealand, namely Lord Howe Is and the Kermadec Is, and differ from M. excelsa by a single base pair change for ITS. Further east, taxa from Tahiti and Rarotonga are identical to M. excelsa or differ by only 1-bp change. All five Hawaiian endemic species, located above the equator ≈7,000 km from New Zealand, are base perfect with M. excelsa. In light of the long fossil record for the genus in New Zealand and the presence of New Zealand species immediately basal to M. excelsa in the consensus analysis (Fig. 1), we identify New Zealand as the likely landmass of origin for all these dispersed taxa.
The resolution of ITS sequence data is insufficient to allow us to determine the order of the dispersal event or events from New Zealand into remote Polynesia. It remains unclear whether there were multiple dispersals from New Zealand to colonize the various island groups separately or whether dispersal followed a stepwise pathway from the landmass of origin to increasingly distant lands. The lack of any common nucleotide change between the various remote taxa is somewhat surprising and suggests that if sequential steps were involved in the dispersal, they must have all occurred in quick succession. Regardless of the details of the dispersal, the ITS similarities between all of the Group 3 species clearly indicate that that dispersal has occurred relatively recently.
There are limitations to the use of island ages in Oceania in developing hypotheses on the divergence times of DNA sequences (21). However, a minimum mutation rate for ITS in recently allopatric Metrosideros taxa of 1 bp per 1.5 million years (my) can be inferred from the known ages of Rarotonga and Tahiti. Rarotonga has a K-Ar age range of 1.1–2.3 my (22), such that the single base pair change separating M. excelsa and M. collina var. collina probably arose some time in the last 2.3 my. Likewise, on the island of Tahiti-nui, there is a single base pair insertion for M. collina var. villosa relative to M. excelsa, whereas M. collina var. collina is identical to M. excelsa. Tahiti-nui lies within an isolated complex of closely adjacent high islands whose maximum age is ≈1.5 my (23). This minimal rate estimate is similar to that calculated for Cucurbitaceae by Jobst et al. (24), who suggest a nucleotide substitution rate range for ITS1–ITS2 of 0.8–1.6 per my. Given that one of the two taxa in Tahiti and all of the taxa in Hawaii remain identical to M. excelsa, it may be that a general dispersal to remote Polynesia occurred more recently within the time frame we suggest. Therefore, a somewhat higher rate of substitution than the minimum described is possible. We note that many species in the subgenus, including M. excelsa, are pioneers in the primary succession on lava, such that it is unlikely that new volcanic landforms were of themselves barriers to successful colonization (25–27). This reasoning in total is the basis for our interpretation of a Pleistocene dispersal event for Group 3 and of more ancient dispersal events for Groups 1 and 2.
The recent nature of the Group 3 dispersal is the most perplexing aspect of the data, because Metrosideros has occupied New Zealand for most of the Tertiary and because some of the islands in remote Polynesia where the subgenus occurs date to the Miocene. It is surprising that earlier dispersal events to the region do not seem to have occurred. With a likely time frame for dispersal of <2 my, any hypothesis that attempts to explain the recent arrival of subg. Metrosideros in remote Polynesia needs to provide some explanation for conditions facilitating its dispersal that are unique to the Pleistocene.
Several authors (e.g., those of refs. 28 and 29) propose a more powerful west wind drift across cool temperate New Zealand during the latter part of the last glacial than occurs today, together with weaker subtropical/tropical trade easterlies (Fig. 3). Associated with stronger middle latitude westerlies over New Zealand during contemporary El Niño events, a corresponding weakened easterly flow in the tropics sometimes also sees the establishment of westerly winds in the lower latitudes. Rodbell et al. (30) point to an increased periodicity of El Nino events during episodes of cooler climates over the past 15,000 years. These are the conditions, presumably even more frequent and marked in coldest glacial climates, that might have seen wind-dispersed Metrosideros finally break out of a more restricted, western Pacific, Tertiary distribution.
We therefore suggest that the subgenus was confined to the western Pacific by warm Tertiary climates that produced strong southeasterly trade wind flows in the subtropics/tropics and which probably also produced only weak intermittent westerly flows in the latitudes of New Zealand (Fig. 3). Climate change during glacial times in the southern hemisphere then seems to have led to a switch of circumstance that allowed wind-borne seed to move finally westward from New Zealand into remote Polynesia on strong westerlies. This dispersal sequence ultimately also included Hawaii to the north of the equator (Fig. 3)—most probably with a trade-wind-mediated dispersal from east Polynesia by way of the Marquesas Is. The recent nature of the dispersal of Group 3 species in Oceania, as indicated by the lack of sequence differences, suggests that jet stream winds are unlikely to have played a role in the dispersal of the genus (1). If these high altitude winds, which flow westward in the tropical Pacific, had been important in the dispersal of Metrosideros, the genus would likely have entered remote Polynesia much earlier.
A Southwestern Pacific Group from New Zealand (Group 2).
This group is located on the islands of Samoa, Vanuatu, and Fiji in the southwestern Pacific (Fig. 3). The six sequences were from four taxa within the M. collina complex, an unnamed species from Vanuatu, and Metrosideros gregoryi, which is a Samoan endemic (5, 12). Both the consensus and bootstrapped topologies (Figs. 1 and 2) indicate that these taxa form a monophyletic sister clade to the New Zealand species in the subgenus. From the position of its basal node (see Figs. 1 and 2), this clade seems to be derived from an extinct New Zealand ancestor, transitional between M. umbellata and M. bartlettii.
The two varieties of M. collina sampled from Fiji have identical sequences. The unnamed species from Vanuatu is 1 bp divergent from M. collina var. collina from the same island group. In contrast to the sequence similarity within the island groups, the sequences of M. collina compared between these two Melanesian island groups and with Samoa are all quite distinctive. Further, the three taxa of M. collina within the Group 3 sample are very distantly related to these Group 2 sequences. This relation indicates that M. collina is probably not monophyletic in Oceania (Fig. 1).
Assuming a rate of mutational change for allopatric taxa of 1 bp per 1–2 my (see above) and given that the closest species in New Zealand is separated by six nucleotide changes from the most like of these dispersed species, we suggest a mid/late Tertiary dispersal for this southwest Pacific oceanic clade. Further, because the Samoan species fall into Group 2 rather than Group 3, Samoa is unlikely to have acted as the springboard for dispersal into remote Polynesia for subg. Metrosideros (6).
Species from New Caledonia in the Western Pacific (Group 1).
These three species are widely separated across the region (Fig. 3): M. boninensis is endemic to the Bonin Is (Japan); M. ochrantha is endemic to Vanua Levu (Fiji); and M. salomonensis occurs in the Solomon Is and Bismarck Archipelago (6, 12). In the consensus topology, the three species occur among the seven-species grouping that is endemic to New Caledonia. This result conflicts with the hypothesis that identified New Zealand as the landmass of origin for all dispersed species of the subgenus in Oceania (6).
At finer scale, the consensus topology (Fig. 1) suggests that M. boninensis and M. ochrantha are more closely related to each other than to any of the seven species in New Caledonia or to M. salomonensis. We therefore propose a stepwise colonization sequence as the dispersal pathway for M. boninensis, from New Caledonia initially to Fiji and subsequently to the Bonin Is. This dispersal pathway may have been facilitated by intervening colonization of (extant or extinct) islands where the subgenus no longer occurs. M. salomonensis does not ally with the M. ochrantha–M. boninensis pair in the strict consensus; thus, it is unclear whether there have been one or two dispersal events from New Caledonia for these three species. Emergent land in the Solomon Is arc region seems to date from at least the Oligocene (31). Fiji is likely to have emerged earlier during the Eocene (32). Again, first dispersal from New Caledonia may have occurred some time during the mid/late Tertiary. Sequence change that distinguishes the closest congener in New Caledonia from the most like of the three vagrant species (M. ochrantha) is 7 bp.
Origins: New Zealand or New Caledonia?
Hawaii, New Zealand, and New Caledonia are the three landmasses with highest diversity for subg. Metrosideros with, respectively, five, four, and seven endemic species (6). Because the five Hawaiian species are identical for ITS and seem to represent a recently established monophyly, the question of origin for the subgenus focuses on which of the two large Gondwanan landmasses in the southwestern Pacific supported initial differentiation. Wilson (6) suggests New Zealand is the landmass where the two subgenera first diverged and that it was also the site for the initial radiation and dispersal of subg. Metrosideros. The long fossil record for Metrosideros in New Zealand and the absence of known fossils on adjacent Gondwanan lands lends weight to that reasoning. The consensus and bootstrapped topologies (Figs. 1 and 2) support Wilson's hypothesis that M. umbellata of New Zealand is basally located to all other taxa within subg. Metrosideros. M. umbellata, together with the seven New Caledonian species, does not show the 6-bp deletion found in all other New Zealand species in the subgenus and in most of the taxa scattered across Oceania (Fig. 4). The apparent basality of M. umbellata suggests founder dispersal to New Caledonia from New Zealand at some time before the emergence of the lineage that produced the other extant New Zealand species. M. umbellata differs from its closest congener in New Caledonia by 7-bp changes and one indel (15-bp deletion), again suggesting a mid/late Tertiary timing for a likely founder event or events from New Zealand to New Caledonia.
Conclusions
Phylogenetic analysis of the ITS region of rDNA suggests that the widely dispersed group of Pacific plants, Metrosideros subg. Metrosideros, separates into three clades with M. umbellata of New Zealand basal to all. The taxa located in the island Pacific seem to have derived from at least three distinct dispersal events, two from New Zealand and one from New Caledonia. The most spectacular of these has occurred quite recently (probably in the Pleistocene) and has involved movement of the M. excelsa lineage from New Zealand to a number of islands in Oceania, some located adjacent to New Zealand and some in remote Polynesia, at the extremes of the range of the subgenus. The unexpectedly recent nature of the last dispersal series from New Zealand is interpreted by us as resulting from climate change during Pleistocene glaciations (28, 29). We hypothesize that this wind-dispersed group of plants was able to increase its range dramatically by using the unusually powerful westerly weather systems that seem to have developed in the middle and lower latitudes of the southern hemisphere during periods of maximum glaciation. This hypothesis may have broader relevance to the biogeography of the central Pacific if this climatic shift also acted to increase the range of other wind-dispersed terrestrial organisms such that total biodiversity was enhanced in those remote islands. Climate change during periods of worldwide glaciation is recognized as having produced conditions that limited the distribution of many woody plants and of entire plant communities (e.g., refs. 33 and 34). The data presented herein suggest that, in the Pacific, glacial climates may have instead had a beneficial effect on the distribution of plants by enhancing dispersal capability across some of the largest distances of hostile marine habitat on earth.
Acknowledgments
We thank Richard Bellamy, David Penny, Peter Wilson, and James Watson for their support of the project; Tony and Vivian Whitaker, Rhys Gardner, Michael Heads, Lyn Craven, Ewen Cameron, John Braggins, Michael Doyle, Sakiusa Masi, Marika Tuiwawa, Fuyuo Nobushima, Moffet Hane, Gerald McCormack, Myknee Sirikolo, David Taylor, Ian Hankin, Art Whistler, James Atherton, Channel Sam, Karen Trickleback, Natalie Patenaude, Tanguy Jaffre, Jean-Marie Veillon, Roger Pouityela, and Michel Guerin for assistance with sample collection; Jeannette Keeling, Stephen Wichman, and Keith Richards for assistance in the laboratory; Richard Newcombe, Dianne Gleason, Allen Rodrigo, and David Saul for assistance with phylogenetic analysis; Jim Sallinger for discussions on climate; Vivian Ward for graphics assistance; and Clifford Morden and Susan Oviatt Grose for independently confirming the ITS sequence of M. polymorpha. We thank the two anonymous referees whose comments have improved this presentation substantially. Research funding was provided by the University of Auckland Research Fund and the Project Crimson Fund sponsored by Carter Holt Harvey Ltd.
Abbreviations
- Is
Island
- my
million years
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF172732–AF172770).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050351197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050351197
References
- 1.Carlquist S J. Bioscience. 1972;22:221–225. [Google Scholar]
- 2.Mueller-Dombois D, Fosberg F R. Vegetation of the Tropical Pacific Islands. New York: Springer; 1998. [Google Scholar]
- 3.Dawson J W. Blumea. 1976;23:7–11. [Google Scholar]
- 4.Balgooy M M J. Blumea Suppl. 1971;6:1–222. [Google Scholar]
- 5.Dawson J W. Blumea. 1970;18:441–445. [Google Scholar]
- 6.Wilson P G. In: The Origin and Evolution of Pacific Island Biotas, New Guinea to Eastern Polynesia: Patterns and Processes. Keast A, Miller S E, editors. Amsterdam: Academic; 1996. pp. 233–246. [Google Scholar]
- 7.Wilson P G. Aust Syst Bot. 1993;6:251–259. [Google Scholar]
- 8.Mildenhall D C. Palaeogeogr Palaeoclimatol Palaeoecol. 1980;31:197–233. [Google Scholar]
- 9.Pole M. J R Soc N Z. 1993;23:313–328. [Google Scholar]
- 10.Corn C A. In: Challenging Biological Problems: Directions Toward Their Solution. Behnke J A, editor. New York: Oxford Univ. Press; 1972. pp. 422–435. [Google Scholar]
- 11.Dawson J W. Adansonia. 1984;4:465–489. [Google Scholar]
- 12.Smith A C. Am J Bot. 1973;60:479–490. [Google Scholar]
- 13.Dawson J W, Stemmerman L. In: Manual of the Flowering Plants of Hawaii. Wagner W L, Herbst D R, Somer S H, editors. Vol. 1. Honolulu: Univ. of Hawaii Press; 1990. pp. 964–970. [Google Scholar]
- 14.Briggs B G, Johnson L A S. Proc Linn Soc N S W. 1979;102:157–256. [Google Scholar]
- 15.Doyle J J, Doyle J L. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 16.Wendel J F, Schnabel A, Seelanan T. Proc Natl Acad Sci USA. 1995;92:280–284. doi: 10.1073/pnas.92.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsenstein J. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawson J W. Flore de la Nouvelle-Caledonie et Dependances 18.Myrtacees Leptospermoidees. Paris: Museum National D'Histoire Naturelle; 1976. [Google Scholar]
- 19.Lloyd D G, Calder V L. J Evol Biol. 1991;4:9–21. [Google Scholar]
- 20.Tajima F. Genetics. 1993;135:599–607. doi: 10.1093/genetics/135.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldwin B G, Sanderson M J. Proc Natl Acad Sci USA. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner D L, Jarrard R D. J Volcanol Geotherm Res. 1982;12:187–220. [Google Scholar]
- 23.Duncan R A, McDougall I. J Volcanol Geotherm Res. 1976;1:197–227. [Google Scholar]
- 24.Jobst J, King K, Hemleben V. Mol Phylogenet Evol. 1998;9:204–219. doi: 10.1006/mpev.1997.0465. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson I A E. Pac Sci. 1970;24:387–400. [Google Scholar]
- 26.Clarkson B D. N Z J Ecol. 1990;14:59–71. [Google Scholar]
- 27.Drake D R, Mueller-Dombois D. Ecology. 1993;74:1012–1019. [Google Scholar]
- 28.Harrison S P, Metcalfe S E, Street-Perrot F A, Pittock A B, Roberts C N, Sallinger M J. In: SASQUA International Symposium, Swaziland 1983. Vogel J C, editor. Rotterdam, The Netherlands: Belkama; 1983. pp. 21–34. [Google Scholar]
- 29.McGlone M S, Salinger M J, Moar N T. In: Global Climate Since the Last Glacial Maximum. Wright H E, Kutzbach J E, Webb T, Ruddiman W F, Street-Perrot F A, Bartlein P J, editors. Minneapolis: Univ. of Minnesota Press; 1993. pp. 294–317. [Google Scholar]
- 30.Rodbell D T, Seltzer G O, Anderson D M, Abbott M B, Enfield D B, Newman J H. Science. 1999;283:516–520. doi: 10.1126/science.283.5401.516. [DOI] [PubMed] [Google Scholar]
- 31.Falvey D A, Colwell J B, Coleman P J, Greene H G, Vedder J G, Bruns T R. Apea. 1995;19:191–206. [Google Scholar]
- 32.Yan C Y, Kroenke L W. In: Proceedings Ocean Drilling Program, Scientific Results, 130. Berger W H, Kroenke L W, Mayer L A, editors. College Station, TX: Texas A & M; 1993. pp. 697–709. [Google Scholar]
- 33.Tzedakis P C. Philos Trans R Soc London B. 1994;345:403–432. [Google Scholar]
- 34.Van der Kaas W A, Dam M A C. Palaeogeogr Palaeoclimat Palaeoecol. 1995;117:55–72. [Google Scholar]