Abstract
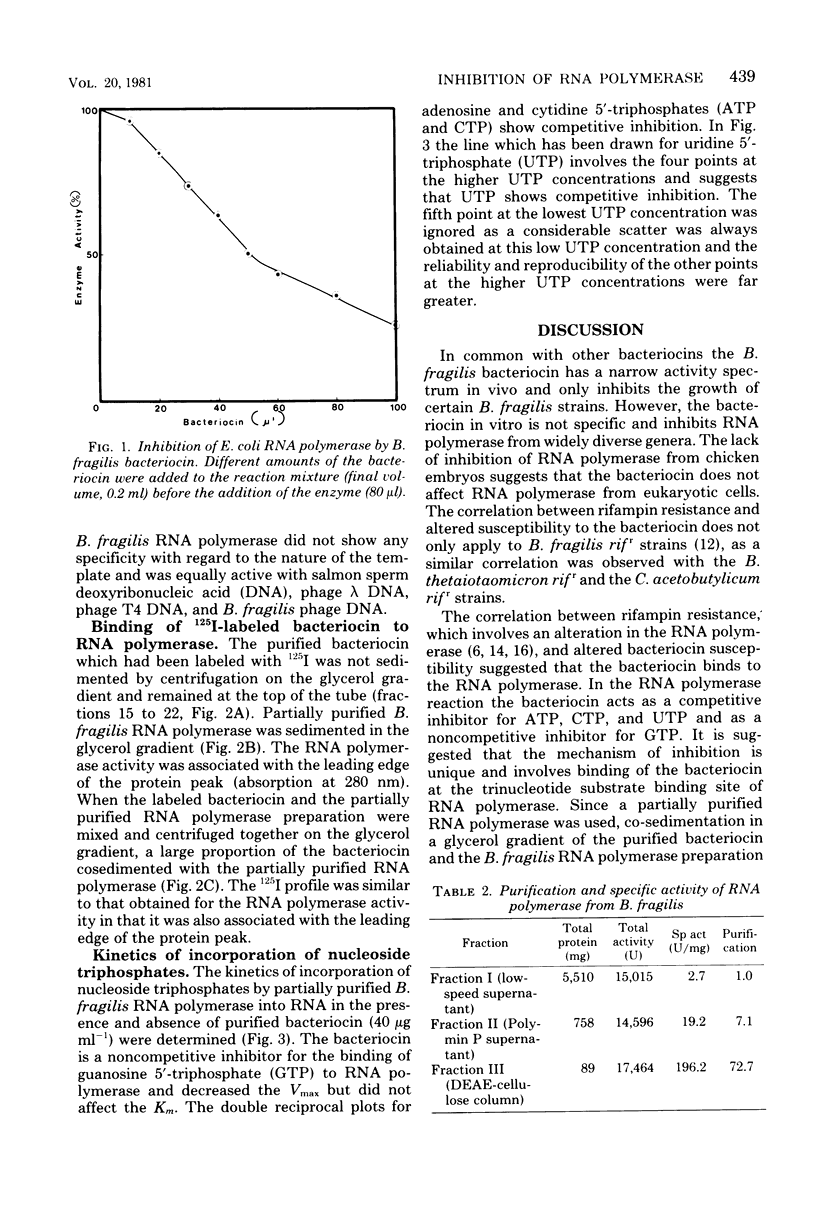
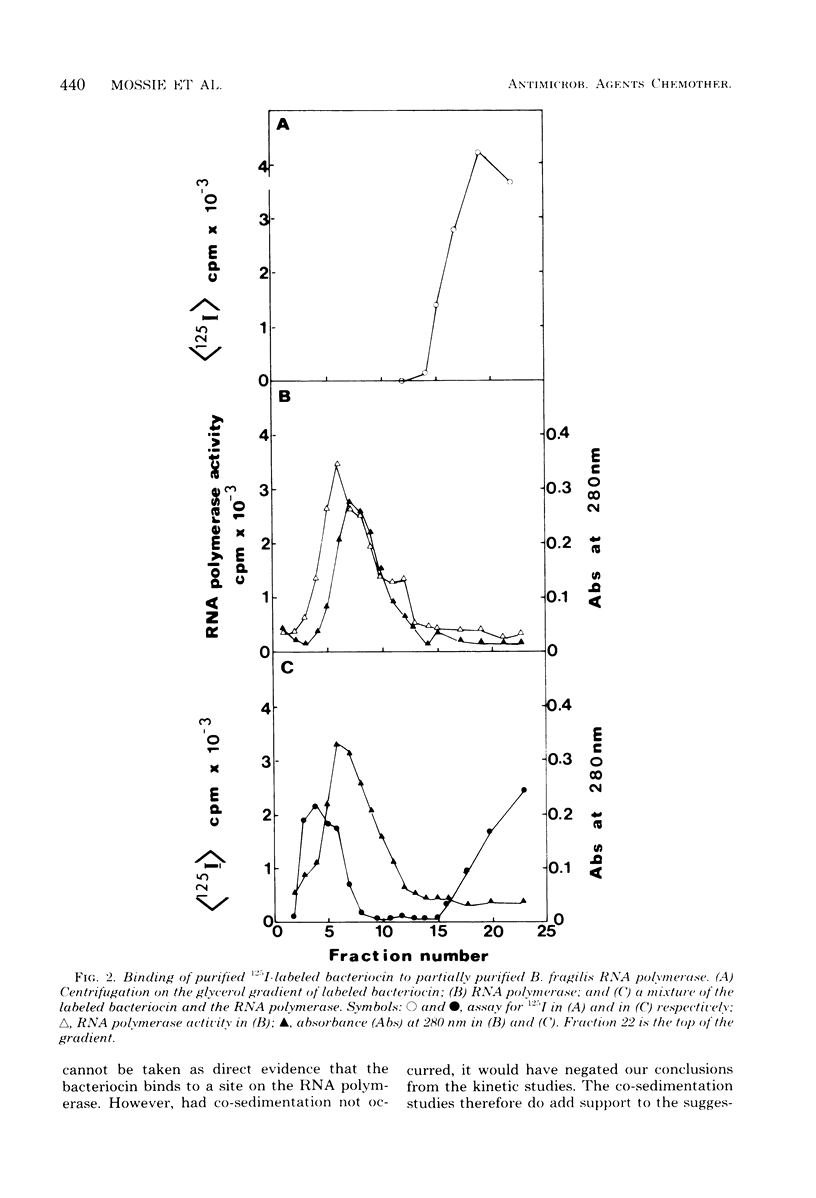
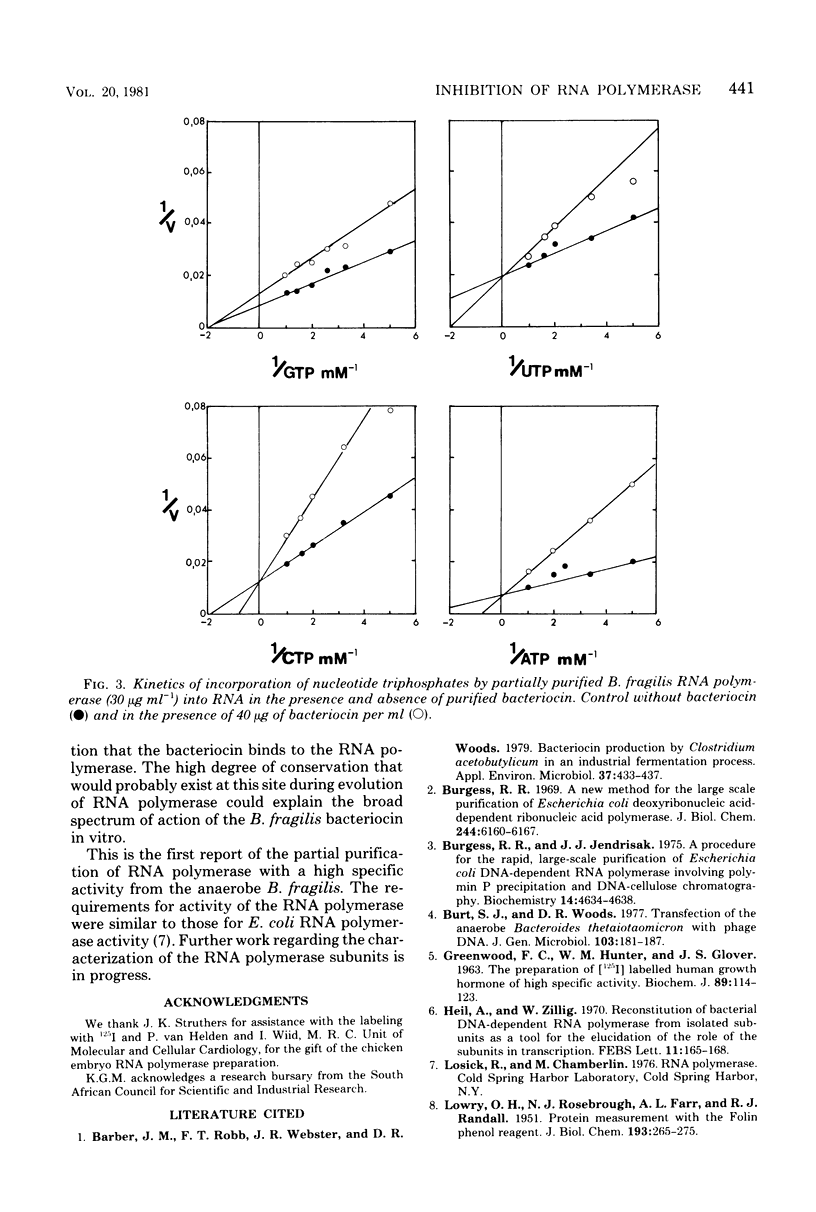
The Bacteroides fragilis bacteriocin which inhibits ribonucleic acid (RNA) polymerase activity had a narrow activity spectrum in vivo and only inhibited the growth of certain B. fragilis strains. In vitro the bacteriocin was not specific and inhibited RNA polymerases from widely diverse bacterial genera. RNA polymerases from rifampin-resistant strains of Bacteroides thetaiotaomicron and Clostridium acetobutylicum were resistant to the bacteriocin in vitro. Purified bacteriocin bound to partially purified RNA polymerase, and both proteins were cosedimented in a glycerol gradient. In the RNA polymerase reaction, the bacteriocin acted as a competitive inhibitor for adenosine, cytidine, and uridine 5'-triphosphates and as a noncompetitive inhibitor for guanosine 5'-triphosphate. The bacteriocin did not inhibit RNA polymerase from chicken embryos.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. M., Robb F. T., Webster J. R., Woods D. R. Bacteriocin production by Clostridium acetobutylicum in an industrial fermentation process. Appl Environ Microbiol. 1979 Mar;37(3):433–437. doi: 10.1128/aem.37.3.433-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moodie H. L., Woods D. R. Anaerobic R factor transfer in Escherichia coli. J Gen Microbiol. 1973 Jun;76(2):437–440. doi: 10.1099/00221287-76-2-437. [DOI] [PubMed] [Google Scholar]
- Moodie H. L., Woods D. R. Isolation of obligate anaerobic faecal bacteria using an anaerobic glove cabinet. S Afr Med J. 1973 Sep 29;47(38):1739–1742. [PubMed] [Google Scholar]
- Mossie K. G., Jones D. T., Robb F. T., Woods D. R. Characterization and mode of action of a bacteriocin produced by a Bacteroides fragilis strain. Antimicrob Agents Chemother. 1979 Dec;16(6):724–730. doi: 10.1128/aac.16.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossie K. G., Jones D. T., Robb F. T., Woods D. R. Rifampin and bacteriocin resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1980 May;17(5):838–841. doi: 10.1128/aac.17.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. C., Woods D. R., Robb F. T. Peptone induction and rifampin-insensitive collagenase production by Vibrio alginolyticus. J Bacteriol. 1980 May;142(2):447–454. doi: 10.1128/jb.142.2.447-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Zechel K., Halbwachs H. J. A new method of large scale preparation of highly purified DNA-dependent RNA-polymerase from E. coli. Hoppe Seylers Z Physiol Chem. 1970 Feb;351(2):221–224. doi: 10.1515/bchm2.1970.351.1.221. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R. DNA-dependent RNA polymerases in skeletal muscle cells differentiating in vitro. Dev Biol. 1979 Jan;68(1):280–286. doi: 10.1016/0012-1606(79)90259-8. [DOI] [PubMed] [Google Scholar]


