Abstract
Canine hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis (RCND) is a rare, naturally occurring inherited cancer syndrome observed in dogs. Genetic linkage analysis of an RCND-informative pedigree has identified a linkage group flanking RCND (CHP14-C05.377-C05.414-FH2383-C05.771-[RCND-CPH18]-C02608-GLUT4-TP53-ZuBeCa6-AHT141-FH2140-FH2594) thus localizing the disease to a small region of canine chromosome 5. The closest marker, C02608, is linked to RCND with a recombination fraction (θ) of 0.016, supported by a logarithm of odds score of 16.7. C02608 and the adjacent linked markers map to a region of the canine genome corresponding to portions of human chromosomes 1p and 17p. A combination of linkage analysis and direct sequencing eliminate several likely candidate genes, including tuberous sclerosis 1 and 2 genes (TSC1 and TSC2) and the tumor suppressor gene TP53. These data suggest that RCND may be caused by a previously unidentified tumor suppressor gene and highlight the potential for canine genetics in the study of human disease predisposition.
The identification of cancer-susceptibility genes has been facilitated by the study of high-risk families characterized by multiple generations of affected individuals (1–4). The general assumption is that identification of genetic mutations causing the highly penetrant phenotypes studied in these rare families will provide a foundation on which to understand cancer susceptibility in the general population.
Informative high-risk cancer families, however, are uncommon in human populations, making it difficult to identify all but a few highly penetrant cancer-susceptibility genes. In addition, inherent limitations in the structure of human families, such as small size and long generation time, mean that genes that are weakly penetrant give rise to variable phenotypes and that genes that act in a recessive fashion are difficult to study. Finally, the high frequency of phenocopies for some cancers confounds linkage results and further complicates analysis of common cancers.
We have proposed that cancer-susceptibility genes could be mapped more easily in animals, where large families can be generated, directed matings are possible, and multiple generations are easily collected. In addition, because of the shorter life span, clinical symptoms often manifest in relatively short periods of time (5). In a search for appropriate animal models in which to study the genetics of cancer susceptibility, we have elected to focus on the domestic dog, which we believe offers two specific advantages over rodents. First, many types of spontaneous canine cancers are more similar to human tumors in histopathological appearance, biological behavior, and response to therapy than the corresponding rodent tumors (6). Second, purebred dogs have a relatively high frequency of autosomal recessive and genetically complex disorders, many of which, including cancer, tend to be breed specific (7). Indeed, distinct dog breeds often differ significantly in the type and frequency of specific tumors (8, 9).
Although there are many animal models for sporadic cancer, currently, there are few well described examples of naturally occurring inherited cancers in nonhuman mammals. The single exception is the well characterized renal carcinoma syndrome found in the Eker rat, which seems to be caused by germ-line mutations in the tsc2 gene (10–13).
Recently, we described a second hereditary mammalian cancer syndrome called hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis (RCND; refs. 14 and 15), a rare, inherited, naturally occurring cancer syndrome in German Shepherd Dogs. The syndrome is characterized by bilateral, multifocal tumors in kidneys and numerous firm nodules, consisting of dense collagen fibers in the skin and subcutis. Most females develop uterine leiomyomas. Pedigree analysis of a canine family with RCND strongly indicates an autosomal dominant pattern of inheritance (16). There is no precisely corresponding human disease known, but the clinical patterns are reminiscent of diseases associated with mutations in tumor suppressor genes, such as tuberous sclerosis (17), which is associated with inherited mutations in the TSC1 and TSC2 genes, and Li–Fraumeni syndrome, which is associated with germ-line mutations in TP53 (18, 19).
Rapid progress on the development of a canine genome map containing both type I (gene) and II (microsatellite) markers (20–23) has been highlighted by the recent mapping of several canine disease genes, including those associated with two forms of hereditary blindness, progressive rod-cone degeneration (prcd), and early retinal degeneration (erd) (refs. 24 and 25), as well as genes for copper toxicosis (26) and neuronal ceroid lipofuscinosis (27). The results reported herein represent, to our knowledge, the first localization of a mammalian cancer syndrome to be mapped in a species other than human and rat and localize RCND to canine chromosome 5 (CFA5). A combination of linkage analysis and direct mutation screening eliminate several likely candidate genes, including TSC1, TSC2, and TP53, suggesting that RCND may be caused by either a previously unidentified tumor suppressor gene or a previously identified gene with no known association with inherited cancer syndromes.
Methods
Canine Pedigree Development and Phenotypic Assessment.
A canine colony segregating RCND was established by breeding one affected male (German Shepherd/Flat Coated Retriever cross) with six unaffected females (one German Shepherd and five English Setters) to yield 67 F2 offspring from nine litters (Fig. 1). All of the dams were unrelated to the proband; the five English Setter females were related. The male used to produce the litters was diagnosed at 1 year of age, and the offspring were diagnosed at 8–12 weeks of age.
Figure 1.
The canine pedigrees segregating RCND. Affected dogs are represented by black; unaffected are represented by white; and those with an unknown diagnosis are indicated with a question mark. Genotypes of all typed markers are shown, but the bar indicating crossovers is shown only for the affected father's side. The RCND locus is shown as + for the wild-type allele and − for the mutant.
Dogs were examined for the presence of multiple microscopic renal cysts by exploratory laparotomy, kidney biopsy or necropsy, and histologic examination as described (28). Microscopic renal cysts are considered to be a precursor of the renal cystadenocarcinomas that are typically observed in mature dogs with RCND and are therefore used as early diagnostic criteria (29). Other diagnostic criteria and their application to early diagnosis have been reported (28).
The Norwegian Animal Research Authority approved colony development, maintenance, and sample collection. Canine DNA samples were handled as specified by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee.
DNA Extraction and Microsatellite Typing.
Genomic DNA was extracted from aliquots of EDTA-anticoagulated whole blood or from samples of muscle tissue by using standard protocols (30). Microsatellites spanning the canine genome at approximately 10-centimorgan (cM) resolution were selected for an initial low-resolution genome screen (31). The majority of markers selected were based on tetranucleotide repeats, which are highly polymorphic in dogs and easy to genotype (32). The primer sequences, amplification conditions, and product sizes are available on our web site: http://www.fhcrc.org/science/dog_genome/markers/all.html. PCR was carried out by using published conditions (33). PCR products were separated by 4–6% PAGE run at 55°C, visualized by autoradiography, and scored manually.
Linkage Analysis.
Each marker was checked for Mendelian segregation by using the prepare option of the multimap program (34). The disease trait was assumed to be fully penetrant and was coded as autosomal dominant. Two-point linkage analysis was carried out between RCND and each marker and between each pair of markers. The most likely order and spacing of markers within the linkage group was calculated by using multipoint analysis and the get-likelihoods function of multimap.
Radiation Hybrid (RH) Analysis.
Markers were typed on DNA isolated from a canine/hamster RH panel (Research Genetics, Huntsville, AL). Canine-specific TSC1 primers were typed on the RH panel described by Vignaux et al. (35) by using previously described protocols (22). The most likely order and spacing of markers within each RH linkage group was calculated by multipoint analysis with the program multimap (34).
Sequencing of Canine TP53.
mRNA was isolated from blood of three affected and three unaffected dogs by using a Quickprep Micro mRNA Purification Kit (Amersham Pharmacia). First-strand cDNA was synthesized with a Ready To-Go T Primed First-Strand Kit (Amersham Pharmacia). Five pairs of primers were designed from a published canine TP53 cDNA sequence (GenBank accession no. AF060514) to amplify overlapping fragments of canine TP53. The positions of the amplified nucleotides were as follows: nucleotides 4–335, 236–529, 441–781, 677–990, and 893–1,145. Fragments of cDNA were amplified for direct sequencing from 3.5 μg of cDNA. All PCR products were bidirectionally sequenced with the Thermo Sequenase Dye Terminator Cycle Sequencing PreMix Kit (Amersham Pharmacia) according to the manufacturer's instructions and analyzed on an Applied Biosystems 373 fluorescence DNA sequencer.
Sequencing of Canine TSC1.
Consensus TSC1 primers were designed from conserved regions of human and rat TSC1 cDNA (GenBank accession nos. AF013168 and AB011821, respectively). The 5′–3′ sequences of the forward and reverse consensus primers are as follows: forward, CATCGCCTTTATGGAATGTA, and reverse, GAGGGTCCAGTTCATGGTC. The consensus primers were used to amplify canine and hamster genomic DNA, and the resulting PCR products were bidirectionally sequenced by using the same primers. Both canine and hamster sequences were confirmed by blast searches as TSC1. The canine and hamster sequences were aligned, and canine-specific primers were designed in regions of mismatch between the canine and hamster sequences: forward, GTGCACAGGCTACACTTGGGT, and reverse, GAACCCTGAAAAATTCCACCA.
Results
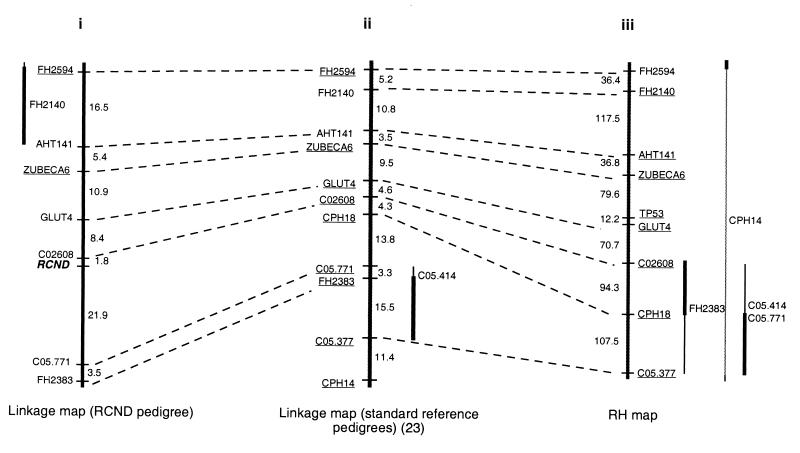
A set of mapped canine-specific microsatellite markers, distributed at ≈10-cM intervals throughout the genome, was selected for typing on a set of canine pedigrees segregating RCND. The set of pedigrees used is shown in Fig. 1. Two-point linkage was detected initially between the RCND locus and ZuBeCa6, a microsatellite marker that has been localized previously to CFA5q12-q13 (36), at a recombination fraction (θ) of 0.161, supported by a logarithm of odds (lod) score of 4.6. Several other polymorphic markers known to be on CFA5 (23, 37, 38) were then genotyped on the same set of pedigrees. Two-point analyses showed the disease locus to be linked to five additional markers on CFA5 with odds of at least 1,000:1 (Table 1). A maximum lod score of 16.7 was observed with C02608 at a θ of 0.016. Thus, we concluded that the RCND gene is located on CFA5. Multipoint analyses indicate that RCND maps to the interval between C05.771 and C02608 (Fig. 2), ≈2 cM from C02608.
Table 1.
Recombination fractions and lod scores for markers flanking the RCND locus on CFA5
| FH2383 | C05.771 | RCND | C02608 | GLUT4 | ZuBeCa6 | AHT141 | FH2140 | FH2594 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| FH2383 | θ | — | ||||||||
| lod | ||||||||||
| C05.771 | θ | 0.034 | — | |||||||
| lod | 20.207 | |||||||||
| RCND | θ | 0.205 | 0.160 | — | ||||||
| lod | 4.964 | 6.707 | ||||||||
| C02608 | θ | 0.273 | 0.218 | 0.016 | — | |||||
| lod | 4.140 | 6.770 | 16.728 | |||||||
| GLUT4 | θ | 0.312 | 0.250 | 0.097 | 0.087 | — | ||||
| lod | 2.504 | 4.926 | 10.103 | 17.707 | ||||||
| ZuBeCa6 | θ | 0.369 | 0.300 | 0.161 | 0.142 | 0.085 | — | |||
| lod | 0.754 | 2.062 | 4.587 | 6.539 | 10.325 | |||||
| AHT141 | θ | 0.387 | 0.379 | 0.233 | 0.257 | 0.168 | 0.039 | — | ||
| lod | 0.843 | 0.919 | 3.905 | 5.227 | 10.525 | 11.672 | ||||
| FH2140 | θ | 0.500 | 0.500 | 0.322 | 0.273 | 0.212 | 0.119 | 0.100 | — | |
| lod | 0.00 | 0.00 | 1.432 | 2.187 | 4.001 | 4.593 | 7.992 | |||
| FH2594 | θ | 0.480 | 0.459 | 0.311 | 0.307 | 0.258 | 0.118 | 0.157 | 0.014 | — |
| lod | 0.026 | 0.106 | 1.777 | 2.828 | 4.706 | 8.088 | 9.054 | 16.081 |
Figure 2.
Comparison of maps of CFA5 constructed with (i) linkage data from the RCND pedigrees, (ii) the standard reference pedigrees (23), and (iii) an RH panel. The distances on the linkage map are shown in centimorgans, and the distances on the RH map are shown in centiRays. All markers were assigned to CFA5 with odds of at least 100,000:1 and ordered with odds of at least 10:1. The most likely position or positions of markers that could not be positioned with odds of at least 10:1 are indicated with vertical bars to the sides of the maps. Thickened portions of bars indicate an interval that is favored over other intervals with odds of at least 10:1. Markers that can be ordered with odds of at least 1,000:1 are underlined.
The canine homolog of the tumor suppressor gene TP53 is located on CFA5 (39). Germ-line mutations in TP53 are associated with Li–Fraumeni syndrome (18, 19), and RCND has several clinical features in common with Li–Fraumeni syndrome (15, 40, 41) making TP53 a potential candidate for RCND. To date, no polymorphisms within canine TP53 have been reported; thus, to determine the position of TP53 relative to RCND and the other markers on CFA5, we constructed an RH map of CFA5. Whole-genome RH maps of the canine genome have been published (22, 31), which include markers on CFA5. The RH panel developed for those studies, however, lacked the region of the genome containing TP53 (22, 42). A distinct RH panel (Research Genetics), therefore, was used for this analysis. DNA was amplified from the RH panel with the canine-specific TP53 primer sequence (GenBank accession no. AF060514), as well as with primers for all available CFA5 markers. Multipoint analysis indicated that TP53 is located between ZuBeCa6 and GLUT4 (odds of at least 1,000:1), on the opposite side of GLUT4 from RCND (Fig. 2). The linkage map of CFA5, constructed with linkage data from the RCND pedigree, shows the same order of markers as the third generation linkage map (23) and the RH map of CFA5 (Fig. 2).
In parallel with linkage analysis, direct sequencing of TP53 was carried out. cDNA from the whole coding sequence of TP53 was sequenced in three unaffected and three affected dogs with primers that amplified the entire coding sequence of the gene. No differences were observed between affected and unaffected individuals.
Other strong candidate genes for RCND include TSC1 and TSC2. Germ-line mutations in both genes have been associated with tuberous sclerosis in humans, another inherited renal cancer syndrome with clinical similarities to RCND (43, 44). To investigate the potential role of TSC1 in RCND, canine-specific primers were designed that amplified a region of the canine but not the hamster TSC1 gene. Primers were typed on the canine/hamster RH panel used to construct published whole-genome RH maps of the canine genome (22, 31, 35). TSC1 was linked significantly to seven markers, all of which localize to CFA9 (results not shown), allowing us to exclude TSC1 as a potential candidate for RCND. Similar, previously reported experiments localize TSC2 to CFA6 and additionally allow us to exclude it as a candidate for RCND (33).
Discussion
Few naturally occurring nonhuman mammalian models for inherited cancer syndromes have been well defined, and the underlying locus has been identified only for the Eker rat (12, 13, 45, 46). The linkage and RH data presented herein firmly establish that the canine cancer syndrome RCND maps to a linkage group containing 13 markers on CFA5. Linkage and RH data suggest that the most likely order of markers is CHP14-C05.377-C05.414-FH2383-C05.771-[RCND-CPH18]-C02608-GLUT4-TP53-ZuBeCa6-AHT141-FH2140-FH2594. We are unable to determine the orientation of RCND and CPH18 with respect to the rest of the chromosome, because CPH18 was not informative in the RCND pedigree.
CFA5 contains homologs of genes located on at least four different human chromosomes (HSA; ref. 31). However, the RCND locus is located in a region most likely homologous to either HSA17p or HSA1p. The breakpoint between these two chromosomes seems to be very close to the RCND locus and thus cannot be mapped precisely until more genes from each HSA have been positioned on the canine map.
The possibility of synteny between RCND and either HSA1 or HSA17 suggests several provocative candidate genes, including the tumor suppressor gene TP53, which is associated with germ-line mutations in Li–Fraumeni syndrome (18, 19). Li–Fraumeni disease is characterized by a wider array of early onset tumors than is observed in RCND, including sarcomas, leukemias, and later in life, breast cancer (40, 41). TP53 has not been associated with any polymorphisms in dogs and thus can be placed only on the RH map, whereas RCND is localized on the linkage map. The position of these two loci, relative to one another, was made by comparing their positions relative to other markers that have been placed on both maps. The RH map places TP53 about 12.2 centiRays (equivalent to about 2 cM) from GLUT4. The third generation linkage map of CFA5 positions GLUT4 4.6 cM from C02608, which is 1.8 cM from RCND, on the opposite side of GLUT4 from TP53. Thus, because there are no discrepancies between the order of markers on the two maps and because we find no mutations in the coding region of TP53 in affected dogs, TP53 is unlikely to be the relevant disease gene. An exception could occur if significant undetected microrearrangements were in this region of the map and if TP53 mutations were located outside the coding region of the gene within promoters, enhancers, or downstream regulatory elements.
Linkage analysis places the RCND locus close to marker CPH18, which Mellersh et al. (31) have placed 21.2 centiRays or about 3.5 cM from the canine homolog of DIO1, which maps to HSA1p32. The tumor suppressor gene p73 maps to HSA1p36 (47) and thus is also a good candidate gene for RCND that will be worth further investigation.
Phenotypic similarities between RCND and some human disorders have suggested several other candidate genes. Tuberous sclerosis complex (TSC) is a syndrome characterized by a high incidence of cutaneous neoplastic nodes and multiple renal neoplasms, with a complex and sometimes inconsistent phenotype. The syndrome has an autosomal dominant mode of inheritance and is caused by mutations in the TSC1 and TSC2 genes (43, 44). Although RCND bears some similarities to TSC, the renal carcinomas associated with TSC are typically hamartomas or angiomyolipomas, whereas those associated with RCND are cystadenocarcinomas. Interestingly, the clinical syndrome presented by the Eker rat also bears some similarity to RCND, with kidney tumors and reproductive tract leiomyomas being common in both diseases (17, 48). RCND, however, is distinct in that it includes skin tumors and lacks vascular neoplasms. Both TSC genes are excluded by our linkage studies. The present study localizes canine TSC1 to CFA9, and we have previously localized TSC2 to CFA6 (33).
One additional candidate we have considered is KRT9, mutations of which are associated with epidermolytic palmoplantar keratoderma (49). This rare syndrome is usually characterized by skin tumors, but few families have been described recently that also have different malignancies (50). Canine KRT9 has been cloned (51) and mapped to CFA9 (22); therefore, it is excluded as the RCND gene.
It is noteworthy that there are several other genes associated with inherited renal cancers. These include the Wilms' tumor gene (WT1; ref. 52), the von Hippel Lindau gene (VHL; refs. 53–56), the neurofibromatosis gene (NF1; ref. 57), and polycystic kidney disease genes 1 and 2 (PKD1 and PKD2; refs. 58 and 59). The canine homologs of WT1 and NF1 have been mapped to CFA9 (22, 38), and PKD1, which is involved in 85% of autosomal dominant polycystic kidney disease in humans, has been mapped to CFA6 (33). These can therefore be excluded from further consideration as candidates. Von Hippel Lindau syndrome also shares several clinical features with RCND, commonly results in bilateral renal cysts and tumors, and may therefore represent an additional candidate worthy of study. Patients with Von Hippel Lindau syndrome, however, also experience tumors in the central nervous system and cysts in the pancreas and central nervous system, which are not observed in RCND. Neither of the canine homologs PDK2 or VHL has been cloned or mapped, and thus, neither can be excluded formally at this time.
The mapping of a unique cancer syndrome to CFA5 shows the potential of the dog to map genes for intractable human genetic disorders. Canine lineages provide a tool with which to overcome problems of locus heterogeneity that often confound human studies. In addition, large pedigrees enhance the statistical power of linkage analysis studies. Placement of additional genes and informative markers on the canine map will be necessary before identification of a disease gene by means other then candidate gene analysis can begin in earnest; however, results published herein indicate that investigation of other canine inherited disease syndromes could make a unique contribution to our understanding of mammalian biology and cancer susceptibility.
Acknowledgments
The authors wish to thank Lise Høgeli, Anita Stensby, and Rita Jørgensen for technical assistance and the members of our laboratories for their continued support. This work was supported by Norwegian Research Council Grant 110642/122 and by a grant from the Norwegian Kennel Club (to F.L., L.M., and T.J.J.), by American Kennel Club Canine Health Foundation Grants 1608 and 1268 (to E.A.O.), and by a grant from the American Cancer Society (to E.A.O.). C.S.M. was supported by a postdoctoral fellowship from Ralston Purina.
Abbreviations
- CFAn
Canis familiaris chromosome n
- HSAn
Homo sapiens chromosome n
- RCND
hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis
- cM
centimorgan
- RH
radiation hybrid
- lod
logarithm of odds
- TSC
tuberous sclerosis complex
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070053397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070053397
References
- 1.Hall J M, Lee M K, Newman B, Morrow J E, Anderson L A, Huey B, King M-C. Science. 1990;250:1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 2.Smith J R, Freije D, Carpten J D, Grönberg H, Xu J, Isaacs S D, Brownstein M J, Bova G S, Guo H, Bujnovszky P, et al. Science. 1996;274:1371–1374. doi: 10.1126/science.274.5291.1371. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, Ewing C, Wilkens E, Bujnovszky P, Bova G S, et al. Nat Genet. 1998;20:175–179. doi: 10.1038/2477. [DOI] [PubMed] [Google Scholar]
- 4.Wooster R, Neuhausen S L, Mangion J, Quirk Y, Ford D, Collins N, Nguyen K, Seal S, Tran T, Averill D, et al. Science. 1994;265:2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 5.Ostrander E A, Giniger E. Am J Hum Genet. 1997;61:475–480. doi: 10.1086/515522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knapp D W, Waters D J. Mol Med Today. 1997;3:8–11. doi: 10.1016/s1357-4310(96)20031-0. [DOI] [PubMed] [Google Scholar]
- 7.Patterson D F. Canine Genetic Disease Information System: A Computerized Knowledgebase of Genetic Diseases in Dogs. St. Louis: Mosby-Harcourt; 2000. , in press. [Google Scholar]
- 8.Nordstoga K, Arnesen K, Gamlem H, Glattre E, Grøndalen J, Moe L. Eur J Comp Anim Pract. 1997;7:41–47. [Google Scholar]
- 9.Priester W A, McKay F W. Natl Cancer Inst Monogr. 1980;54:1–210. [PubMed] [Google Scholar]
- 10.Eker R, Mossige J. Nature (London) 1961;189:858–859. [Google Scholar]
- 11.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. Nat Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- 12.Hino O, Kobayashi T, Tsuchiya H, Kikuchi Y, Kobayashi E, Mitani H, Hirayama Y. Biochem Biophys Res Commun. 1994;203:1302–1308. doi: 10.1006/bbrc.1994.2324. [DOI] [PubMed] [Google Scholar]
- 13.Yeung R S, Xiao G-H, Jin F, Lee W-C, Testa J R, Knudson A G. Proc Natl Acad Sci USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moe L, Lium B. J Small Anim Pract. 1997;38:498–505. doi: 10.1111/j.1748-5827.1997.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 15.Lium B, Moe L. Vet Pathol. 1985;22:447–455. doi: 10.1177/030098588502200503. [DOI] [PubMed] [Google Scholar]
- 16.Moe L Norwegian School of Veterinary Science, editors. Norwegian School of Veterinary Science Publications, 1997. Oslo: Bibliotekets Arsmelding; 1998. pp. 1–24. [Google Scholar]
- 17.Everitt J I, Goldsworthy T L, Wolf D C, Walker C L. Toxicol Lett. 1995;82–83:621–625. doi: 10.1016/0378-4274(95)03506-0. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava S, Zou Z, Pirollo K, Blattner W, Chang E H. Nature (London) 1990;348:747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- 19.Malkin D, Li F P, Strong L C, Fraumeni J F, Jr, Nelson C E, Kim D H, Kassel J, Gryka M A, Bischoff F Z, Tainsky M A, et al. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 20.Mellersh C S, Langston A A, Acland G M, Fleming M A, Ray K, Wiegand N A, Francisco L V, Gibbs M, Aguirre G D, Ostrander E A. Genomics. 1997;46:326–336. doi: 10.1006/geno.1997.5098. [DOI] [PubMed] [Google Scholar]
- 21.Neff M W, Broman K W, Mellersh C S, Ray K, Acland G M, Aguirre G D, Ziegle J S, Ostrander E A, Rine J. Genetics. 1999;151:803–820. doi: 10.1093/genetics/151.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priat C, Hitte C, Vignaux F, Renier C, Jiang Z, Jouquand S, Chéron A, André C, Galibert F. Genomics. 1998;54:361–378. doi: 10.1006/geno.1998.5602. [DOI] [PubMed] [Google Scholar]
- 23.Werner P, Mellersh C S, Raducha M G, DeRose S, Acland G M, Prociuk U, Wiegand N, Aguirre G D, Henthorn P S, Patterson D F, et al. Mamm Genome. 1999;10:814–823. doi: 10.1007/s003359901096. [DOI] [PubMed] [Google Scholar]
- 24.Acland G M, Ray K, Mellersh C S, Gu W, Langston A A, Rine J, Ostrander E A, Aguirre G D. Proc Natl Acad Sci USA. 1998;95:3048–3053. doi: 10.1073/pnas.95.6.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acland G M, Ray K, Mellersh C S, Langston A A, Rine J, Ostrander E A, Aguirre G D. Genomics. 1999;59:134–142. doi: 10.1006/geno.1999.5842. [DOI] [PubMed] [Google Scholar]
- 26.Yuzbasiyan-Gurkan V, Blanton S H, Cao Y, Ferguson P, Li J, Venta P J, Brewer G J. Am J Vet Res. 1997;58:23–27. [PubMed] [Google Scholar]
- 27.Lingaas F, Aarskaug T, Sletten M, Bjerkaas I, Grimholt U, Moe L, Juneja R K, Wilton A N, Galibert F, Holmes N G, et al. Anim Genet. 1998;29:371–376. doi: 10.1046/j.1365-2052.1998.295358.x. [DOI] [PubMed] [Google Scholar]
- 28.Moe, L., Gamlem, H., Jónasdóttir, T. J. & Lingaas, F. (2000) J. Comp. Pathol., in press. [DOI] [PubMed]
- 29.Moe L, Lium B. Vet Radiol Ultrasound. 1997;38:335–343. doi: 10.1111/j.1740-8261.1997.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 30.Bell G I, Karam J H, Rutter W J. Proc Natl Acad Sci USA. 1981;78:5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellersh C S, Hitte C, Richman M, Vignaux F, Priat C, Jouquand S, Werner P, André C, DeRose S, Patterson D F, et al. Mamm Genome. 2000;11:120–130. doi: 10.1007/s003350010024. [DOI] [PubMed] [Google Scholar]
- 32.Francisco L V, Langston A A, Mellersh C S, Neal C L, Ostrander E A. Mamm Genome. 1996;7:359–362. doi: 10.1007/s003359900104. [DOI] [PubMed] [Google Scholar]
- 33.Jónasdóttir, T. J., Mellersh, C. S., Moe, L., Vignaux, F., Ostrander, E. A. & Lingaas, F. (2000) Anim. Genet., in press. [DOI] [PubMed]
- 34.Matise T C, Perlin M, Chakravarti A. Nat Genet. 1994;6:384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- 35.Vignaux F, Hitte C, Priat C, Chuat J C, André C, Galibert F. Mamm Genome. 1999;10:888–894. doi: 10.1007/s003359901109. [DOI] [PubMed] [Google Scholar]
- 36.Ladon D, Schelling C, Dolf G, Switonski M, Schläpfer J. Anim Genet. 1998;29:466–467. [PubMed] [Google Scholar]
- 37.Jónasdóttir T J, Dolf G, Sletten M, Aarskaug T, Schelling C, Schläpfer J, Jouquand S, Priat C, Holmes N G, Lingaas F. Anim Genet. 1999;30:366–370. doi: 10.1046/j.1365-2052.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 38.Werner P, Raducha M G, Prociuk U, Henthorn P S, Patterson D F. Genomics. 1997;42:74–82. doi: 10.1006/geno.1997.4723. [DOI] [PubMed] [Google Scholar]
- 39.Guevara-Fujita M L, Loechel R, Venta P J, Yuzbasiyan-Gurkan V, Brewer G J. Mamm Genome. 1996;7:268–270. doi: 10.1007/s003359900080. [DOI] [PubMed] [Google Scholar]
- 40.Lynch H T, Mulcahy G M, Harris R E, Guirgis H A, Lynch J F. Cancer. 1978;41:2055–2064. doi: 10.1002/1097-0142(197805)41:5<2055::aid-cncr2820410554>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Li F P, Fraumeni J F, Jr, Mulvihill J J, Blattner W A, Dreyfus M G, Tucker M A, Miller R W. Cancer Res. 1988;48:5358–5362. [PubMed] [Google Scholar]
- 42.Vignaux F, Priat C, Jouquand S, Hitte C, Jiang Z, Chéron A, Renier C, André C, Galibert F. J Hered. 1999;90:62–67. doi: 10.1093/jhered/90.1.62. [DOI] [PubMed] [Google Scholar]
- 43.Roach E S, Gomez M R, Northrup H. J Child Neurol. 1998;13:624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 44.Franz D N. Semin Pediatr Neurol. 1998;5:253–268. doi: 10.1016/s1071-9091(98)80004-1. [DOI] [PubMed] [Google Scholar]
- 45.Hino O, Mitani H, Nishizawa M, Katsuyama H, Kobayashi E, Hirayama Y. Jpn J Cancer Res. 1993;84:1106–1109. doi: 10.1111/j.1349-7006.1993.tb02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeung R S, Buetow K H, Testa J R, Knudson A G., Jr Proc Natl Acad Sci USA. 1993;90:8038–8042. doi: 10.1073/pnas.90.17.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan J-C, Valent A, Minty A, Chalon P, Lelias J-M, Dumont X, et al. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 48.Everitt J I, Wolf D C, Howe S R, Goldsworthy T L, Walker C. Am J Pathol. 1995;146:1556–1567. [PMC free article] [PubMed] [Google Scholar]
- 49.Reis A, Hennies H-C, Langbein L, Digweed M, Mischke D, Drechsler M, Schrock E, Royer-Pokora B, Franke W W, Sperling K, et al. Nat Genet. 1994;6:174–179. doi: 10.1038/ng0294-174. [DOI] [PubMed] [Google Scholar]
- 50.Stevens H P, Kelsell D P, Leigh I M, Ostlere L S, Macdermot K D, Rustin M H A. Br J Dermatol. 1996;134:720–726. [PubMed] [Google Scholar]
- 51.Lachaume P, Hitte C, Jouquand S, Priat C, Galibert F. Anim Genet. 1998;29:173–177. doi: 10.1046/j.1365-2052.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- 52.Call K M, Glaser T, Ito C Y, Buckler A J, Pelletier J, Haber D A, Rose E A, Kral A, Yeger H, Lewis W H, et al. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 53.Fleming S. Forum (Genova) 1998;8:176–184. [PubMed] [Google Scholar]
- 54.Greene L F, Rosenthal M H. N Engl J Med. 1951;244:633–634. doi: 10.1056/NEJM195104262441704. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan C, Sayre G P, Greene L F. J Urol. 1961;86:36–42. doi: 10.1016/S0022-5347(17)65102-4. [DOI] [PubMed] [Google Scholar]
- 56.Horton W A, Wong V, Eldridge R. Arch Intern Med. 1976;136:769–777. doi: 10.1001/archinte.136.7.769. [DOI] [PubMed] [Google Scholar]
- 57.Stone N N, Atlas I, Kim U S, Kwan D, Leventhal I, Waxman J S. Urology. 1993;41:66–71. doi: 10.1016/0090-4295(93)90249-a. [DOI] [PubMed] [Google Scholar]
- 58.The European Polycystic Kidney Disease Consortium. Cell. 1994;70:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 59.Mochizuki T, Wu G, Hayashi T, Xenophontos S L, Veldhuisen B, Saris J J, Reynolds D M, Cai Y, Gabow P A, Pierides A, et al. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]