Abstract
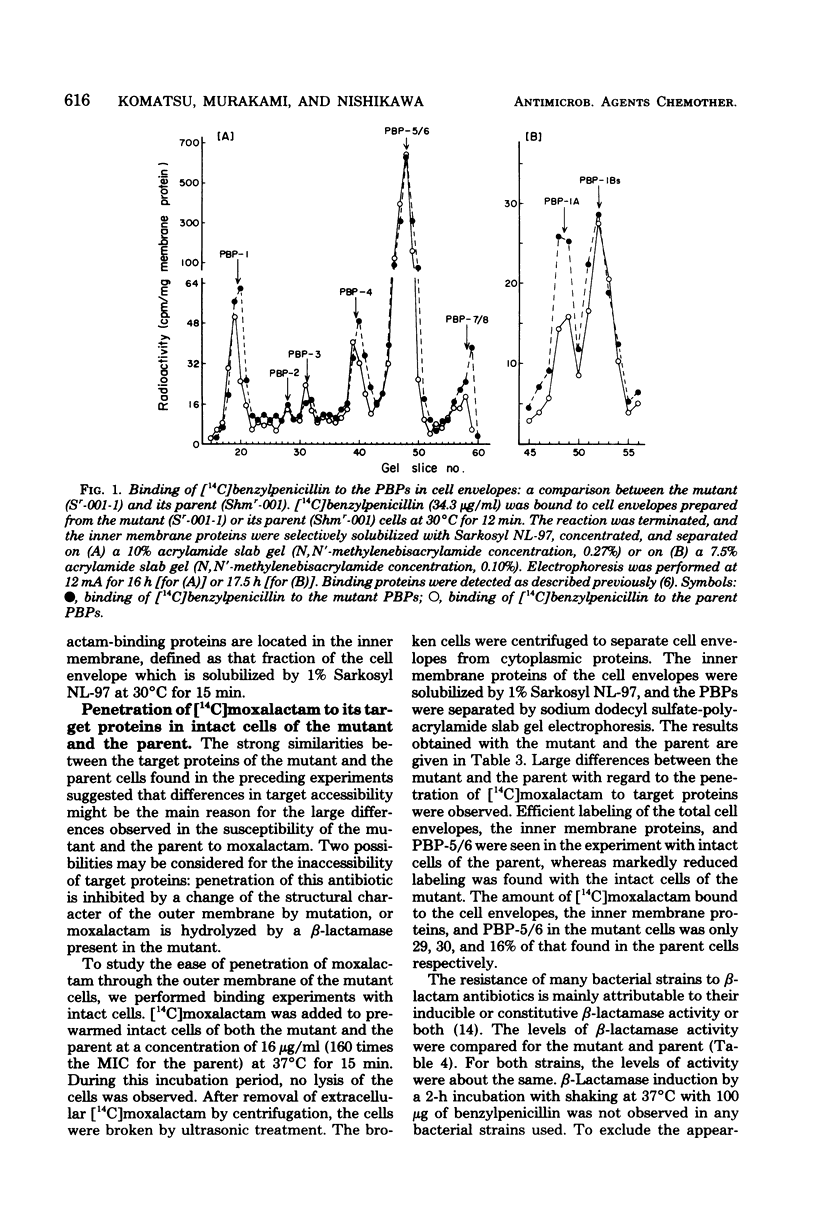
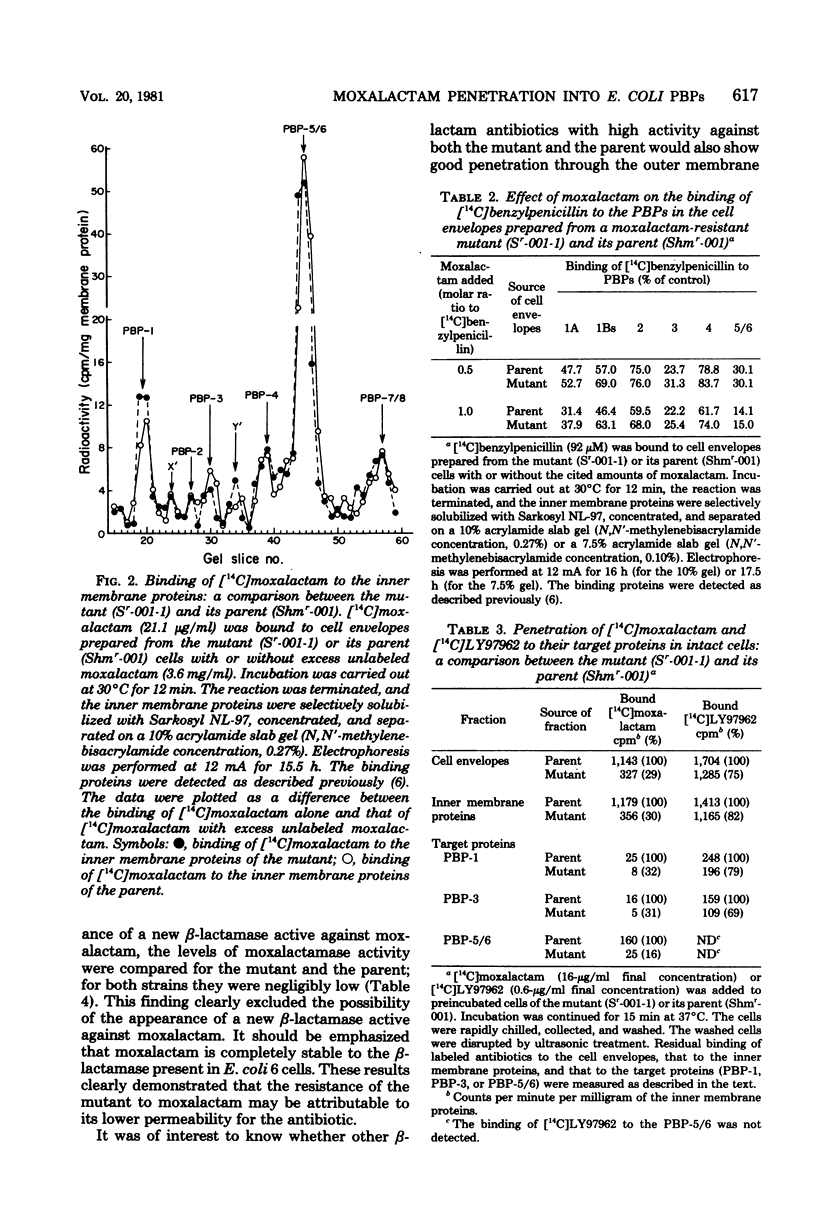
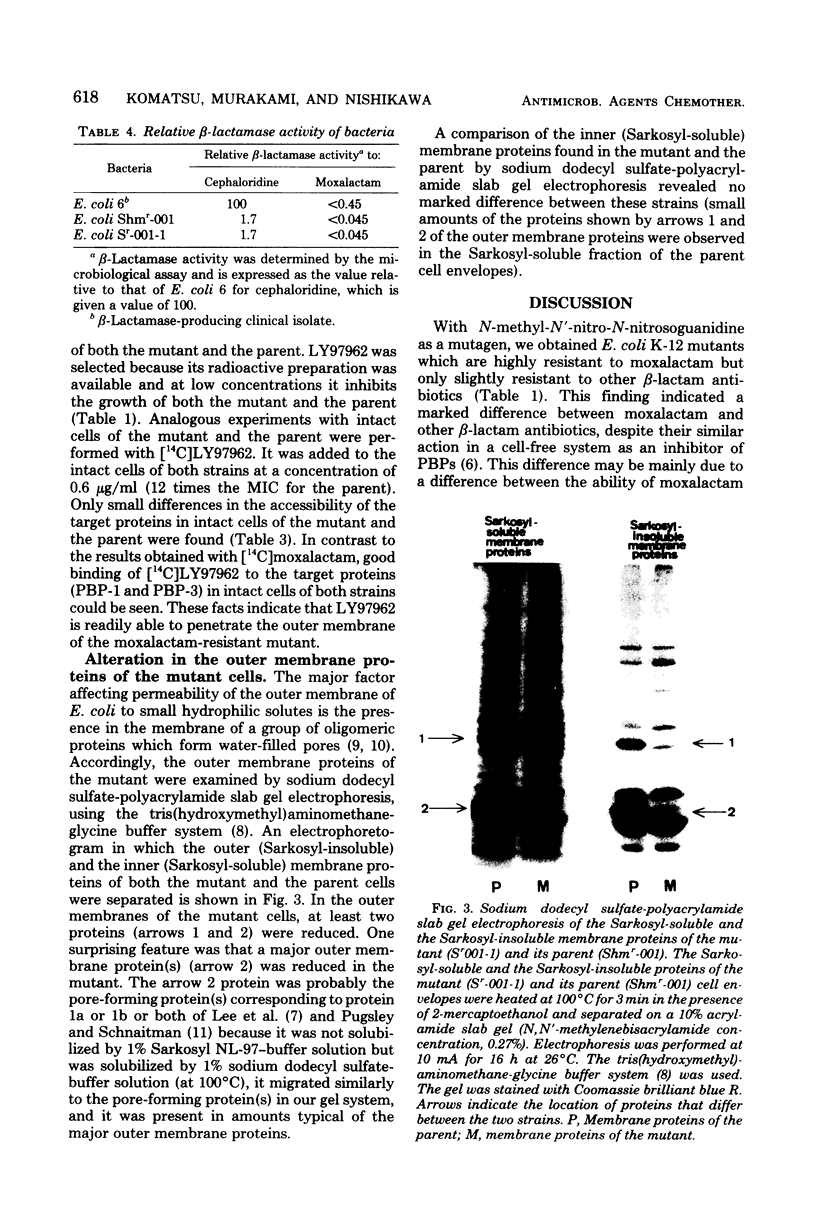
An eschericia coli K-12 mutant highly resistant to moxalactam but only slightly resistant to other beta-lactam antibiotics was obtained by mutagen treatment. The affinity of moxalactam for its target penicillin-binding proteins was unchanged, as was the level of beta-lactamase activity. The penetration of [14C] moxalactam, however, was markedly reduced in the mutant. Electrophoretic analysis revealed alterations of the outer membrane proteins. A reduction in the amount of one of the pore-forming proteins (porins) was especially noteworthy. These data suggest that moxalactam resistance is the result of an alteration in the outer membrane structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ichihara S., Mizushima S. Arrangement of proteins O-8 and O-9 in outer membrane of Escherichia coli K-12. Existence of homotrimers and heterotrimers. Eur J Biochem. 1979 Oct 15;100(2):321–328. doi: 10.1111/j.1432-1033.1979.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Komatsu Y. Adenosine uptake by isolated membrane vesicles from Escherichia coli K-12. Biochim Biophys Acta. 1973 Dec 13;330(2):206–221. doi: 10.1016/0005-2736(73)90226-5. [DOI] [PubMed] [Google Scholar]
- Komatsu Y. Mechanism of action of showdomycin. V. Reduced ability of showdomycin-resistant mutants of Escherichia coli K-12 to take up showdomycin and nucleosides. J Antibiot (Tokyo) 1971 Dec;24(12):876–883. [PubMed] [Google Scholar]
- Komatsu Y., Nishikawa T. Moxalactam (6059-S), a new 1-oxa-beta-lactam: binding affinity for penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1980 Mar;17(3):316–321. doi: 10.1128/aac.17.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. R., Schnaitman C. A., Pugsley A. P. Chemical heterogeneity of major outer membrane pore proteins of Escherichia coli. J Bacteriol. 1979 Jun;138(3):861–870. doi: 10.1128/jb.138.3.861-870.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., Chanter K. V., Harris A. M., Kirby S. M., Marshall M. J., O'Callaghan C. H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1973 Jul;54(1):9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Matsuura S., Mayama M., Kameda Y., Kuwahara S. Moxalactam (6059-S), a novel 1-oxa-beta-lactam with an expanded antibacterial spectrum: laboratory evaluation. Antimicrob Agents Chemother. 1980 Mar;17(3):302–312. doi: 10.1128/aac.17.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]




