Abstract
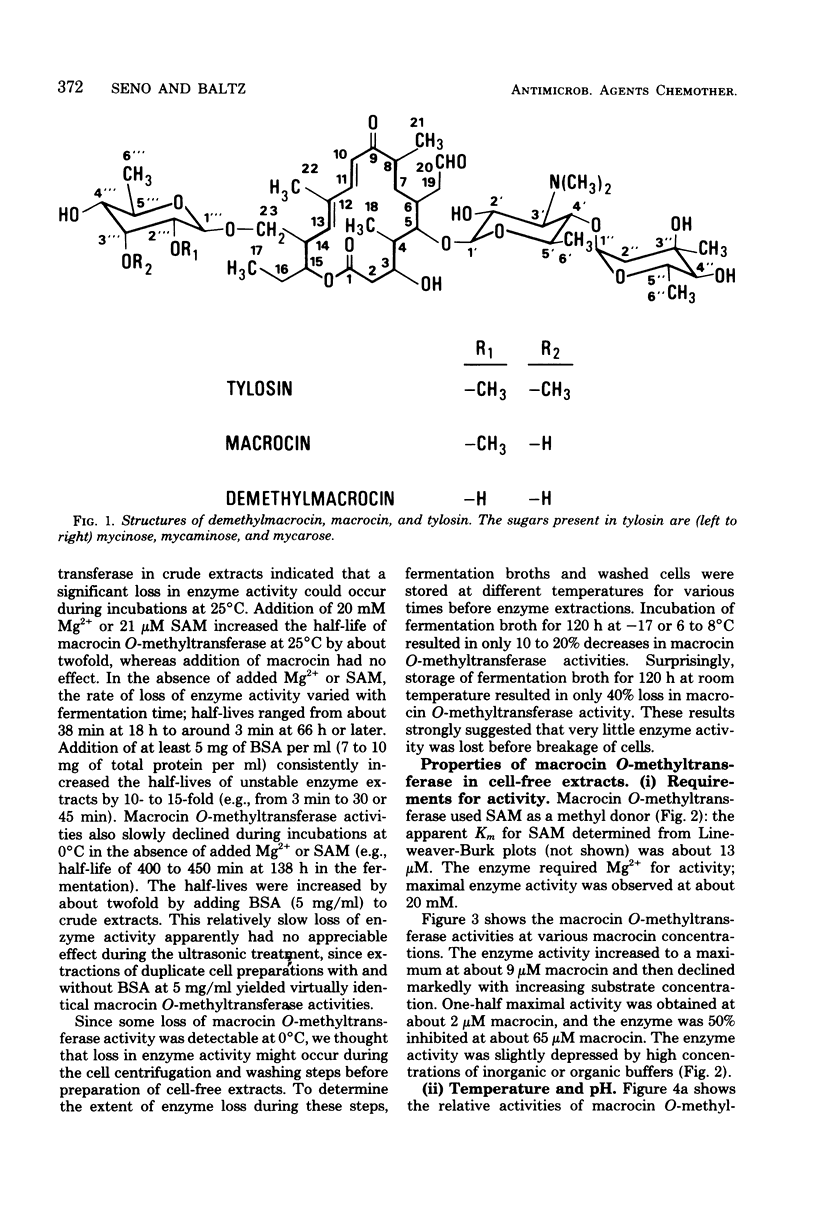
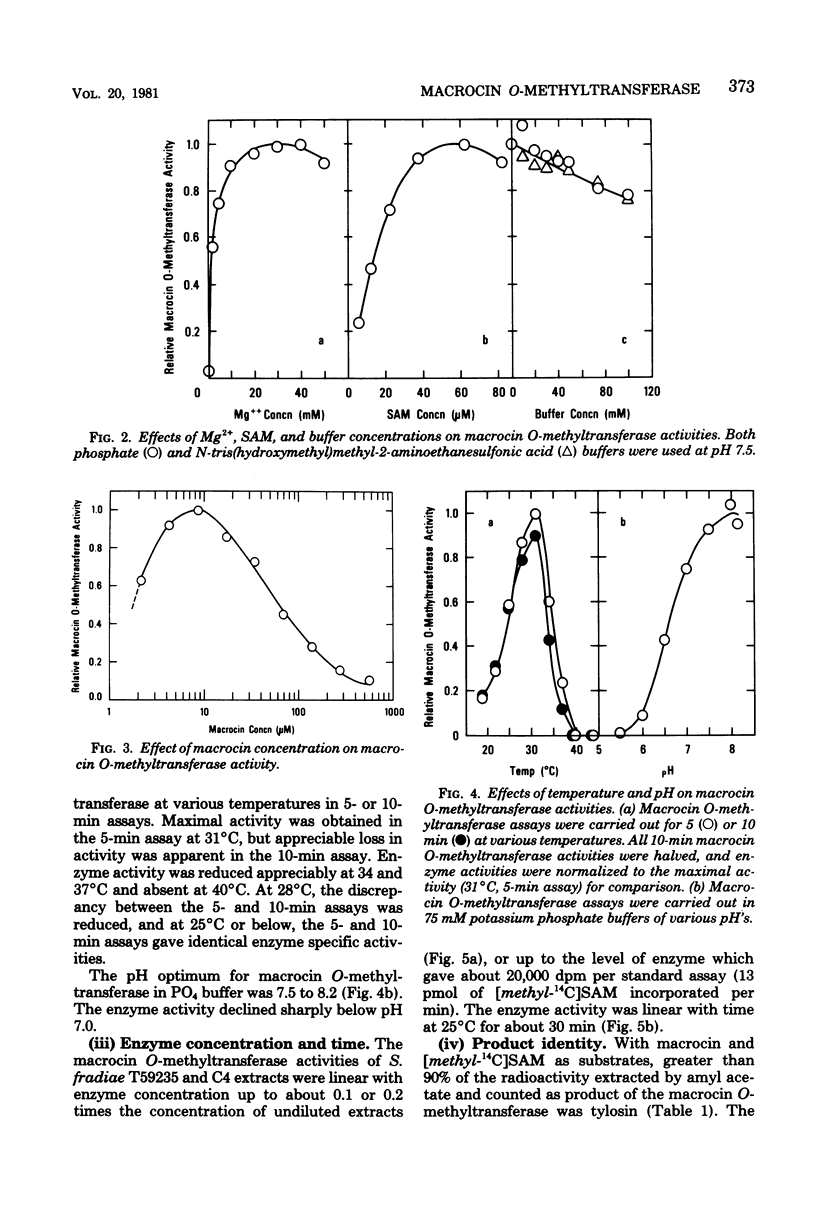
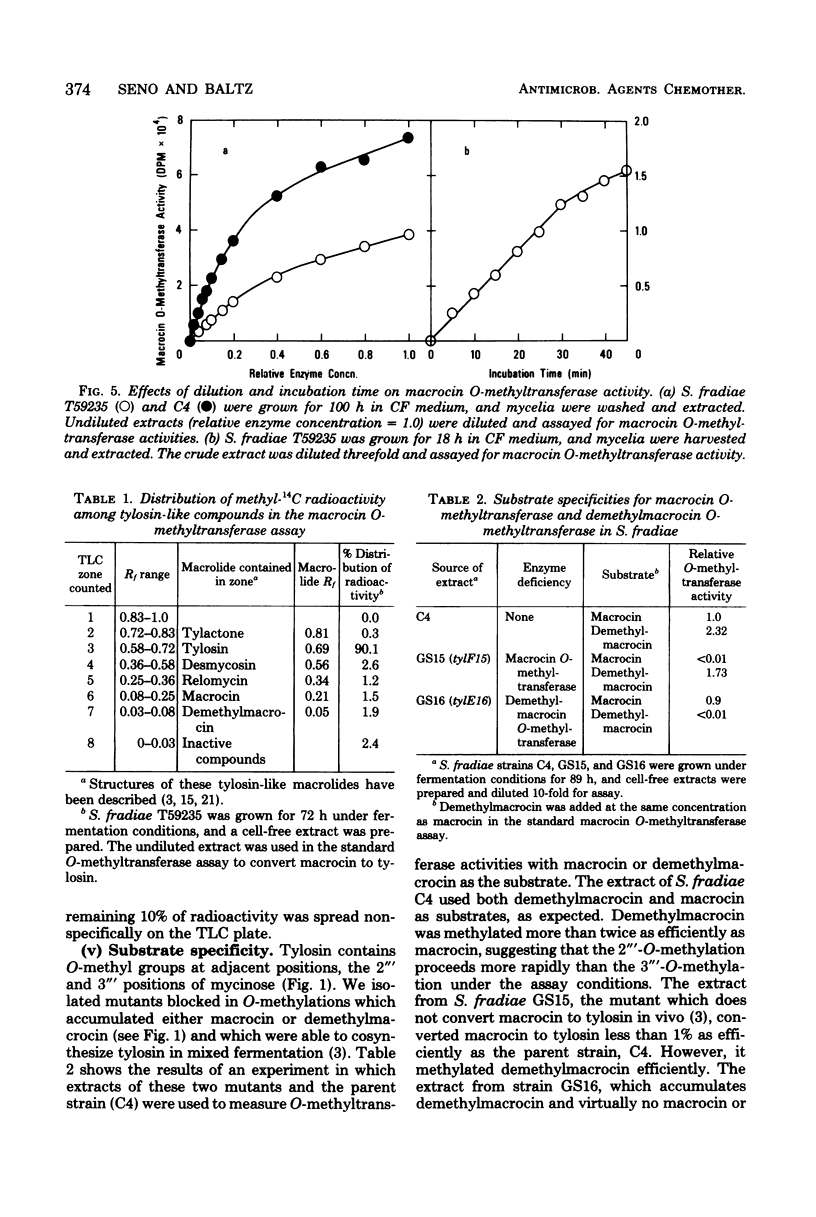
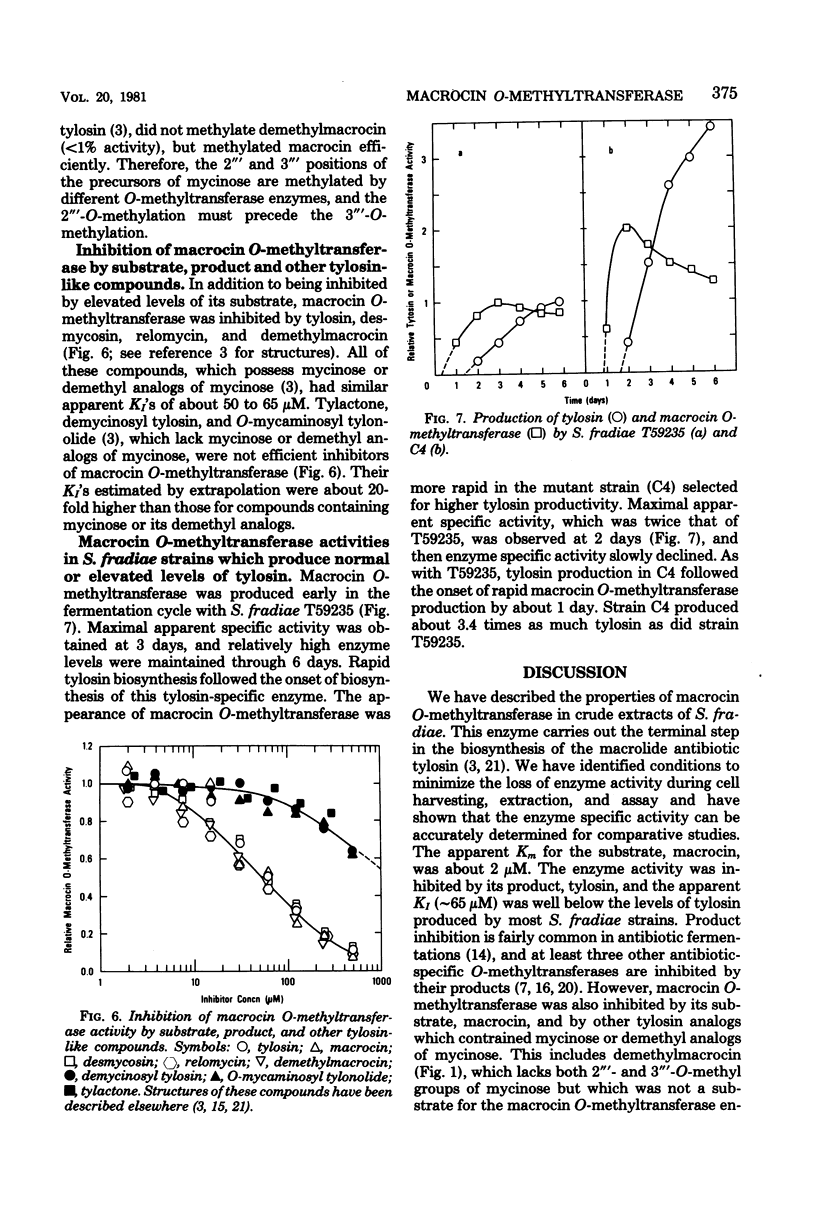
An efficient assay for S-adenosyl-L-methionine:macrocin O-methyltransferase, the enzyme which carries out the terminal step in tylosin biosynthesis, is described. Macrocin O-methyltransferase requires Mg2+ and S-adenosyl-L-methionine for activity, has a temperature optimum of about 31 degrees C, and has a pH optimum of 7.5 to 8.2. Macrocin O-methyltransferase specifically converts macrocin to tylosin by O-methylation of the 3" ' position of macrocin. In vitro methylation studies with extracts from a tylosin-producing Streptomyces fradiae strain and from mutant strains blocked in 2" '- or 3" '-O-methylations indicated that: (i) the 2" '- and 3" '-O-methylations occur after 6-deoxy-D-allose is attached to the macrolide ring; (ii) the 2" '- and 3" '-O-methylations are carried out by separate enzymes; and (iii) the 2" '-O-methylation precedes the 3" '-O-methylation. Macrocin O-methyltransferase was inhibited by high levels of its substrate, macrocin, by its product, tylosin, and by other tylosin analogs which contained mycinose or demethyl analogs of mycinose. Macrocin O-methyltransferase was produced early in the tylosin fermentation cycle by S. fradiae and preceded the onset of rapid tylosin biosynthesis by about 24 h. The enzyme specific activity reached maximum at about 72 h and then slowly declined. A mutant strain of S. fradiae selected for increased tylosin production synthesized macrocin O-methyltransferase more rapidly and accumulated a higher enzyme specific activity than a wild-type strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Seno E. T. Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob Agents Chemother. 1981 Aug;20(2):214–225. doi: 10.1128/aac.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Corcoran J. W. S-adenosylmethionine:erythromycin C O-methyltransferase. Methods Enzymol. 1975;43:487–498. doi: 10.1016/0076-6879(75)43109-3. [DOI] [PubMed] [Google Scholar]
- Godfrey O., Ford L., Huber M. L. Interspecies matings of Streptomyces fradiae with Streptomyces bikiniensis mediated by conventional and protoplast fusion techniques. Can J Microbiol. 1978 Aug;24(8):994–997. doi: 10.1139/m78-163. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978 Jul 4;162(3):307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M., Bibb M. J., Cohen S. N. Genetic recombination through protoplast fusion in Streptomyces. Nature. 1977 Jul 14;268(5616):171–174. doi: 10.1038/268171a0. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Factors affecting recombinant frequency in protoplast fusions of Streptomyces coelicolor. J Gen Microbiol. 1979 Mar;111(1):137–143. doi: 10.1099/00221287-111-1-137. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Muth W. L., Nash C. H., 3rd Biosynthesis of mycophenolic acid: purification and characterization of S-adenosyl-L-methionine: demethylmycophenolic acid O-methyltransferase. Antimicrob Agents Chemother. 1975 Sep;8(3):321–327. doi: 10.1128/aac.8.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Hitchcock M. J., Katz E. High-frequency fusion of Streptomyces parvulus or Streptomyces antibioticus protoplasts induced by polyethylene glycol. J Bacteriol. 1979 Sep;139(3):984–992. doi: 10.1128/jb.139.3.984-992.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H., Brillinger G. U. Stoffwechselfprodukte von Mikroorganismen. 113. Biosynthese von Thymidin-diphospho-mycarose durch ein zellfreies System aus Streptomyces rimosus. Arch Mikrobiol. 1973;88(1):25–35. [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase. Antimicrob Agents Chemother. 1975 Dec;8(6):721–732. doi: 10.1128/aac.8.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno E. T., Pieper R. L., Huber F. M. Terminal stages in the biosynthesis of tylosin. Antimicrob Agents Chemother. 1977 Mar;11(3):455–461. doi: 10.1128/aac.11.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. DNA cloning in Streptomyces: a bifunctional replicon comprising pBR322 inserted into a Streptomyces phage. Nature. 1980 Jul 31;286(5772):527–529. doi: 10.1038/286527a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]



