Abstract
Trogocytosis is a fast uptake of membranes and associated molecules from one cell by another. Trogocytosis between natural killer (NK) cells and tumors is already described, but the functional relevance of NK–tumor targets material exchange is unclear. We investigated whether the immunosuppressive molecule HLA-G that is commonly expressed by tumors in vivo and known to block NK cytolytic function, could be transferred from tumor cells to NK cells, and if this transfer had functional consequences. We show that activated NK cells acquire HLA-G1 from tumor cells, and that upon this acquisition, NK cells stop proliferating, are no longer cytotoxic, and behave as suppressor cells. Such cells can inhibit other NK cells' cytotoxic function and protect NK-sensitive tumor cells from cytolysis. These data are the first demonstration that trogocytosis of HLA-G1 can be a major mechanism of immune escape that acts through effector cells made to act as suppressor cells locally, temporarily, but efficiently. The broader consequences of membrane sharing between immune and non-immune cells on the function of effectors and the outcome of immune responses are discussed.
Keywords: HLA-G, natural killer cells, suppressor cells, trogocytosis, tumor cells
Introduction
HLA-G is a non-classical MHC Class I molecule characterized by limited polymorphism and a restricted expression to immuno-privileged sites (reviewed in Carosella et al, 2003). HLA-G has immunosuppressive properties first evidenced at the fetal–maternal interface, where its expression by classical HLA Class I-negative cytotrophoblast cells protects the fetus from destruction by maternal natural killer (NK) cells. In pathological contexts, HLA-G was detected in virus infections, allotransplantation, and inflammatory diseases (reviewed in Carosella et al, 2003). Furthermore, ectopic expression of HLA-G was observed in tumor biopsies and it was shown that HLA-G expression confers tumor cells resistance to cytolysis in vitro (Rouas-Freiss et al, 2005). This has positioned HLA-G as a molecule capable of contributing to immune escape of tumors (Paul et al, 1998; Wiendl et al, 2002; Nuckel et al, 2005).
NK may serve as a first line of defense against tumors (reviewed in Moretta et al, 2005). Indeed, NK cells lyse MHC Class I-negative targets, such as some tumor cells (reviewed in Long, 2002). Yet, tumors can escape NK cytotoxicity by delivering inhibitory signals, which counterbalance triggering signals. One such inhibitory signal can be delivered by HLA-G (Menier et al, 2002). Indeed, HLA-G expressed by tumors may inhibit NK cytotoxicity by interacting with NK cell ILT-2 and/or KIR2DL4 receptors (Riteau et al, 2001a).
However, protection of tumors by direct interaction between tumor HLA-G and NK cell ILT-2 and/or KIR2DL4 receptors is not sufficient to explain HLA-G-dependent immune escape. Indeed (i) HLA-G expression in tumors is heterogeneous and does not concern all cells (Wiendl et al, 2002; Rouas-Freiss et al, 2005), and (ii) it was shown in vitro that only 10% of HLA-G-expressing tumor cells are sufficient to inhibit CTL cytotoxicity and prevent destruction of HLA-G-negative tumor cells (Wiendl et al, 2002, 2003). Thus, HLA-G-mediated immune escape can be extended to HLA-G-negative tumor cells. We chose to investigate whether HLA-G could be transferred from HLA-G-expressing tumor cells to NK cells and if this transfer would explain the protective effect of HLA-G-positive tumor cells toward HLA-G-negative tumor cells. This hypothesis is based on recent reports showing that molecules expressed by tumor cells can be acquired by NK cells (Carlin et al, 2001; Sjostrom et al, 2001; Tabiasco et al, 2003). This transfer was demonstrated in humans and mice and was recently named trogocytosis (Joly and Hudrisier, 2003).
Trogocytosis is a fast, cell-to-cell contact-dependent uptake of membranes and associated molecules. To date, most of the work on trogocytosis was carried out on murine T cells and shows that CD4+ and CD8+ T cells can respectively acquire MHC Class II and MHC Class I molecules from antigen-presenting cells (APC) in an antigen-specific manner (Patel et al, 1999; Hwang et al, 2000; Hudrisier et al, 2001). Trogocytosis is a transfer of membrane fragments and not individual molecules. Consequently, during trogocytosis, all molecules contained within a certain membrane area are transferred, including some that do not participate in cell-to-cell crosstalk, and thus transfer nonspecifically. Indeed, CD8+ T cells can acquire MHC Class II molecules along with the MHC Class I complexes they are specific for (Lorber et al, 1982; Hwang et al, 2000), and the reverse is true for CD4+ T cells (Lorber et al, 1982). Recently, trogocytosis of HLA-DR, CD80, and HLA-G1 from APC by T cells was evidenced in humans, and shown to follow the same rules as in the murine system (Tatari-Calderone et al, 2002; Game et al, 2005; LeMaoult et al, 2007). Functionally, (i) CD8+ T cells that acquired their MHC Class I ligands became susceptible to ‘fratricide' antigen-specific cytolysis (Huang et al, 1999; Hudrisier et al, 2001). Furthermore, (ii) after HLA-DR and CD80 acquisition, T cells stimulated resting T cells in an antigen-specific manner, behaving as APCs themselves (Sabzevari et al, 2001; Tatari-Calderone et al, 2002; Game et al, 2005) whereas acquisition of HLA-G1 rendered T cells immunosuppressive (LeMaoult et al, 2007). This might constitute a cheap and efficient way of modulating presentation/stimulation capabilities of the immune system. Comparatively less work was carried out on trogocytosis by NK cells. However, it was shown that NK cells can acquire MHC Class I proteins (Carlin et al, 2001; Sjostrom et al, 2001; Zimmer et al, 2001; Tabiasco et al, 2003; Vanherberghen et al, 2004) and viral receptors (Tabiasco et al, 2003) from their targets. As HLA-G is commonly expressed by tumor cells in vivo, we investigated whether it could be acquired by NK cells, and if this altered their function and constituted a mechanism of immune escape.
Our results show that HLA-G1 can indeed be acquired from tumor cells by activated but not resting NK cells by trogocytosis. In our system, almost all activated NK cells acquire detectable levels of HLA-G1 in a matter of minutes by a cell-to-cell contact-dependent mechanism. Yet, away from HLA-G1-expressing cells, NK cell-surface expression of acquired HLA-G1 is temporary as these cells do not transcribe HLA-G. Functionally, NK cells acquired HLA-G1 stop proliferating, are no longer cytotoxic, and behave as suppressor cells capable of inhibiting cytotoxic functions of other NK cells. All these functional changes are directly due to acquired HLA-G1, as they could systematically be abrogated by masking HLA-G1 or its receptor ILT2 at the NK cell surface.
This report is the first demonstration that transfer of HLA-G from tumor cells to activated, cytotoxic NK cells can be a mechanism of immune escape for HLA-G-negative tumor cells.
This mechanism differs from others because (i) it makes effectors act as suppressors through a molecule they do not even express and (ii) it is instant, temporary, and does not involve maturation. This is also the demonstration that flexibility of the immune system is such that the natural function of an immune cell and the actual function it performs might be opposite and unrelated to the repertoire of molecules it synthesizes.
Results
Activated NK cells acquire HLA-G1 from tumor cells
During interaction between human NK cells and tumor cells, NK cells can acquire membrane fragments and associated molecules, including MHC Class I molecules from target cells (Carlin et al, 2001). This transfer was reported after cell-to-cell interaction during short co-cultures between target cells and IL-2-activated NK cells from PBMC. As HLA-G is expressed on various tumor cells, we investigated whether it could transfer from tumor to NK cells. For this purpose, we used adherent HLA-G1-transfected melanoma M8-HLA-G1 line and non-adherent NK line (NKL) as the HLA-G1-expressing target:effector pair in coincubation experiments. Thus, we set up 1-h co-cultures between non-activated or IL-2-activated NKL cells and M8-HLA-G1 or their HLA-G1-negative controls, M8-pcDNA. Non-adherent NKL cells were then collected and their level of HLA-G1 cell-surface expression was investigated by flow cytometry using their CD45 expression to exclude any eventual contamination by CD45− M8 cells. As shown in Figure 1, cell-surface HLA-G1 was absent on non-activated NKL cells, IL-2-activated NKL cells before coincubation, or IL-2-activated NKL cells after coincubation with M8-pcDNA cells. By contrast, almost all IL-2-activated NKL displayed cell-surface HLA-G1 after coincubation with HLA-G1+ M8-HLA-G1 cells.
Figure 1.

HLA-G1 is found on activated NKL cells after coincubation with HLA-G1-positive melanoma cells. Non-activated and IL-2-activated NKL cells were coincubated for 1 h with adherent HLA-G1-negative M8-pcDNA cells and HLA-G1-positive M8-HLA-G1 cells. After coincubation, non-adherent NKL cells were recovered and their HLA-G1 cell-surface expression was evaluated. NK cells were distinguished from potential M8 contamination by size and CD45 expression. CD45 versus HLA-G1 expression is shown for M8 donor cells and for activated and non-activated NKL cells before and after a 1 h coincubation with M8 cells and isolation. Results shown are from one representative experiment out of five.
In order to validate these results in a more physiologically significant system and to rule out possible culture artifacts, we reproduced these experiments using polyclonal NK (pNK) cells from PBMC and other HLA-G1-expressing tumor cells, that is, HLA-G1-transfected LCL721.221 cells (LCL-HLA-G1) and their HLA-G1− controls (LCL-RSV). For these experiments, pNK cells were identified by CD16 expression on CD45+-gated cells. (Figure 2). As observed with NKL cells, non-activated pNK cells never expressed cell-surface HLA-G1 even after coincubation with M8-HLA-G1 or LCL-HLA-G1 cells. By contrast, IL-2-activated pNK cells all became cell-surface HLA-G1-positive after incubation with either M8-HLA-G1 or LCL-HLA-G1 cells, but not with their HLA-G1− counterparts (Figure 2). In these examples, transferred HLA-G1 was of recombinant origin as M8-HLA-G1 and LCL-HLA-G1 cells are tranfected cells. However, this played no part in HLA-G1 transfer as HLA-G1 endogenously produced by monocytes after IFNG treatment (LeMaoult et al, 2007) was also efficiently acquired by NKL cells (Supplementary Figure 2).
Figure 2.
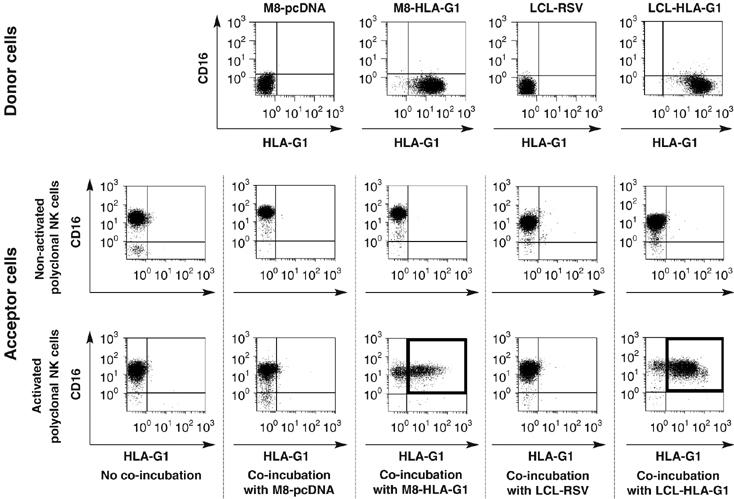
HLA-G1 is found on activated polyclonal NK cells (pNK) after coincubation with HLA-G1 tumor cells. Non-activated and IL-2-activated pNK cells were coincubated for 1 h with HLA-G1-negative M8-pcDNA or LCL-RSV cells, and HLA-G1-positive M8-HLA-G1 or LCL-HLA-G1 cells. After coincubation, pNK cells were recovered and their HLA-G1 cell-surface expression was evaluated. pNK cells were distinguished from potential donor cell contamination by size and CD45 and CD16 expression. CD16 versus HLA-G1 expression is shown for donor cells and for CD45-positive activated and non-activated pNK cells before and after coincubation with donor cells. Results shown are from one representative experiment out of eight.
These results show that activated, but not resting NK cells can express cell-surface HLA-G1 after an encounter with HLA-G1-expressing tumors. Given that NK cells were reported as not expressing HLA-G mRNA, this strengthened the possibility of a molecular transfer between tumor and NK cells. Thus, we investigated whether the mechanism by which NK cells became HLA-G1-positive was trogocytosis, using activated NKL or activated polyclonal NK cells.
Transfer of HLA-G1 from tumor cells to NK cells is a trogocytosis process
Acquisition of a tumor cell surface molecule by an NK effector could be due to (i) surface neo-display of endogenously produced HLA-G following effector–target interaction (LeMaoult et al, 2005), (ii) detection of shed tumor cell-produced HLA-G1 that is attached to NK HLA-G receptors, as can be HLA-G5 (Le Rond et al, 2006), or (iii) uptake of membrane fragments by NK cells following cell-to-cell contact (i.e. trogocytosis) (Carlin et al, 2001).
In order to show that HLA-G1 displayed on NK cells after coincubation with M8-HLA-G1 cells was not endogenously produced, we purified HLA-G1-positive NK cells from co-cultures with M8-HLA-G1 cells and measured their transcription of HLA-G1 by PCR. The oligonucleotides we used are specific for native HLA-G mRNA and cannot amplify HLA-G1 mRNA derived from transfected M8-HLA-G1 cells. As shown in Figure 3A, (i) native HLA-G1 was detected in the JEG-3 control cells as mRNA and cell-surface protein. By contrast, (ii) no native HLA-G1 mRNA was detected in HLA-G1-transfected M8-HLA-G1 cells despite high recombinant HLA-G1 cell-surface expression, and (iii) no native HLA-G1 mRNA or cell-surface protein was detected in unmanipulated NKL cells. After coincubation with M8-HLA-G1 cells, NKL cells displayed cell-surface HLA-G1, but still did not transcribe HLA-G mRNA. This shows that HLA-G1 displayed on NK cells was of M8-HLA-G1 origin and not endogenously produced.
Figure 3.

Parameters of HLA-G1 trogocytosis mechanism. (A) NK cell-surface HLA-G1 is acquired from tumor cells and not endogenously produced: activated NKL cells were incubated or not with M8-HLA-G1 cells and their HLA-G1 cell-surface expression and corresponding transcription of endogenous HLA-G1 were investigated. Plots: cell-surface expression of HLA-G1 (native or recombinant) by flow cytometry. Gels: transcription levels of native (not transfected) HLA-G by RT–PCR. JEG-3 cells were used as positive controls and M8-HLA-G1 cells were used as negative controls for native HLA-G transcription. β-Actin was used as an internal standard. Data are representative of three independent experiments. (B) Acquisition of HLA-G1 by NKL cells is cell-to-cell contact dependent and not due to HLA-G1 shedding. NKL cells and M8-HLA-G1 cells were coincubated for 30 min together (no transwell) or separated by a semipermeable membrane (transwell) before analysis of NKL HLA-G1 cell-surface expression. Data are mean±s.d. of 10 independent experiments. Coincubation with M8-HLA-G1-GFP: M8 cells transfected with HLA-G1 fused to EGFP at its intracellular part were used as HLA-G1 donors. After 1 h of coincubation, the presence of HLA-G1-EGFP was detected on NKL cells by flow cytometry. (C) HLA-G1 does not require interaction with its receptors to transfer from target cells to NK cells. HLA-G1 interactions with its receptors on NKL cells were prevented by masking HLA-G1 or ILT2 with blocking antibodies before coincubation. Transfer of HLA-G1 to NKL cells was analyzed by flow cytometry. Data are mean±s.d. of three independent experiments. (D) HLA-G1 acquisition kinetics by activated NKL and pNK cells. Activated NKL or activated pNK cells were coincubated with M8-HLA-G1 cells for the indicated times and NK-HLA-G1 expression was determined by flow cytometry. The results are mean±s.d. of five independent experiments. (E) Lifetime of acquired HLA-G1 at the surface of activated NKL cells. NKL cells that had acquired HLA-G1 from M8-HLA-G1 cells were purified, put back in culture, and their cell-surface HLA-G1 levels were analyzed at the indicated times. Data are representative of seven independent experiments.
In order to prove that HLA-G1 displayed on NK cells after coincubation with M8-HLA-G1 cells was not M8-produced soluble HLA-G1 released by shedding (Park et al, 2004) and bound to NK HLA-G receptors, we performed transwell experiments. In these, M8-HLA-G1 and NKL cells were separated by a semipermeable membrane (transwell) or were in contact with each other for 30 min (Figure 3B). At the end of the experiment, shed HLA-G1 concentrations in supernatants in the absence of transwell or in either compartment of the transwell were measured by ELISA and shown to be all identical (data not shown). Yet, prevention of cell-to-cell contact between M8-HLA-G1 cells and NKL cells also prevented HLA-G1 cell-surface neo-expression by NKL cells. Finally, when M8 cells transfected with an HLA-G1-EGFP fusion protein were used as donor cells, EGFP fluorescence (intracellular side of M8 membrane) transferred as well as HLA-G extracellular domains, indicating that a complete and not shed HLA-G1 protein was transferred from tumor cells to NK cells. These results show that cell-to-cell contact is necessary for HLA-G1 neo-display by NK cells, and rule out a possible detection of shed HLA-G1 bound to NK HLA-G receptors. Lastly, as reported (Hudrisier et al, 2005), HLA-G1 transfer was signaling-dependent as it could be prevented by the Src kinase inhibitor PP2 (data not shown).
To determine if HLA-G was transferred onto NKL cells as a result of its interaction with its receptors, we used antibodies against HLA-G and/or ILT2 to block HLA-G/ILT2 interaction. This had no effect on the proportion of cells that acquired HLA-G1 (Figure 3C) or on the amount of HLA-G1 acquired per NK cell (data not shown). These data indicate that interaction of HLA-G1 with one of its receptors did not drive its transfer. This has been reported for other molecules during membrane fragment transfers between cells (Lorber et al, 1982; Hwang et al, 2000), and was recently named trogocytosis.
The main parameters of trogocytosis are (i) cell-to-cell contact dependence, (ii) fast kinetics of the order of minutes, and (iii) limited lifetime of the transferred molecules. To prove that HLA-G1 was transferred from tumor cells to NK cells by trogocytosis, we performed transfer kinetics experiments and measured the lifetime of HLA-G1 at the NKL cell surface. Figure 3D shows that after 5 min of coincubation, 50% of activated NKL or polyclonal NK cells had already acquired HLA-G1, and that 30 min was sufficient for all NK cells to acquire HLA-G1. Additionally, the amount of HLA-G1 acquired by NK cells, as measured by HLA-G MFI on NK cells, rapidly reached a plateau and coincubation times longer than 30 min to 1 h did not cause more HLA-G to be acquired per cell (data not shown).
Furthermore, half of the NK cells that had acquired HLA-G1 from M8-HLA-G1 cells no longer displayed it 12 h after they had been purified and separated from M8-HLA-G1 cells, and no NK cells displayed it after 24 h (Figure 3E). This time is compatible with the 14-h half-life of HLA-G1 at the cell surface (Park et al, 2001).
Thus, these results show that activated NK cells acquire membrane fragments that contain HLA-G1 from HLA-G1-expressing tumor cells, by trogocytosis. NK cells that have so acquired HLA-G1, will thereafter be referred to as NK-HLA-G1acq+ cells and their negative counterparts generated with M8-pcDNA will be named NK-HLA-G1acq−.
A confocal visualization of trogocytosis of HLA-G1 is shown in Figure 4: NKL cells were conjugated for different times with M8-HLA-G1 cells and then fixed and stained with anti-HLA-G1 mAb. After 1 min of coincubation, contact areas between M8-HLA-G1 cells and NKL cells were clearly visible and no intracellular HLA-G1 was detected in NKL cells. After 5 min, and more so after 30 min of contact, multiple HLA-G1-positive areas were seen on the surface of NKL cells, some of which located outside of the contact area with M8-HLA-G1 cells, whereas intracellular HLA-G was still not detectable. These additional patches might indicate multiple contacts with M8-HLA-G1 cells and/or membrane movements.
Figure 4.
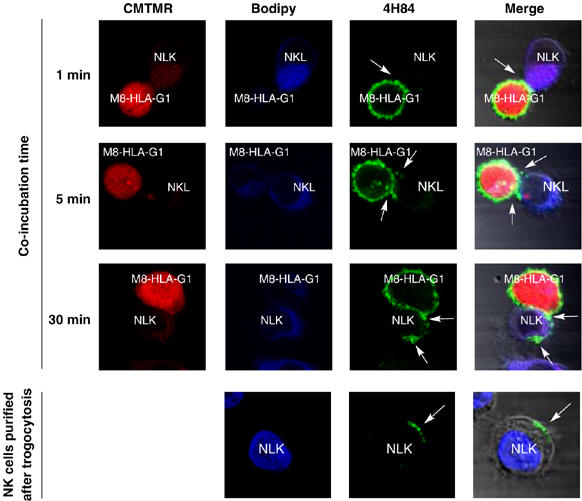
Visualization of HLA-G1 transfer from M8-HLA-G1 cells to NKL cells by confocal microscopy. Red: CMTMR cytoplasmic labeling of M8-HLA-G1 cells; blue: BODIPY cytoplasmic labeling of NKL cells; green: HLA-G labeling by anti-HLA-G antibody 4H84. Pictures were taken at the indicated time after the beginning of coincubation. Arrows indicate areas of interest. M8-HLA-G1/NKL conjugates (top lines) and isolated NKL cells (bottom line) are shown. Data are representative of three independent experiments.
Having demonstrated that it occurred, we next investigated whether trogocytosis of HLA-G1 had functional consequences, and, given HLA-G1 immunosuppressive functions, whether it could constitute an immune escape mechanism for tumor cells.
Acquisition of HLA-G1 by NK cells stops their proliferation
Activation of NK cells by IL-2 enhances their proliferation and cytolytic response against tumor cells (Trinchieri et al, 1984). HLA-G is known to inhibit cell-cycle progression of T lymphocytes by lowering expression of cyclins (Bahri et al, 2006). Thus, we investigated whether acquisition of HLA-G1 by NK cells blocked their IL-2-induced proliferation. To this end, we generated and purified IL-2-activated NKL-HLAG1acq+ or pNK-HLAG1acq+, and NKL-HLA-G1acq− or pNK-HLAG1acq− from co-culture with M8-HLA-G1 or M8-pcDNA cells. NK-HLA-G1acq− and NK-HLA-G1acq+ cells were then stimulated for 24 h with 200 IU/ml of IL-2 and their proliferation was measured by [3H]thymidine incorporation. As shown in Figure 5A for NKL or in 5B for polyclonal NK cells, unmanipulated NK and NK-HLA-G1acq− cells proliferated in response to IL-2 stimulation to the same extent. By contrast, NK-HLA-G1acq+ cells did not proliferate 12 h after IL-2 stimulation. To demonstrate that proliferation block was due to acquired HLA-G1 on NK cells, we masked HLA-G1 at all times, even before acquisition, using anti-HLA-G1 blocking antibodies. Additionally, we used Fab blocking antibodies to account for ADCC impact, non-blocking anti-HLA-G antibodies to account for steric hindrance effects, and blocking anti-ILT2 on NK cells to show the involvement of this receptor in the effects of HLA-G. None of these treatments prevented HLA-G1 uptake by NK cells. The results show that masking HLA-G1 at the surface of NK-HLA-G1acq+ cells or masking HLA-G receptor ILT2 restored their proliferation to levels identical to those of unmanipulated NK or NK-HLA-G1acq− cells. Fab HLA-G1 blocking antibody yielded the same results, ruling out any impact of ADCC, and non-blocking anti-HLA-G1 antibody had no effect, ruling out any impact of steric hindrance on these data. Moreover, 24 and 48 h after HLA-G acquisition, that is, at times when HLA-G is no longer present at the cell surface of NK-HLA-G1acq+ cells, proliferative capacity of these cells was restored, confirming that HLA-G1 proliferation block was dependent on cell-surface HLA-G1 and temporary.
Figure 5.

Acquisition of HLA-G1 by NK cells stops their proliferation. (A) NKL, NKL-HLA-G1acq−, and NKL-HLA-G1acq+ cells, and (B) pNK, pNK-HLA-G1acq−, and pNK-HLA-G1acq+ cells were generated in the presence of the indicated antibodies, isolated, and put back in culture. Proliferation was evaluated for the 12-h period before the indicated times. Data are representative of seven independent experiments.
Taken together, these results show that acquisition of HLA-G1 by activated NK cells blocks their proliferation. This blockade is strong enough so that ongoing cytokine-induced proliferation is stopped, and addition of 200 IU/ml of IL-2 has no effect. Yet, proliferation was restored by using anti-HLA-G or anti-ILT2 blocking antibodies, demonstrating that acquired HLA-G1 was responsible for proliferation inhibition. We next investigated whether transferred HLA-G1 altered NK cell functions.
Acquisition of HLA-G1 by NK cells abrogates their cytolytic function
HLA-G1 inhibits the cytolytic function of NK cells (Riteau et al, 2001a). Thus, we investigated whether acquisition of HLA-G1 from tumors by activated NK cells affected their cytotoxic function.
For this purpose, we generated and purified IL-2-activated NKL-HLA-G1acq+ or pNK-HLA-G1acq+, and NKL-HLA-G1acq− or pNK-HLA-G1acq− from co-cultures with M8-HLA-G1 or M8-pcDNA cells, and used them as effectors in a cytotoxicity assay against M8-pcDNA cells, as previously described (Riteau et al, 2001a). As shown in Figure 6A for NKL cells or Figure 6B for polyclonal NK cells, cytokine-activated NK cells taken before trogocytosis assay were cytotoxic and lysed M8-pcDNA cells efficiently. NK-HLA-G1acq− cells lysed M8-pcDNA with the same efficiency as unmanipulated NK cells, proving that coincubation with target cells did not alter NK cytolytic function. By contrast, NK-HLA-Gacq+ cells no longer lysed their targets. Masking HLA-G1 from before trogocytosis assay until cytotoxicity assay prevented function loss of NK-HLA-G1acq+ cells, which then killed M8-pcDNA cells as efficiently as unmanipulated NK cells or NK-HLA-G1acq− cells. Fab HLA-G1 blocking antibody yielded the same results, ruling out any impact of ADCC, and non-blocking anti-HLA-G1 antibody had no effect, ruling out any impact of steric hindrance on these data. As shown in Figure 6, masking HLA-G1 receptor ILT2 at the surface of NK-HLA-G1acq+ cells restored NK-HLA-G1acq+ cytolytic function as well, demonstrating the direct involvement of acquired-HLA-G1/ILT2 interaction in NK-HLA-G1acq+ loss of function. Finally, HLA-G1 effect was temporary, as NK cells fully recovered cytotoxic function once acquired HLA-G1 cell-surface expression was lost.
Figure 6.

HLA-G1 acquisition makes activated NK cells non-cytotoxic. The capability to lyse M8-pcDNA target cells was investigated by 51Cr release assay for (A) NKL-HLA-G1acq− and NKL-HLA-G1acq+ cells, and (B) pNK-HLA-G1acq− and pNK-HLA-G1acq+ taken immediately or 24 h after HLA-G1 acquisition (NKL only). When indicated, isotypic control or blocking mAb was added before trogocytosis and was kept present throughout the experiment. Results shown for NKL cells are mean±s.d. of three independent experiments, and those for pNK are representative of three experiments.
Taken together, these data show that acquisition of HLA-G1 from tumor cells by activated, cytotoxic NK effectors makes them temporarily non-cytolytic. As it is clear that NK-HLA-G1acq+ cells were not constitutively non-cytolytic, and that HLA-G acted through ILT2, we next investigated the possibility that NK-HLA-G1acq+ cells were prevented from performing their cytolytic function by HLA-G1-positive NK cells in their vicinity. This mechanism could be called cross-inhibition, each NK-HLA-G1acq+ cell inhibiting others, and would be that of regulatory/suppressive cells acting through a cell-surface molecule.
Acquisition of HLA-G1 from tumors by cytotoxic NK effectors makes them act as immunosuppressive NK cells
In order to prove that acquisition of HLA-G by cytotoxic NK cells made them act as regulatory/suppressor cells, we investigated whether NK-HLA-G1acq+ cells blocked the cytotoxic responses of autologous NK cells. For this purpose, we generated and purified IL-2-activated NKL-HLAG1acq+ or pNK-HLAG1acq+, and NKL-HLA-G1acq− or pNK-HLAG1acq− from co-cultures with M8-HLA-G1 or M8-pcDNA cells, and added them as third-party cells to a cytotoxicity assay between activated NKL or pNK effectors and M8-pcDNA targets. This assay was set up at an effector:target ratio of 50:1, which was determined to lead to maximum cytolysis, and for various effector:suppressor ratios.
As shown in Figure 7A for NKL and in 7B for pNK cells, addition of third-party NK-HLA-G1acq− cells to the cytotoxicity assay had no effect at any effector:suppressor ratio tested. Thus, NK-HLA-G1acq− cells had no regulatory/suppressor function. By contrast, addition of third-party NK-HLA-G1acq+ cells to the cytotoxicity assays at a 1:0.5 effector:suppressor ratio (i.e. 50:1:25 effector:target:suppressor ratio) inhibited target cell lysis.
Figure 7.

NK-HLA-G1acq+ cells are immunosuppressive. (A) NKL-HLA-G1acq− and NKL-HLA-G1acq+ cells, and (B) pNK-HLA-G1acq− and pNK-HLA-G1acq+ cells taken immediately or 24 h after HLA-G1 acquisition (NKL only), were added as third-party cells to cytotoxic assay between autologous activated NK and M8-pcDNA cells. E:T:S, effector:target:suppressor ratio. When indicated, isotypic control or blocking mAb was added before trogocytosis and kept present throughout the experiment. Results shown for NKL cells are mean±s.d. of three independent experiments, and those for pNK are representative of three experiments.
The regulatory function of NK-HLA-G1acq+ cells was abrogated by masking HLA-G1 at their surface, which demonstrates that they mediated their regulatory/suppressive function directly through acquired HLA-G1. Fab HLA-G1 blocking antibody yielded the same results, ruling out any impact of ADCC. Furthermore, masking HLA-G1 receptor ILT2 at the surface of effector NK cells rendered them insensitive to the suppressive effect of NK-HLA-G1acq+ cells. Finally, preventing cell-to-cell contact between NK-HLA-G1acq+ cells and effector cells in transwell experiments abrogated their suppressive function, as illustrated in Supplementary Figure 3. These data demonstrate that NK-HLA-G1acq+ cells mediated their suppressive function through cell-to-cell contact and interaction of acquired HLA-G1 at their surface with ILT2 receptors on effector NK cells.
As shown in Figure 7, inhibition of the cytotoxic function of NK cells by NK-HLA-G1acq+ cells was dependent on the effector:suppressor ratio. Indeed, immunosuppressive function was strong at a 50:1:25, intermediate at a 50:1:12, but not observable at a 50:1:6 effector:target:suppressor ratio. This is consistent with a cell-to-cell contact-dependent regulatory function.
Finally, aquired-HLA-G1-mediated suppressive function of NK cells was temporary, and no longer observable 24 h after acquisition, when acquired HLA-G1 cell-surface expression was lost.
Thus, these results show that upon acquisition of tumor cell-produced HLA-G1, activated cytotoxic NK cells become temporarily immunosuppressive and capable of protecting tumor cells from destruction by NK cells. They also show that interaction of HLA-G1 with ILT2 receptors on effector NK cells is directly responsible for this functional switch. This raises the possibility that it is because NK-HLA-G1acq+ cells become suppressors that they are non-cytolytic, being both regulatory cells and the regulatory cell targets.
Discussion
The data presented here show that activated but not resting NK cells can acquire the immunosuppressive molecule HLA-G from target cells. This was true for the NK cell line NKL as well as for pNK cells from peripheral blood. Similar transfers from target to NK cells have been reported before, but only for classical MHC Class I molecules (Carlin et al, 2001). The mechanism by which tumor cell-expressed HLA-G1 is acquired by activated NK cells is trogocytosis, that is, an intercellular transfer of a membrane patch and all the proteins it contains. As such, acquisition of HLA-G1 by activated NK cells is cell-to-cell contact dependent and occurs within minutes. Furthermore, as NK cells do not transcribe HLA-G, its cell-surface expression after acquisition is transitory and should depend on cell metabolism, membrane dynamics, recycling, shedding, or even acquisition by other cells. Thus, in a more physiological setting, HLA-G1-positive NK cells might only be found in the vicinity of HLA-G1-expressing cells.
Functionally, acquired HLA-G1 blocked the on-going proliferation of activated NK cells, and inhibited their cytolytic function toward NK-sensitive tumor cells. Furthermore, acquisition of HLA-G1 by activated cells rendered them capable of inhibiting other NK cells' cytolytic functions through interaction of acquired HLA-G1 with its receptor ILT2. It has to be noted that while this is no conceptual challenge when 100% ILT2-positive effector NK cells such as NKL cells are used, it becomes one when polyclonal NK cells are used as effectors, as only about 20% of peripheral NK cells express ILT2. One can argue that flow cytometry is dependent on a precise combination of antibody epitope versus target protein structure, and that any variation of the structure of targeted proteins might yield false/incomplete expression data. Even though this is a possibility, given the scope of this paper, we favor the hypothesis that ILT2 could also be transferred from ILT2-positive NK cells to ILT2-negative activated NK cells as reported in other systems (LeMaoult et al, 2007) and other inhibitory receptors (Vanherberghen et al, 2004). Thus, borrowed HLA-G may act through borrowed ILT2. Regardless, our data show that acquisition of HLA-G1 by NK cells amounts to the acquisition of an immunosuppressive function, albeit a temporary one due to the limited lifetime of acquired HLA-G at the NK cell surface.
At first sight, these findings suggest that trogocytosis of HLA-G1 profoundly alters activated NK cell functions, as would cell maturation. However, the fast kinetics of HLA-G1 acquisition and NK functional switch is incompatible with cell maturation events. This implies that trogocytosis does not alter the real nature of the acquirer cell, that is, a cytotoxic NK cell that has acquired HLA-G1 is still a cytotoxic NK cell by nature. Yet, as shown here, such a cell might perform a function that is other than that it is made for. In our case, upon acquisition of HLA-G1, NK cells stopped proliferating, stopped being cytotoxic, and even acted as suppressor cells, thus protecting the targets they were set to kill. This very dramatic outcome looks like an alteration of the natural function of activated NK cells and not like the addition of a new function with no alteration of the acquirer cell's natural function, as reported before (Sabzevari et al, 2001; Tatari-Calderone et al, 2002; Game et al, 2005). However, this is most likely because HLA-G1 acts on a broad array of immune cells including the NK cells themselves, which allows NK cells that have acquired HLA-G1 to act on each other. Hence, (i) NK cells that have acquired HLA-G1 inhibit the proliferation of other NK cells through HLA-G1, and the NK cell population as a whole stops proliferating. Similarly, (ii) NK cells that have acquired HLA-G1 inhibit the cytolytic function of other NK cells, and the NK population as a whole stops being cytotoxic, whether it is constituted of HLA-G1acq+ NK cells only or contains HLA-G1-negative effectors.
We did not definitely prove the physiological relevance of trogocytosis of tumor-expressed HLA-G1 by NK cells. Yet, the requirements for HLA-G1 capture by NK cells seem to be within physiological range, those being (i) cell-to-cell contact between target and NK cell and (ii) activation such as that by 200 IU of IL-2, which is a widely used activation protocol. It is then possible that all activated NK cells take membrane fragments from their targets and possibly other cells. Although trogocytosis by NK cells was evidenced in vivo in mice (Sjostrom et al, 2001; Zimmer et al, 2001), it might often go unnoticed for several reasons: (i) molecules transferred were not investigated; (ii) transfer might concern molecules expressed by both provider and acquirer cells, in which case a purely quantitative approach such as labeling could not have detected it. This would be the case of the transfer of MHC Class I molecules causing qualitative-only changes in MHC Class I:peptide repertoire of the acquirer cells, albeit of functional importance (Huang et al, 1999; Hudrisier et al, 2001). (iii) Trogocytosis-induced changes are temporary, and thus can be detected only at the right place, at the right time, and most likely not in peripheral blood. All this makes trogocytosis detection in humans a difficult matter, and a functional relevance assessment even more so.
Yet, one can hypothesize how trogocytosis of HLA-G can constitute an efficient immune escape mechanism: by transferring HLA-G1 onto activated NK cells, a few HLA-G1-expressing tumor cells might protect a comparatively larger area from cytolytic destruction by (i) quickly increasing the number of regulatory HLA-G1-positive cells locally without time-consuming and potentially hazardous unlocking of HLA-G expression and (ii) spread HLA-G1 presence to a larger area than that covered by the few HLA-G1-expressing cells. This would (iii) block the function of any effector cell that would be recruited to that area, including that of the HLA-G1acq+ cells themselves, and (iv) dampen/stop the local reaction, thus preventing damage to tissues in the vicinity of HLA-G1-expressing cells. This would constitute a cheap and efficient emergency immune suppression mechanism: triggered by and using cytotoxic effectors, limited in space because of the requirement for NK cell continuous access to HLA-G1-expressing cells, and temporary because not involving cell maturation.
Such an emergency immune suppression might find its physiological meaning in situations where immune reactions are deleterious, such as the fetal–maternal interface. It might also find a perverted meaning as a tumor immune escape mechanism of particular efficiency, as HLA-G1 presented by NK cells might inhibit not only the cytotoxic function of NK cells, but also the maturation of dendritic cells and proliferation of CD4+ T cells (Riteau et al, 1999; Horuzsko et al, 2001; LeMaoult et al, 2004).
Finally, our data highlight the key role of the cellular microenvironment in immune responses, not only because of what it may contain as soluble factors, but also because of what it contains as membrane-bound molecules. Indeed in the case presented here, it is the surrounding cells that made activated NK cells perform a function that was the opposite of their natural one. The results presented here are very dramatic because HLA-G is a molecule that is not ubiquitously expressed, easy to follow, and broadly immunosuppressive. However, other less evident transfers such as those inducing qualitative changes, or some that might alter reactivity thresholds of the acceptor cells, or even induce transcriptional changes, or apoptosis, might have equally significant consequences. Thus, immune responses might sometimes depend not on the stimulation–activation–recruitment–maturation processes that are so precisely controlled by the immune system, but on what surrounding cells make effectors do.
Materials and methods
Cells and cell lines
NKL cells were maintained in culture with 50 IU/ml of IL-2 (Sigma). Non-activated NKL cells were NKL cells deprived of IL-2 for 6 days before experiments. Activated NKL cells were obtained by adding 200 IU/ml of IL-2 (Sigma) 18 h before experiments. Adherent melanoma cell line M8 transfected with pcDNA3.1 (Invitrogen) alone (M8-pcDNA) or containing HLA-G1 cDNA (M8-HLA-G1), and lymphoblastoid LCL-721.221 cells (LCL; ATCC, Rockville, MD) transfected with pRc/RSV (Invitrogen) alone (LCL-RSV) or containing HLA-G1 cDNA (LCL-HLA-G1) have been previously described (Riteau et al, 2001b; LeMaoult et al, 2005). M8-EGFP cells were transfected with the pN3-EGFP vector alone (Clontech), and M8-HLA-G1-EGFP cells with the pN3-EGFP vector containing HLA-G1 cDNA fused to EGFP (M8-HLA-G1-EGFP). M8, LCL, and NKL cells were cultured in RPMI 1640 (Invitrogen) supplemented with 2 mM L-glutamine, 1 μg/ml gentamicin and fungizone (Sigma), and 10% heat-inactivated FCS (Invitrogen).
Choriocarcinoma cell line JEG-3 (ATCC, Rockville, MD) was used and maintained in DMEM with glutamax-I supplemented with 4.5 mg/l glucose (Invitrogen).
Monocytes were obtained from PBMC by adherence on plastic plates. HLA-G1 cell-surface expression was induced by a 48-h stimulation with 500 IU of IFNG, as previously described (LeMaoult et al, 2007).
Polyclonal NK isolation and activation
To obtain polyclonal NK cells, PBMCs from healthy donors were incubated with 20 μg/ml of anti-CD3 (OKT3), anti-CD14 (Immunotech, France), and anti-CD19 (Immunotech, France) mAbs (37°C, 30 min), followed by goat anti-mouse-coated Dynabeads (Dynal) and immunomagnetic depletion. The purity of polyclonal NK cells was systematically verified by flow cytometry on the basis of CD16 and CD56 expression. Polyclonal NK cells were used only if more than 80% CD16+CD56+ and devoid of CD3+ cells. Purified polyclonal NK cells were cultured in RPMI 1640 (Invitrogen) supplemented with 2 mM L-glutamine, 1 μg/ml gentamicin and fungizone (Sigma), and 10% heat-inactivated FCS. Activation of polyclonal NK cells was performed by adding 200 UI/ml of IL-2 (Sigma) in the culture medium for 48 h.
Trogocytosis experiments
For trogocytosis assays, non-adherent NK cells were coincubated at 37°C for various times on a layer of HLA-G1-positive or HLA-G1-negative adherent tumor cells. NK cells were then separated from adherent tumor cells, placed on ice, and all further steps were performed at 4°C or on ice. Acquisition of tumor cell-derived molecules by acquirer NK cells was investigated by flow cytometry using CD45 expression to exclude tumor cells, and additional CD16 expression in the case of pNK cells.
Transwell experiments
Transwell experiments were performed using Transwell culture system (Greiner Bio-One): NK cells were cultured in the upper chamber of a 12-well plate separated from the M8-pcDNA or M8-HLA-G1 cells by a 0.4-μm-pore semipermeable membrane.
Antibodies and flow cytometry
FITC-conjugated anti-CD45, anti-CD16, anti-CD14; PE-conjugated anti-CD56; and ECD-conjugated anti-CD19 were from Immunotech (France). Purified, FITC- and PE-conjugated anti-HLA-G1 MEM-G/9 and purified or Fab blocking anti-HLA-G1 87G were from Exbio (Czech Republic).
For flow cytometry analyses, Fc receptors were blocked by a 30-min incubation in 20% human serum, all steps were performed on ice, and isotype-matched control antibodies were systematically used. Flow cytometry analyses were performed on an Epics XL cytometer (Beckman Coulter) using EXPO32 software (Beckman Coulter).
RT–PCR analysis
After trogocytosis, RNA were extracted from NKL cells, reverse transcribed, and standard PCR amplification of HLA-G and β-actin cDNAs was performed as described (pan-HLA-G primers: G.257F/G.1250; Moreau et al, 1999). These HLA-G primers are specific for native HLA-G mRNA and do not amplify HLA-G1 mRNA from HLA-G1-transfected cells. JEG-3 cells were used as a positive control and M8-HLA-G1 cells were used as a negative control for native HLA-G1 expression.
Blocking assays
Fc receptors were first blocked using human serum and cells were incubated for 30 min at 37°C with 10 μg/ml of blocking anti-HLA-G 87G or 87G Fab, non-blocking anti-HLA-G1 MEM-G/9, or blocking anti-ILT2 before use. These concentrations are those that induced a complete block of HLA-G-mediated inhibition of NKL and pNK cytolytic function in our system (Supplementary Figure 1). All blocking conditions were maintained as they were before trogocytosis assay and throughout all subsequent steps, including purifications and washes.
Confocal microscopy
Activated NK cells and M8-HLA-G1 cells were respectively loaded (10 min, 37°C) with 0.5 μM BODIPY 630 or 0.5 μM Orange-CMTMR Cell Tracker (Molecular Probes, The Netherlands). Activated NK cells were then conjugated with M8-HLA-G1 at 37°C for different times and left adhered on poly-L-lysine-coated slides for 1 min at 37°C. Cells were then fixed for 10 min with 3% paraformaldehyde and permeabilized for 5 min with 0.1% Triton X-100. Staining was performed with anti-HLA-G1 (4H84) mAb followed by FITC-labeled goat anti-mouse Ab (Immunotech, France). Samples were finally mounted and analyzed using a Carl Zeiss LSM 510 confocal microscope (Zeiss, Germany).
Proliferation assays
Proliferation of NK cells was measured on 105 purified NK cells by 3H-labeled thymidine incorporation (1 μCi per well, Amersham Biosciences). 3H-labeled thymidine was added 12 h before incorporation and measured on a β-counter (Wallac 1450, Amersham Biosciences). Samples were collected and proliferation measured before, or 12, 24, and 48 h after trogocytosis assay.
Cytotoxicity assays
Cytolytic activity of NKL and polyclonal NK cells was assessed against 51Cr-labeled M8-pcDNA target cells in a standard 4-h 51Cr-release assay (Riteau et al, 2001b). For each experiment, triplicate samples were used.
Suppressive assays
NKL and polyclonal NK cells after trogocytosis were purified as described above and used as third-party cells autologous to effector cells in such a cytotoxic assay as above. Effector:target ratio was 50:1 in order to ensure maximum cytolysis. Final effector:target:suppressor cell ratios were 50:1:6, 50:1:12, and 50:1:50.
Statistical analyses
Data are presented as means±s.d. Student's t-test was used and a P<0.05 was taken to be significant.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Legends to supplemental Figures
References
- Bahri R, Hirsch F, Josse A, Rouas-Freiss N, Bidere N, Vasquez A, Carosella ED, Charpentier B, Durrbach A (2006) Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol 176: 1331–1339 [DOI] [PubMed] [Google Scholar]
- Carlin LM, Eleme K, McCann FE, Davis DM (2001) Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med 194: 1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N (2003) HLA-G molecules: from maternal–fetal tolerance to tissue acceptance. Adv Immunol 81: 199–252 [DOI] [PubMed] [Google Scholar]
- Game DS, Rogers NJ, Lechler RI (2005) Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant 7: 1614–1625 [DOI] [PubMed] [Google Scholar]
- Horuzsko A, Lenfant F, Munn DH, Mellor AL (2001) Maturation of antigen-presenting cells is compromised in HLA-G transgenic mice. Int Immunol 13: 385–394 [DOI] [PubMed] [Google Scholar]
- Huang J-F, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z (1999) TCR-mediated internalization of peptide–MHC complexes acquired by T cells. Science 286: 952–954 [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Riond J, Garidou L, Duthoit C, Joly E (2005) T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol 35: 2284–2294 [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E (2001) Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol 166: 3645–3649 [DOI] [PubMed] [Google Scholar]
- Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J (2000) T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med 191: 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Hudrisier D (2003) What is trogocytosis and what is its purpose? Nat Immunol 4: 815. [DOI] [PubMed] [Google Scholar]
- Le Rond S, Azema C, Krawice-Radanne I, Durrbach A, Guettier C, Carosella ED, Rouas-Freiss N (2006) Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol 176: 3266–3276 [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella E (2007) Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 109: 2040–2048 [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED (2004) HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA 101: 7064–7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED (2005) HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J 19: 662–664 [DOI] [PubMed] [Google Scholar]
- Long EO (2002) Tumor cell recognition by natural killer cells. Semin Cancer Biol 12: 57–61 [DOI] [PubMed] [Google Scholar]
- Lorber M, Loken M, Stall A, Fitch F (1982) I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol 128: 2798–2803 [PubMed] [Google Scholar]
- Menier C, Riteau B, Carosella ED, Rouas-Freiss N (2002) MICA triggering signal for NK cell tumor lysis is counteracted by HLA-G1-mediated inhibitory signal. Int J Cancer 100: 63–70 [DOI] [PubMed] [Google Scholar]
- Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P (1999) IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol 11: 803–811 [DOI] [PubMed] [Google Scholar]
- Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A (2005) Human natural killer cells: molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett 100: 7–13 [DOI] [PubMed] [Google Scholar]
- Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H (2005) HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood 105: 1694–1698 [DOI] [PubMed] [Google Scholar]
- Park B, Lee S, Kim E, Chang S, Jin M, Ahn K (2001) The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity 15: 213–224 [DOI] [PubMed] [Google Scholar]
- Park GM, Lee S, Park B, Kim E, Shin J, Cho K, Ahn K (2004) Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem Biophys Res Commun 313: 606–611 [DOI] [PubMed] [Google Scholar]
- Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD (1999) Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol 163: 5201–5210 [PubMed] [Google Scholar]
- Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG, Carosella ED (1998) HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA 95: 4510–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau B, Menier C, Khalil-Daher I, Martinozzi S, Pla M, Dausset J, Carosella ED, Rouas-Freiss N (2001a) HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol 13: 193–201 [DOI] [PubMed] [Google Scholar]
- Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, Carosella ED (1999) HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol 43: 203–211 [DOI] [PubMed] [Google Scholar]
- Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED (2001b) HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol 166: 5018–5026 [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED (2005) HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res 65: 10139–10144 [DOI] [PubMed] [Google Scholar]
- Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge JW, Bernon J, Schlom J (2001) Acquisition of CD80 (B7-1) by T Cells. J Immunol 166: 2505–2513 [DOI] [PubMed] [Google Scholar]
- Sjostrom A, Eriksson M, Cerboni C, Johansson MH, Sentman CL, Karre K, Hoglund P (2001) Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med 194: 1519–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournie J-J (2003) Acquisition of viral receptor by NK cells through immunological synapse. J Immunol 170: 5993–5998 [DOI] [PubMed] [Google Scholar]
- Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H (2002) Acquisition of CD80 by human T Cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol 169: 6162–6169 [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Matsumoto-Kobayashi M, Clark S, Seehra J, London L, Perussia B (1984) Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med 160: 1147–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanherberghen B, Andersson K, Carlin LM, Nolte-'t Hoen EN, Williams GS, Hoglund P, Davis DM (2004) Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci USA 101: 16873–16878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M (2002) A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 168: 4772–4780 [DOI] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Weiss EH, Dichgans J, Lochmuller H, Hohlfeld R, Melms A, Weller M (2003) The non-classical MHC molecule HLA-G protects human muscle cells from immune-mediated lysis: implications for myoblast transplantation and gene therapy. Brain 126: 176–185 [DOI] [PubMed] [Google Scholar]
- Zimmer J, Ioannidis V, Held W (2001) H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function. J Exp Med 194: 1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Legends to supplemental Figures


