Abstract
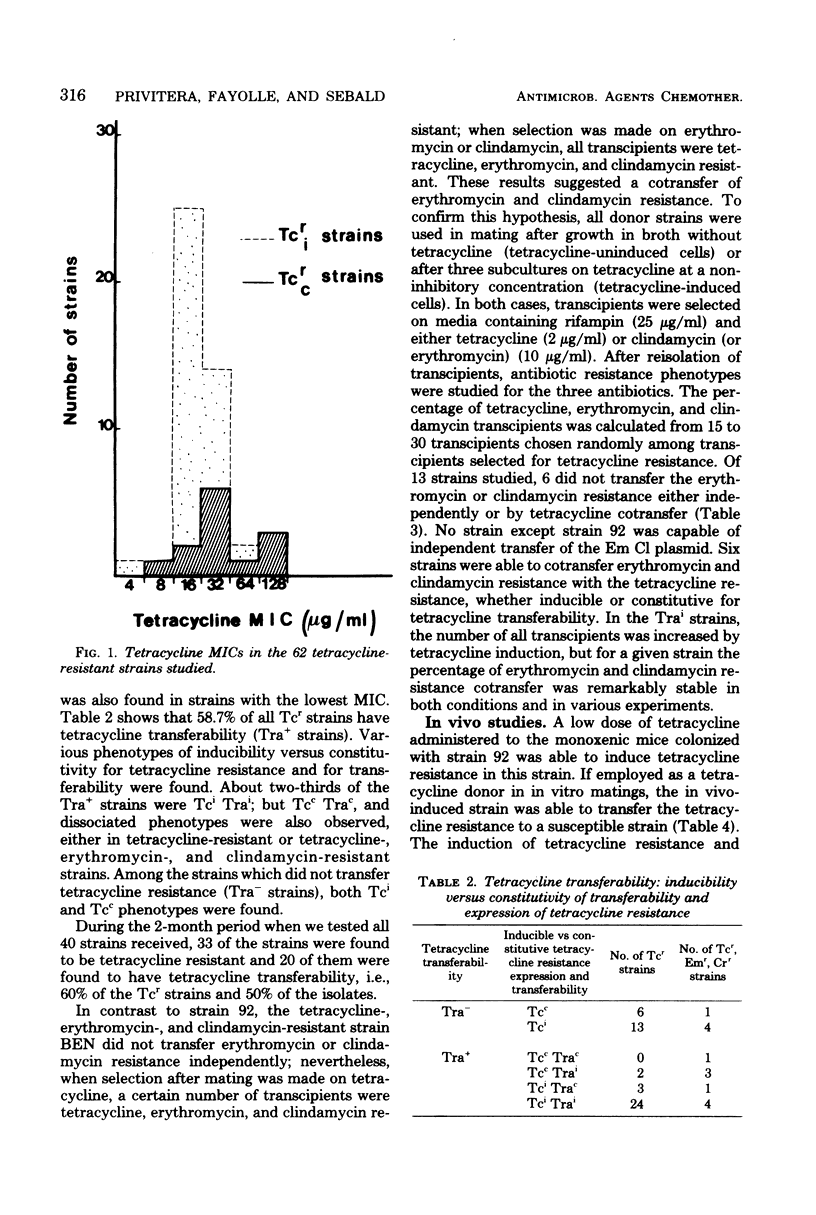
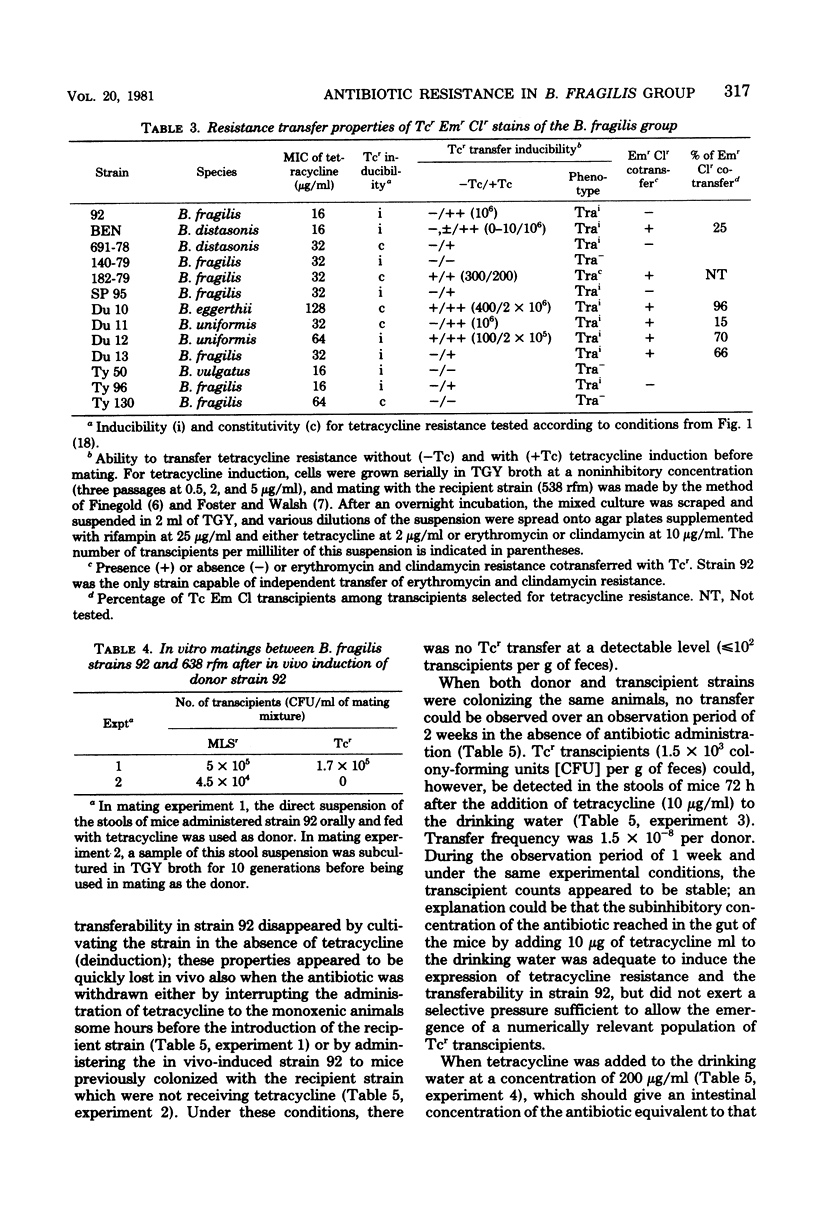
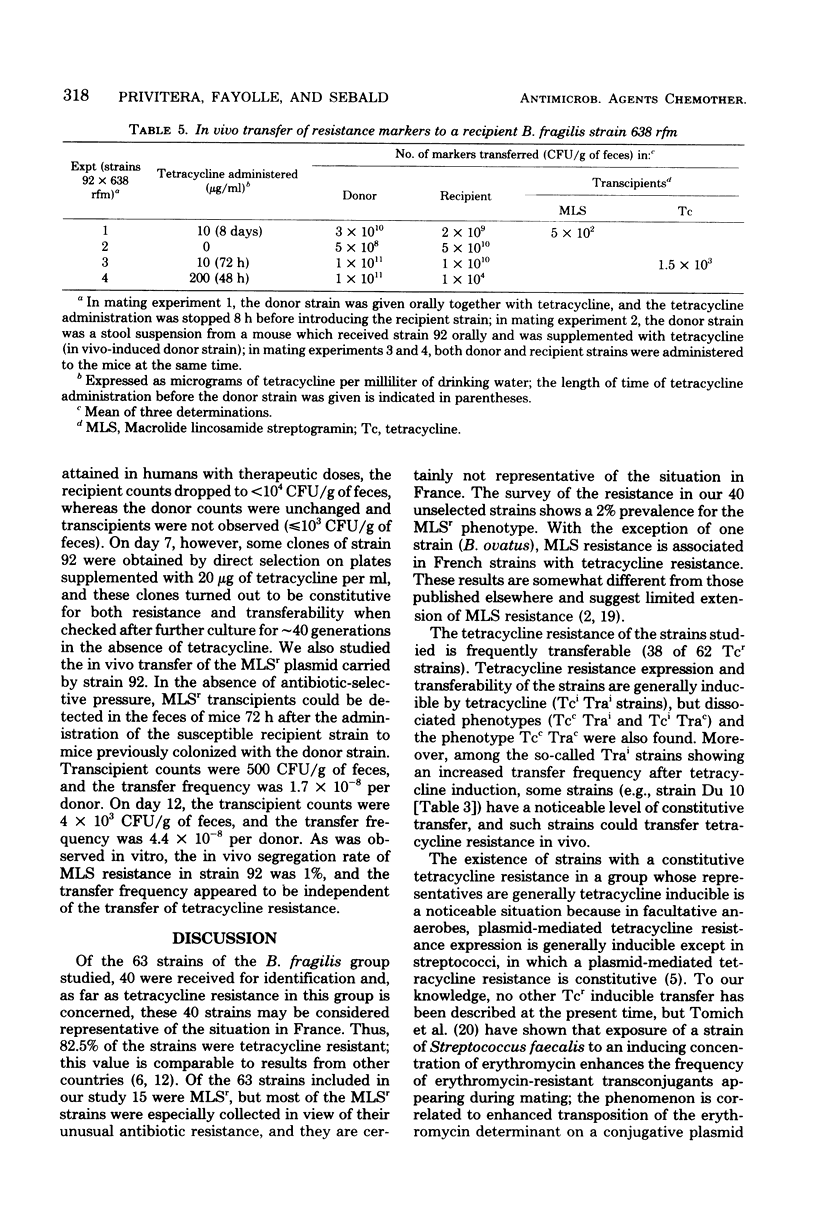
The transferability of plasmid-mediated tetracycline, erythromycin, and clindamycin resistance was studied in 63 clinical isolates of the Bacteroides fragilis group. Of 48 strains which were tetracycline resistant (Tcr), the regulation of both the expression of Tcr and its transferability was shown to be under inducible control by tetracycline. In 29 of the strains, Tcr was transferable; in the majority of these (26 strains), transferability was inducible (Trai) and it was constitutive (Trac) in only 3 strains. All four possible phenotypes were found (Tci Trai, Tci Trac, Tcc Trai, and Tcc Trac), which indicates independent control of both Tcr expression and its transferability. Resistance to erythromycin and clindamycin was cotransferred with Tcr in 14 of the 48 Tcr strains and transferred independently of Tcr in only 1 strain.
Full text
PDF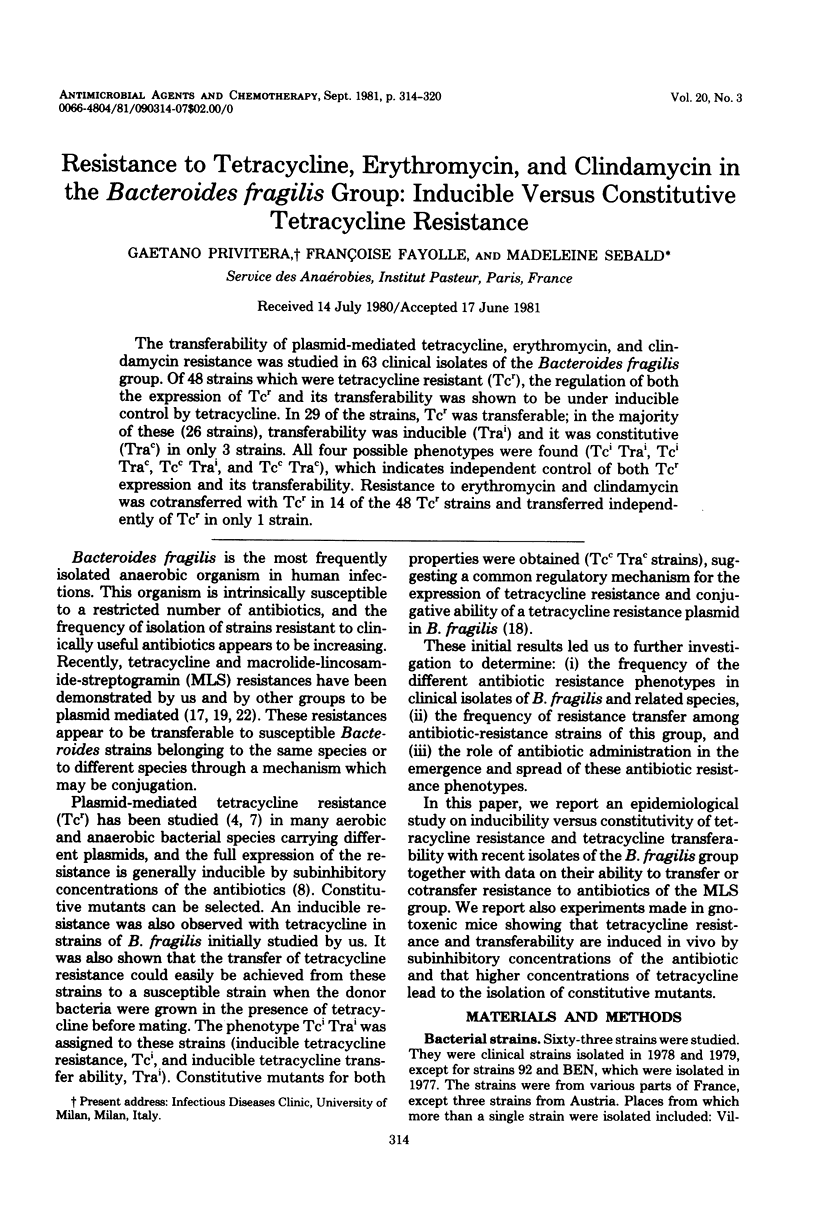



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawdon R. E., Rozmiej E., Palchaudhuri S., Krakowiak J. Variability in the susceptibility pattern of Bacteroides fragilis in four Detroit area hospitals. Antimicrob Agents Chemother. 1979 Nov;16(5):664–666. doi: 10.1128/aac.16.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. J., Woods D. R. R factor transfer to obligate anaerobes from Escherichia coli. J Gen Microbiol. 1976 Apr;93(2):405–409. doi: 10.1099/00221287-93-2-405. [DOI] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Walsh A. Phenotypic characterization of R-factor tetracycline resistance determinants. Genet Res. 1974 Dec;24(3):333–343. doi: 10.1017/s0016672300015330. [DOI] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Jr, Davis C. E. Identification of a conjugative R plasmid in Bacteroides ochraceus capable of transfer to Escherichia coli. Nature. 1978 Jul 13;274(5667):181–182. doi: 10.1038/274181a0. [DOI] [PubMed] [Google Scholar]
- Guinée P. A. Transfer of multiple drug resistance from Escherichia coli to Salmonella typhi murium in the mouse intestine. Antonie Van Leeuwenhoek. 1965;31(3):314–322. doi: 10.1007/BF02045911. [DOI] [PubMed] [Google Scholar]
- Mancini C., Behme R. J. Transfer of multiple antibiotic resistance from Bacteroides fragilis to Escherichia coli. J Infect Dis. 1977 Oct;136(4):597–600. doi: 10.1093/infdis/136.4.597. [DOI] [PubMed] [Google Scholar]
- Moller J. K., Bak A. L., Stenderup A., Zachariae H., Afzelius H. Changing patterns of plasmid-mediated drug resistance during tetracycline therapy. Antimicrob Agents Chemother. 1977 Mar;11(3):388–391. doi: 10.1128/aac.11.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Dublanchet A., Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Gorbach S. L., Malamy M. H. Plasmid-mediated, transferable resistance to clindamycin and erythromycin in Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):83–88. doi: 10.1093/infdis/139.1.83. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. In vivo transfer of infectious drug resistance. Nature. 1966 Jul 16;211(5046):312–313. doi: 10.1038/211312a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]


