Abstract
The caudal ventral respiratory column (cVRC) contains premotor expiratory neurons that play an important role in cough-related expiratory activity of chest wall and abdominal muscles. Microinjection of D,L-homocysteic acid (DLH) was used to test the hypothesis that local activation of cVRC neurons can suppress the cough reflex. DLH (20-50 mM, 10-30 nl) was injected into the region of cVRC in 9 anesthetized spontaneously breathing cats. Repetitive coughing was elicited by mechanical stimulation of the intrathoracic airways. Electromyograms (EMG) were recorded bilaterally from inspiratory parasternal and expiratory transversus abdominis (ABD) and unilaterally from laryngeal posterior cricoarytenoid and thyroarytenoid muscles. Unilateral microinjection of DLH (1-1.5 nmol) elicited bilateral increases in tonic and phasic respiratory ABD EMG activity, altered the respiratory pattern, and laryngeal motor activities. However, DLH also decreased cough frequency by 51±7% compared to control (p<0.001) and the amplitude of the contralateral (−35±3%, p<0.001) and ipsilateral (−34±5%, p<0.001) ABD EMGs during post injection coughs compared to control. The cough alterations were much less pronounced after microinjection of a lower dose of DLH (0.34-0.8 nmol). No cough depression was observed after microinjections of vehicle. These results suggest that an endogenous cough suppressant neuronal network in the region of the cVRC may exist and this network may be involved in the control of cough reflex excitability.
Keywords: excitatory amino acid, brainstem, ventral respiratory group, abdominal, laryngeal
INTRODUCTION
Considerable progress has been made in the understanding of central cough mechanisms since the first evidence that respiratory neurons in the ventrolateral medulla participated in coughing (14, 21). Our current conceptualization of the neurogenesis of cough holds that there is a common respiratory / cough generating neuronal network in the brainstem (38, 40, 41). However, this concept of cough generation cannot explain alterations of cough excitability induced by the administration of antitussive drugs (6, 7) or the elimination of the cough reflex by kainic acid lesions in several brainstem areas that are not involved in respiratory rhythm generation (22, 23, 35, 36). We have proposed the existence of a functional element in the cough generation system that we have termed a gate (7). This excitatory element enables a reconfigured respiratory pattern generator to produce single or repetitive coughs and provides excitatory input to expiratory premotor neurons in the medulla (8). Whether the gating mechanism is the sole brainstem control element that regulates coughing or is itself modulated by other regulatory elements is unknown. Cough can be under inhibitory control. For example, this behavior can be inhibited voluntarily in awake humans (19). While it is presumed that this voluntary cough suppression is solely due to suprapontine pathways, the role of brainstem mechanisms in mediating this effect is unknown.
The medullary caudal ventral respiratory column (cVRC) is associated with the nucleus retroambigualis and contains a high number of expiratory modulated neurons. A great majority of these cells are premotor expiratory neurons (1, 27) which transmit motor drive to spinal expiratory motoneurons (24, 30) and so play an important role in numerous expiratory-related behaviors involving the chest wall and abdominal muscles such as cough (17, 31, 39). This population of expiratory neurons is thought to have few axon collaterals to other areas of the brainstem (1, 15). As such, this population of neurons is thought to be solely premotor and have not been considered important in respiratory rhythmicity (see also 18, 42, 45). However, earlier studies also revealed that injection of the excitatory amino acid agonist D,L-homocysteic acid (DLH) into the region of cVRC induced abdominal muscle activation (10, 51), increased laryngeal muscle activity and blood pressure (51) and decreased inspiratory neuronal and motor activity which frequently led to the complete apnea (10). Bongianni et al. (10) proposed that axon collaterals to the other areas of the brainstem from the population of cVRC premotor neurons were more common than previously thought and as such this population of neurons could account for the effects that they observed. This group also proposed an alternative hypothesis that other neurons in or near the cVRC were stimulated by the DLH microinjections and that they were responsible for the apnea produced by the intervention. Apnea can also be associated with inhibition of cough (47). We speculated that chemical excitation of neurons in the region of the cVRC would result in suppression of cough. Alternatively, if no population of neurons exists in this area that participates in the inhibition of cough, chemical activation of the expiratory premotor pathway in the cVRC should only increase cough-related expiratory spinal motor activity with no other alteration in the behavior.
METHODS
Experiments were performed on 12 female cats (3.5±0.2 kg). The animals were anesthetized with sodium pentobarbital (35 mg/kg, i.v.) and supplementary doses were administered (1-3 mg/kg, i.v.) as needed. Atropine (0.1 mg/kg, i.v.) was given at the beginning of the experiment to reduce secretions. The trachea, femoral artery and vein were cannulated. The animals were allowed to spontaneously breathe a gas mixture of 40% oxygen, balance nitrogen. Arterial blood pressure (BP), end-tidal CO2 (ETCO2) and body temperature were continuously monitored. Body temperature was controlled by a heating pad and maintained at 37.5±0.5 °C. Periodically samples of arterial blood were removed for blood gas analysis. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the university of Florida IACUC.
Electromyograms (EMG) of respiratory muscles were recorded with bipolar insulated fine wire electrodes by the technique of Basmajian and Stecko (3). EMGs were recorded bilaterally from the expiratory transversus abdominis (ABD) muscles, inspiratory parasternal (PS) muscles and unilaterally from the laryngeal abductor posterior cricoarytenoid (PCA) and laryngeal adductor thyroarytenoid (ThAr) muscles. The PS electrodes were placed at T3 proximal to the sternum after exposing the surface of the muscle. Transversus abdominis electrodes were placed 3-4 cm lateral to the linea alba. The PCA electrodes were inserted along the dorsal surface of the arytenoid cartilage using its dorsal edge as a visual cue after gently elevating the larynx. The ThAr electrodes were inserted directly through the cricothyroid membrane approximately 1 cm rostrally into the larynx. Proper placement of each set of electrodes was confirmed by the appropriate inspiratory or expiratory phased activity during breathing and / or cough. In some cases, placement of laryngeal electrodes also was confirmed postmortem.
Animals were placed prone in a stereotaxic frame and the dorsal surface of medulla was exposed by an occipital craniotomy. The surface of the brainstem was covered by warm parafin oil. Microinjection of the excitatory amino acid agonist DLH (20 or 50 mM) in artificial cerebrospinal fluid (aCSF) was used to excite neurons. These concentrations of DLH allowed us to deliver small volumes of the drug and total doses that were less than or equal to than amounts that had been microinjected in other studies (10). Single or multibarrel (3 or 5 barrel) glass micropipettes (tip diameter 10-40 μm) were used for pressure injection of solutions. The tip of the micropipette was positioned under stereotaxic control into the region of cVRC (2.9-3.5 mm caudal to the obex, 2.8-3.1 mm lateral to the midline, 2.8-3.1 mm below the dorsal medullary surface). The injected volume was monitored by observation of movement of the meniscus in the micropipette barrel with a microscope. Injection sites were labelled by 4% Fast Green, 1% Pontamine Sky Blue dye, or by fluorescent latex beads (Fig. 3A). Moreover, the pipette tip was considered to be placed in or near the cVRC when DLH injections induced increases in ABD activity (10).
Figure 3.
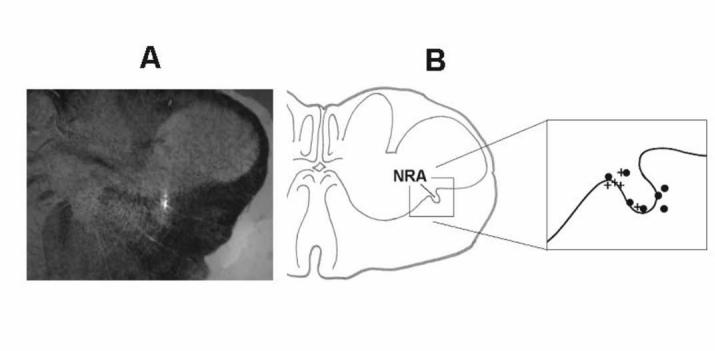
Reconstruction of DLH injection sites. Panel A: An example of a transverse medullary section with a DLH injection site (fluorescent beads, white spot in the middle) in the dorsal part of nucleus retroambigualis (NRA). The shape of the labeled region was partially protracted in the dorso-ventral direction consistent with the track of the micropipette. Injection locations were considered to be the point of maximal intensity of the marker in the most ventral part of the labeled area. Panel B: Summary of injection sites in the caudal medulla highlighting the position of NRA. In the right side of panel B the enlarged area of NRA shows representative locations of 5 identified DLH microinjection (+) and 7 reconstructed aCSF microinjection spots (●).
Tracheobronchial cough was elicited by mechanical stimulation of the intrathoracic airways with a thin polyethylene catheter. This catheter was inserted into the trachea for periods of 10 or 20 s to elicit repetitive coughing. Cough was defined by a large burst of inspiratory-related PS EMG activity immediately followed by a burst of expiratory ABD EMG activity. These criteria separated cough from other airway defensive behaviors such as expiration reflex, augmented breath, and aspiration reflex (26).
All EMGs were amplified, filtered (300-5000 Hz), rectified, and integrated (time constant 200 ms). The number of coughs in response to mechanical stimulation of the trachea (cough number, average number of coughs per 10 s stimulation), amplitudes of PS, ABD and laryngeal muscle EMG moving averages during appropriate phases of cough and breathing, respiratory rate (RR), duration of inspiratory and expiratory phases of breathing (TI and TE) and cough (CTI and CTE), BP, heart rate, and ETCO2 were analyzed in control pre-injection and post-injection periods. Monitored cardio-respiratory parameters were measured in the control cough period just before the last pre-injection cough trial, in post-injection periods right before the second cough trial. RR and respiratory phase durations were calculated from 3-10 consecutive breathing cycles. This range of breathing cycles was used to more accurately represent the maximum response of the transient and variable duration changes in breathing pattern that we observed. The cough inspiratory phase was defined as the period from the onset of PS EMG activity until its maximum during cough. The cough expiratory phase was defined as the interval from the maximum of PS activity to the onset of the next parasternal EMG burst (7). In addition we analyzed the duration of ABD EMG activity during the expiratory phase of cough. The PCA was active during both CTI and CTE. PCA EMG amplitudes were analyzed separately in the inspiratory and expiratory phases of cough. ThAr EMG amplitudes were taken independently during the cough inspiratory-expiratory transition and in the late expiratory period of cough (after the maximum of ABD EMG activity). These separate maxima in activities of laryngeal muscles correspond to the four cough laryngeal phases (34): inspiration (1st maximum of PCA), compression (1st maximum of ThAr), expulsion (2nd maximum of PCA) and subsequent laryngeal constriction (2nd maximum of ThAr).
Experimental protocol
Fifteen to 25 consecutive cough stimulation trials, separated by approximately 1 min, were conducted to establish a stable cough baseline. At this point in time, 3-5 control pre-injection trials were made with each trial separated by approximately 1 min. Another 3-5 trials were performed in the period 0-5 min after the injection followed by an additional 3-5 trials in the 10-15 min post-injection interval. For DLH microinjections (1-1.5 nmol) cough stimuli were also conducted at time points of 25 min or greater after the microinjection to test the recovery of the cough reflex. Magnitudes of the moving averages during coughing were normalized relative to the mean intensities of control pre-injection coughs. All parameters were averaged over each group of 3-5 trials. In some experiments, 20 s stimulation durations were used if 10 s duration trials were ineffective in eliciting repetitive coughing in the initial stimulation protocol.
After the experiment, the caudal medulla was removed for histological processing. The tissue was fixed in 4% paraformaldehyde followed by 30% sucrose solution. Some animals were perfused transcardially by saline and 4% paraformaldehyde before removing the brainstem. The frozen medulla was then cut into transverse slices (thickness 50 or 100 μm) by a freezing microtome. Sections were examined under light and/or UV microscopy for detection and localization of injection sites.
Results are expressed as a mean values ± SEM. For statistical analysis ANOVA was applied with Student-Neuman Kuels post tests. The differences of variables were considered significant if p < 0.05.
RESULTS
Mechanical stimulation of the intrathoracic airways elicited stable repetitive coughing with an average of 6.8±1.4 coughs per 10s stimulation in the control period. Five animals received only DLH microinjections, 3 animals received only microinjections of aCSF (27-60 nl, 37±3 nl), and 4 animals received microinjections of both aCSF and DLH. Of the 9 animals that received DLH, 5 were injected only with a higher dose (50 mM, 20-30 nl, 1.3±0.2 nmol) of this excitatory amino acid agonist. Two cats received only a smaller total dose of DLH (20 or 50 mM, 10-40 nl, 0.5±0.2 nmol) and two animals received both doses of DLH.
DLH microinjection (1.3±0.2 nmol, range 1-1.5 nmol, 11 injections, 7 locations, 7 cats) in or near the region of the cVRC led to the suppression of tracheobronchial cough induced by mechanical stimulation (Fig. 1). The microinjection induced a transient increase of ABD EMG activity (Fig. 1) and significantly reduced cough number by 51±7% (p<0.001) in post-injection trials compared to control (Fig. 2). This intervention also reduced the amplitudes of expiratory-related cough ABD EMG activity in both the contralateral and ipsilateral sides by 35±3% (p<0.001) and by 34±5% (p<0.001), respectively (Fig. 2). The cough suppression lasted 10-15 min after the injection (Fig. 2). There was no significant effect of DLH injection on PS EMG amplitudes during cough (Fig. 2), however there was a high degree of variability in the cough PS EMG responses after DLH injection with cough-related PS EMG responses markedly suppressed in some trials (Fig. 1). In 4 cats when lower doses of DLH (0.5±0.2 nmol, range 0.34-0.8 nmol, 4 injections, 4 locations) were injected into the area of cVRC there was a reduction in the amplitudes of cough ABD bursting activity in the range 17% - 25% for both the ipsi- and contralateral sides in the post-DLH periods. This inhibitory effect was statistically significant (p<0.05) only for the ABD EMG contralateral to the injection site. The number of coughs was not significantly altered under this condition.
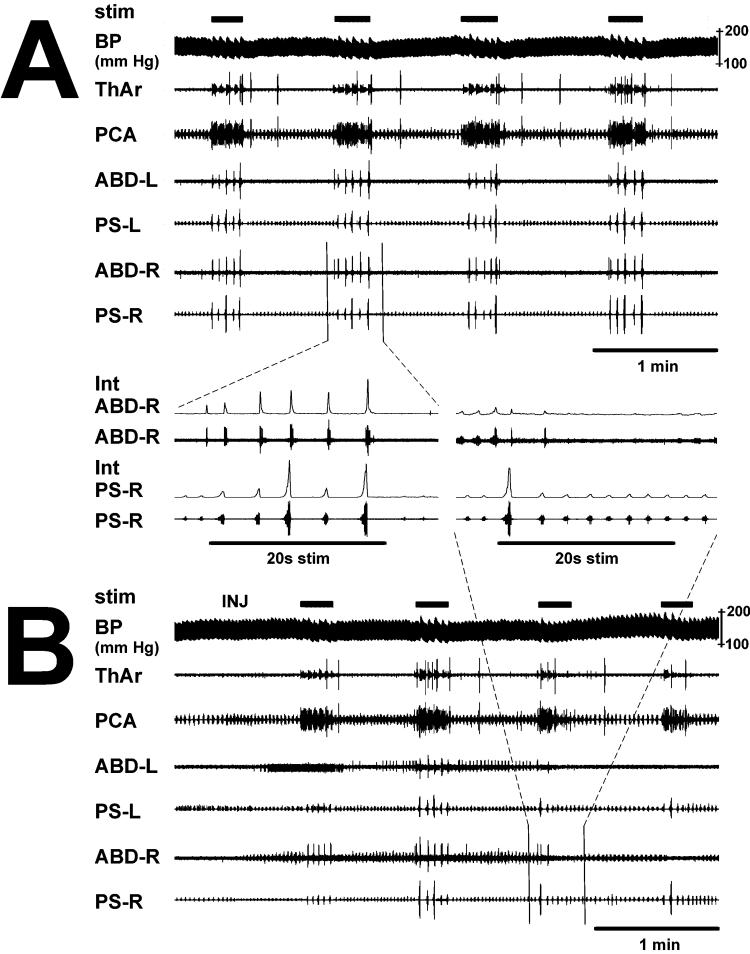
Figure 1.
Example of DLH induced suppression of cough. Tracheobronchial cough was suppressed by DLH microinjection (INJ) into the left cVRC. A- The control record (upper panel) shows 4 control pre-injection trials during which 20 total coughs were induced (5 coughs per trial). B– The post-injection record (lower panel) shows that ABD EMG activity was transiently increased bilaterally due to the DLH microinjection and a short lasting depression of PS inspiratory activity occurred on the contralateral right side (PS-R) immediately after the microinjection. Shown are 4 post-injection airway stimulation trials with 12 total coughs. Cough ABD EMG amplitudes were bilaterally decreased after the DLH microinjection. Between panels A and B are shown two expanded records of ABD and PS EMGs of the third control trial before microinjection and the third post-injection cough trial. An expiration reflex was followed by 5 coughs in the control record (left side of the panel) and by 2 weaker coughs during post-injection airway stimulation (right side of the panel). stim, periods of mechanical stimulation of the airway; BP, arterial blood pressure; ThAr, PCA, ABD, and PS, EMG of thyroarytenoid, posterior cricoarytenoid, abdominal transversus abdominis, and parasternal muscles in the right (R) or left (L) side; Int, moving average of EMG.
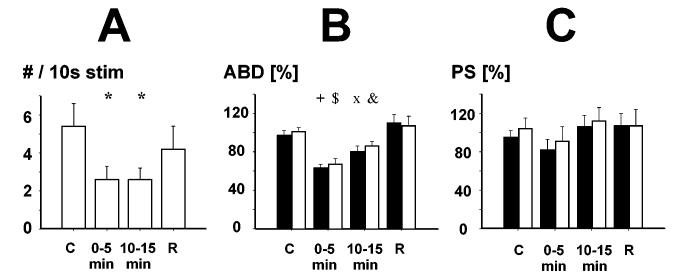
Figure 2.
Influence of DLH microinjection into the region of cVRC on cough. Panel A : Number of coughs per 10s stimulation (#/10s stim). Panels B and C : contralateral (black bars) and ipsilateral (gray bars) expiratory transversus abdominis (ABD) and inspiratory parasternal (PS) muscle EMG amplitudes during control pre-injection (C) and post-injection cough trials in periods 0-5 min, 10-15 min and during recovery period (R) more than 25 min after the injection. *, p<0.001 vs. control, p<0.05 vs. recovery; +, p<0.001 vs. control and recovery, p<0.05 vs. 10-15 min; $, p<0.001 vs. control and recovery, p<0.01 vs. 10-15 min; x, p<0.001 vs. recovery, p<0.05 vs. control and 0-5 min; &, p<0.01 vs. 0-5 min and recovery, p<0.05 vs. control.
Laryngeal abductor (PCA) and adductor (ThAr) activity were recorded during coughing in 5 cats injected with higher dose of DLH (1 – 1.5 nmol). There were no significant differences in laryngeal PCA or ThAr EMG amplitudes in any of four laryngeal phases of cough reflex (Tab. 1), even though the cough ABD EMG amplitudes were significantly reduced after the DLH injection (Fig. 2).
Table 1.
Effect of DLH microinjections in region of cVRC on laryngeal abductor and adductor activity during cough.
| PCA [% of control] | ThAr [% of control] | |||
|---|---|---|---|---|
| inspiration | expiration | I-E transition | late E | |
| Pre-injection | 113 ± 24 | 100 ± 4 | 123 ± 16 | 167 ± 28 |
| 0-5min post DLH | 111 ± 18 | 88 ± 7 | 115 ± 23 | 137 ± 25 |
| 10-15min post DLH | 122 ± 39 | 101 ± 11 | 142 ± 20 | 129 ± 21 |
| Recovery | 149 ± 59 | 107 ± 7 | 163 ± 42 | 178 ± 31 |
Relative amplitudes of EMG activities of posterior cricoarytenoid (PCA) and thyroarytenoid (ThAr) muscles in cough inspiration, expiration, inspiratory-expiratory transition (I-E transition), and the late expiratory period (late E).
To determine the mechanism by which cough number decreased in response to DLH microinjection, we analyzed the effect of this intervention on cough phase durations (Tab. 2). Even though cough number was reduced by 51% (Fig. 2), there was no significant impact of DLH microinjection on the total cough cycle time (CTtot, CTI, or CTE (Tab. 2) even when these durations were normalized to the control CTtot (expressed as percentages of the control CTtot).Similarly, there were no significant alterations in the duration of ABD EMG activity during the cough expiratory phase caused by DLH microinjections (0.83±0.08 s compared to the control 0.82±0.09 s).
Table 2.
Effect of DLH microinjections in the region of cVRC column on cough phase durations.
| CTI | CTE | CTtot | |
|---|---|---|---|
| Pre-injection | 1.00 ± 0.16 s | 1.69 ± 0.46 s | 2.69 ± 0.57 s |
| 40 ± 3 % | 60 ± 3 % | 100 % | |
| 0-5 min post DLH | 1.06 ± 0.14 s | 1.86 ± 0.33 s | 2.92 ± 0.42 s |
| 44 ± 4 % | 72 ± 7 % | 116 ± 10 % | |
| 10-15 min post DLH | 1.01 ± 0.16 s | 1.86 ± 0.43 s | 2.87 ± 0.53 s |
| 41 ± 4 % | 69 ± 5 % | 110 ± 7 % | |
| Recovery | 1.05 ± 0.17 s | 1.97 ± 0.66 s | 3.02 ± 0.78 s |
| 41 ± 3 % | 68 ± 7 % | 109 ± 6 % |
Cough inspiratory (CTI), cough expiratory (CTE) phase and total cough cycle (CTtot) durations are expressed in seconds as well as the percentages of the control pre-injection CTtot.
The control cough parameters in the aCSF treated group of animals (n=7; cough number per 10s stimulation 4.8±0.9, amplitudes of contralateral and ipsilateral ABD EMGs 90±10% and 93±9%, respectively, amplitudes of contralateral and ipsilateral PS EMG 93±10% and 98±11%, respectively) were not significantly different from those measured during the control period in DLH treated cats. Microinjections of the aCSF into the area of cVRC (13 microinjections, 8 locations) had no significant influence on number of coughs (4.5±0.9) or any other parameter of the cough reflex induced by mechanical stimulation of the intrathoracic airways.
Microinjection of DLH in the vicinity of the nucleus retroambigualis produced increases in ABD EMG activity (Fig. 1). We also observed changes in the inspiratory motor pattern consisting of transient reductions in PS EMG amplitude usually associated with increases in respiratory frequency. In addition, alterations in BP, increases in laryngeal adductor ThAr activity, and depression of inspiratory laryngeal abductor PCA EMGs were observed. We did not detect any ABD or ThAr activity in the control period, thus the level of these EMGs in pre-injection control period was considered to be zero and their increases after the DLH microinjection were normalized to the maximum burst during cough. In 7 cats injected with the higher dose of DLH there were transient increases in BP (146±8 from 129±6 mm Hg, p<0.01), RR (37±6 from 30±4 breath per minute, p<0.05), ABD EMG activity (48±15% on the contralateral side, p<0.05 and 18±5% on the ipsilateral side, p<0.05), and suppression of contralateral PS activity (41±14% of pre-injection control, p<0.01). ABD discharge induced on the contralateral side was significantly higher than that on the ipsilateral side (p<0.05). These peak changes typically were completed within one minute and beyond this period of time the parameters were not significantly different from control (Tab. 3). The expiratory ThAr EMG activity recruited by the DLH microinjection (amplitude 38±25%) as well as inspiratory PCA EMG activity (86±12% of pre-injection control) was not statistically significant compared to the control. In 4 cats injected with lower dose of DLH, the alterations of monitored cardio-respiratory parameters were qualitatively similar (and less pronounced) to that observed in the group of animals with the higher dose of DLH.
Table 3.
Mean arterial blood pressure (BP), heart rate (HR), end tidal CO2 (ETCO2), respiratory rate (RR), and duration of inspiratory (TI) and expiratory (TE) phases of breathing before and after the DLH microinjection.
| Pre-injection | 1-5 min post DLH | 10-15 min post DLH | Recovery | |
|---|---|---|---|---|
| BP [mm Hg] | 129±6 | 135±7 | 133±7 | 129±6 |
| HR [# / min] | 224±8 | 225±7 | 227±8 | 222±6 |
| ETCO2 [mm Hg] | 31±1 | 31±1 | 31±1 | 32±1 |
| RR [#/ min] | 32±4 | 33±4 | 30±3 | 31±4 |
| TI [s] | 0.64±0.06 | 0.62±0.05 | 0.65±0.05 | 0.65±0.05 |
| TE [s] | 1.46±0.20 | 1.37±0.19 | 1.44±0.17 | 1.47±0.25 |
In 7 aCSF injected animals (8 locations) in some cases we observed slight (range 0-13%) and non-significant changes in the monitored cardio-respiratory parameters after the microinjection.
We histologically reconstructed (Fig. 3A) the position of 5 injection locations in animals that received the higher dose of DLH (Fig. 3B). All these injections were placed in the ventral and ventromedial caudal nucleus retroambigualis (cNRA) or in its ventromedial border (Fig. 3B). Seven out of 8 aCSF injected locations were reconstructed. They were found in the central, medial, 2 of them in ventral part of cNRA and 3 injections were localized at and close to the lateral edge of cNRA (Fig. 3B).
DISCUSSION
The major finding of this study is that the cough reflex was suppressed by excitation of neurons in the region of the caudal ventral respiratory column with microinjection of the excitatory amino acid agonist D,L-homocysteic acid. This cough suppression manifested as a reduction in cough number as well as in abdominal EMG amplitudes lasted up to 15 min after the injection.
This is the first report to investigate the effects of local microinjection of an excitatory amino acid (EAA) agonist into the cVRC on cough. DLH is a nonspecific EAA agonist and was used in this study to excite neurons in the region of the micropipette tip. Our present experiments were based on excitation of EAA receptors by single injections of relatively small volumes (and doses) of DLH which presumably affected a limited number of neurons in cVRC of the cat. Our microinjections were unlikely to have directly modified the activity of neurons at distance more than 0.5 mm away from the micropipette tip (32). The concentrations and doses of DLH used here also were unlikely to have caused significant longlasting and extensive depolarization block (for a detailed discussion of mechanisms and limitation of the technique see 28, 32). Consistent with this concept was our observation that we could reproduce DLH induced cardiorespiratory responses within 10 min of a prior injection.
The microinjections made in these experiments were in or very near to the nucleus retroambigualis, a region that is associated with a high concentration of expiratory premotor neurons and is considered to be part of the ventral respiratory column (1, 20). The DLH microinjections excited expiratory premotor pathways because we observed increases in ABD motor discharge particularly on the contralateral side to the injection. In the cat, expiratory premotor neurons in cVRC have axons that cross the medullary midline before descending the spinal cord (1, 29). We also observed increases in ABD motor activity on the ipsilateral side, presumably because some of these descending axons cross at the segmental level and/or synapse on interneurons that have crossed axons (24, 30). These observations are consistent with those of Bongianni et al. (10) who employed DLH microinjections in cVRC to study the effects of excitation of neurons in this area on breathing in cats.
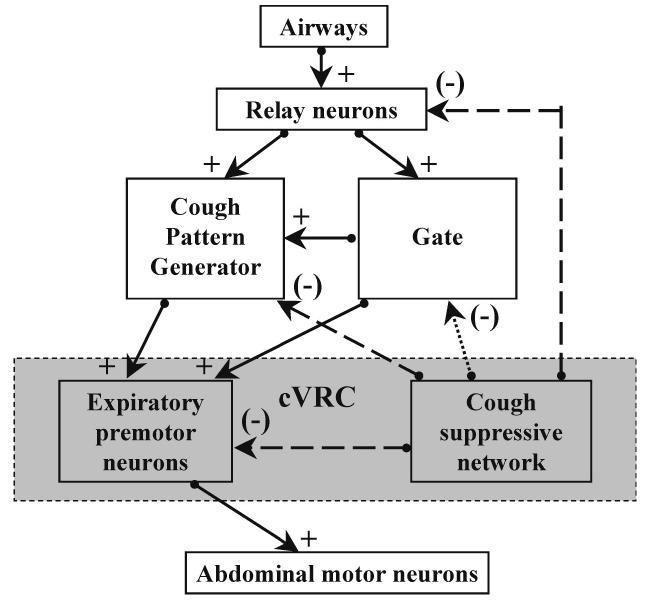
DLH microinjections in cVRC reduced cough number. According to our knowledge of the control of cough (7-9) the number of coughs can be reduced by inhibition of afferent input, by suppression of the excitatory gating mechanism, or by a direct action on the respiratory/cough pattern generator (Fig. 4). These effects will result in either prolongation of individual phases of coughing and/or a reduced ability of the pattern generator to reconfigure for the production of cough (e.g. inhibition of input from the gating mechanism). The fact that no significant alterations of phase durations (including the duration of cough ABD bursts) were detected was similar to the actions of centrally-administered cough suppressant drugs (6, 7). In addition, the relative proportions (and significant difference) of normalized CTI to CTE were preserved after the DLH microinjection. These findings also indicate that the DLH microinjections did not directly alter the excitability of elements of the core cough pattern generator (9, 40) that regulate the temporal features of the behavior. Although we cannot rule out an effect on cough relay neurons, we suggest that DLH microinjections altered the activity of neurons in the cVRC that were involved in a pathway that reduces the excitability of the cough gating mechanism (Fig. 4).
Figure 4.
Model of possible neuronal pathways (solid arrows, excitatory; dot arrow, inhibitory; dashed arrows, possible inhibitory) involved in effects of DLH microinjection into the region of cVRC on cough reflex. Relay neurons transmit afferent information from airway cough-related receptors to the cough pattern generator and gate mechanism. The gate provides excitatory inputs to the cough pattern generator and to the expiratory premotor neurons. We hypothesize an inhibitory influence of the cVRC cough suppressive element on the gating mechanism. However, inhibition of other components of cough network cannot be ruled out.
Any of the above mechanisms that could account for the suppression of cough number induced by DLH microinjection in cVRC presume the existence an axonal pathway from cVRC to other areas of the brainstem. Spontaneously active expiratory premotor neurons have few axon collaterals to the rest of the brainstem (1, 15, 27, for further references see 20). Bongianni et el. (11) have proposed that other neurons in the region of cVRC may mediate the effects of microinjection of pharmacologic agents on the respiratory and cough patterns. Indeed, neurons that were silent during breathing but recruited during cough were found in the medullary respiratory areas, particularly in cVRC (21, 41). Arita et al. (1) described some inspiratory cells intermingled among expiratory neurons in cVRC. These inspiratory units were not antidromically activated from the spinal cord or vagus nerve. This population of neurons, populations of silent neurons, or non-breathing modulated neurons in or near the cVRC could be responsible for the DLH induced cough suppression. Very little is known about possible axonal projections and/or connectivity of such groups of neurons. Anatomical studies report extensive projections from the region of cVRC to the multiple locations in the medulla and pons (44) in spite of the lack of such projections from spontaneously active expiratory bulbospinal neurons. It is possible that the results of the anatomical studies are related primarily to one or more populations of neurons that were activated by DLH in this study. Indeed, there is a well defined neuronal pathway from periaqueductal gray to neuronal populations in cVRC. Silent neurons in the region of the cVRC can be antidromically activated from the contralateral ventral respiratory column rostral to the obex. These neuronal pathways are presumably involved in the expression of vocalization (42, 51) but could be involved in the expression of other behaviors such as cough.
Unilateral microinjection of DLH reduced cough-related ABD discharge and this depression was bilateral. Moreover, cough ABD amplitudes were suppressed even when tonic ABD activity due to the DLH injection was elevated. These observations are consistent with a loss or suppression of presynaptic drive to the expiratory premotor neurons specifically during cough, and not with a generalized suppression of expiratory motor output.
Alternatively, although it is accepted that the reflex coughing is produced by brainstem neuronal network, we cannot rule out the existence of a spinal descending component of the cough suppression mechanism that could have been activated by microinjection of DLH in cVRC. Such a neuronal pathway could directly reduce cough–related ABD motor discharge and/or activate ascending tract neurons that could influence the brainstem cough mechanism.
Taken together, results from this study are consistent with the hypothesis that there is an endogenous cough suppressive neuronal mechanism or network involving neurons located in the cVRC (Fig. 4). In addition to the observations that were already noted, the cough suppression lasted considerably longer (approximately 15 min) than the transient perturbations of breathing and/or BP induced by DLH microinjection. This observation indicates that the prolonged reduction is specific for cough and does not represent a generalized suppression of any respiratory motor output. While we believe that local excitation of neurons in the region of the cVRC is involved in this effect, other more complex mechanisms could play a role. For example, in the immediate region of the micropipette, depolarization block of spontaneously active neurons could occur. This mechanism may lead to disfacilitaion of other neuron groups. Furthermore, the DLH microinjections could lead to suppression of local and/or distant neurons via the excitation of inhibitory interneurons.
A recent study of Bongianni et al. (11) performed on rabbits has shown that multiple microinjections of excitatory amino acid antagonists into the area of cVRC eliminated both the inspiratory and expiratory components of mechanically induced cough. Suppression of coughing by EAA antagonists suggests an existence of an excitatory neuronal population with ionotropic EAA receptors in the region of cVRC involved in cough expression. Multiple injections performed by Bongianni et al. (11) were placed to cover a large proportion of the rabbit cVRC bilaterally, whereas our study was restricted to single microinjections. It is unknown whether EAA antagonists would have similar actions in the cat.
Laryngeal abductor and adductor activities did not change significantly when cough ABD amplitudes were reduced. Furthermore, the laryngeal motor pattern during cough can be variable in the same as well as in different experimental preparations (2, 16, 34, 37, 43). These observations suport the concept that cranial and spinal motor outputs are subject to different regulation specifically during cough (36, 43).
The DLH microinjections into the area of cVRC in our study induced ABD and ThAr discharges, a transient increase of BP, and suppression of inspiratory activity. These effects have been described by others (10, 49). On the other hand we did not observe long apneic responses as reported by Bongianni et al. (10). We typically observed suppression of PS inspiratory amplitudes and increased RR after the microinjection of DLH in contrast to their findings. These differences may be due to the lower doses of DLH in our study (maximum 1.5 nmol) as compared to that (1.6 – 4.8 nmol) in the experiments of Bongianni et al. (10). Moreover, the animals in their experiments were vagotomized and artificially ventilated, which is markedly different from our preparation.
The neuropharmacological mechanisms responsible for control of the putative cough inhibitory population of neurons in the cVRC are currently unknown. These mechanisms are likely to be important in understanding the functional relevance of our results in the regulation of cough. DLH is a nonspecific excitatory amino acid receptor agonist and it was used solely as a tool for excitation of neurons in the cVRC. The use of this drug did not allow us to identify specific excitatory amino acid receptor subtypes that were responsible for the effects that we observed. The role of inhibitory systems such as GABAergic and glycinergic receptor mechanisms may be very important in the regulation of the cough suppressive mechanism. GABA-A receptors participate in gain modulation of cVRC expiratory premotor neurons (48) although the role of these receptors in controlling the activity of other neurons in the region is not well understood. Peripheral or central administration of the specific GABA-B receptor agonist, baclofen, inhibits cough in a dose-dependent manner (5). This cough suppressant action of baclofen was significantly reduced by pretreatment with a specific GABA-B receptor antagonist, but the antagonist alone had no effect on the expression of cough (4). These results suggest that GABA-B receptor mechanisms do not participate in a tonically active control system for cough expression. However, they may have a role in regulating the expression of cough under specific conditions that did not occur in our experiments. Although glycinergic synaptic mechanisms are important in various functions of the brainstem respiratory motor system (12), the role of glycine receptors in the control of neuron excitability in the region of the cVRC is not clear (50). Further work is necessary to more clearly define the neurochemical control of this cough control system.
Results from this study have prompted us to revise our functional model of the cough neurogenic system (7, 8). In addition to an excitatory regulatory element (the gate) that was proposed in earlier versions of the model, we now propose the presence of an inhibitory element (Fig. 4), which we term a cough suppressor. The exact conditions under which this cough suppressive element may act are unknown. However, unlike the gating mechanism, we have identified a particular medullary region which contains at least some of the neural components which contribute to this cough suppressive element. The cough suppressor does share an important characteristic with the gate in that its influence on cough can be manifest in the absence of an action on breathing. In essence, the gate and cough suppressive element are not critical components of the breathing pattern generation system. These concepts are supportive of our recent hypothesis (9) that the neurogenic mechanisms for airway defensive behaviors (such as cough) are functionally organized in a holarchical system (25). That is, the expression of these behaviors is controlled by novel elements, which impart unique regulation to the system. For example, unlike breathing, cough in humans and animals is suppressed by hypoxia (13, 46). Furthermore, hypercapnia and hypocapnia have little effect on cough (33). According to this hypothesis, the breathing pattern generator is important in the production of cough (38-40), but is itself regulated by other control elements (9).
ACKNOWLEDGEMENT
This project was supported by NIH HL 70125.
REFERENCES
- 1.Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- 2.Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol. 2001;534(Pt 2):565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basmajian JV, Stecko The role of muscles in arch support of the foot. J Bone Joint Surg Am. 1963;45:1184–1190. [PubMed] [Google Scholar]
- 4.Bolser DC, Blythin DJ, Chapman RW, Egan RW, Hey JA, Rizzo C, Kuo SC, Kreutner W. The pharmacology of SCH 50911: a novel, orally-active GABA-beta receptor antagonist. J Pharmacol Exp Ther. 1995;274:1393–1398. [PubMed] [Google Scholar]
- 5.Bolser DC, Degennaro FC, O'Reilly S, Chapman RW, Kreutner W, Egan RW, Siegel MI, Hey JA. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulm Pharmacol. 1996;9(56):357–364. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- 7.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86(3):1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 8.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15(3):221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 9.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respiratory Physiology & Neurobiology. 2006;152(3):255–65. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bongianni F, Corda M, Fontana GA, Pantaleo T. Chemical activation of caudal medullary expiratory neurones alters the pattern of breathing in the cat. J Physiol. 1994;474(3):497–507. doi: 10.1113/jphysiol.1994.sp020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bongianni F, Mutolo D, Nardone F, Pantaleo T. Ionotropic glutamate receptors mediate excitatory drive to caudal medullary expiratory neurons in the rabbit. Brain Res. 2005;1056(2):145–157. doi: 10.1016/j.brainres.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Dutschmann M, Paton JF. Glycinergic inhibition is essential for coordinating cranial and spinal respiratory motor outputs in the neonatal rat. J Physiol. 2002;543:643–653. doi: 10.1113/jphysiol.2001.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert DJ, Catcheside PG, Stadler DL, McDonald R, Hlavac MC, McEvoy RD. Acute sustained hypoxia suppresses the cough reflex in healthy subjects. Am J Respir Crit Care Med. 2006;173(5):506–511. doi: 10.1164/rccm.200509-1455OC. [DOI] [PubMed] [Google Scholar]
- 14.Engelhorn R, Weller E. Central representation of cough effecting afferent impulses in the medulla oblongata in the cat [in German] Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;284(3):224–239. [PubMed] [Google Scholar]
- 15.Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog.Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- 16.Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp Brain Res. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- 17.Grélot L, Bianchi AL. Multifunctional medullary respiratory neurons. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. CRC Press; Boca Raton: 1997. pp. 297–304. [Google Scholar]
- 18.Huang Q, John WM. Respiratory neural activities after caudal-to-rostral ablation of medullary regions. J Appl Physiol. 1988;64:1405–1411. doi: 10.1152/jappl.1988.64.4.1405. [DOI] [PubMed] [Google Scholar]
- 19.Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87(5):379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 20.Iscoe S. Control of abdominal muscles. Progress in Neurobiology. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 21.Jakus J, Tomori Z, Stransky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov. 1985;34(2):127–136. [PubMed] [Google Scholar]
- 22.Jakus J, Stransky A, Poliacek I, Barani H, Boselova L. Effects of medullary midline lesions on cough and other airway reflexes in anaesthetized cats. Physiol Res. 1998;47(3):203–213. [PubMed] [Google Scholar]
- 23.Jakus J, Stransky A, Poliacek I, Barani H, Boselova L. Kainic acid lesions to the lateral tegmental field of medulla: effects on cough, expiration and aspiration reflexes in anesthetized cats. Physiol Res. 2000;49(3):387–398. [PubMed] [Google Scholar]
- 24.Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurones in the cat. J Physiol (London) 1995;484:201–225. doi: 10.1113/jphysiol.1995.sp020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koestler A. The Ghost in the Machine. The Macmillan Company; New York: 1967. [Google Scholar]
- 26.Korpas J, Tomori Z. Cough and other respiratory reflexes. S Karger, Basel; New York: 1979. [Google Scholar]
- 27.Lindsey BG, Segers LS, Shannon R. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. II. Evidence for inhibitory actions of expiratory neurons. J Neurophysiol. 1987;57:1101–1117. doi: 10.1152/jn.1987.57.4.1101. [DOI] [PubMed] [Google Scholar]
- 28.Lipski J, Bellingham MC, West MJ, Pilowski P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- 29.Merrill EG. The lateral respiratory neurons of the medulla: their association with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller AD, Ezure K, Suzuki I. Control of abdominal muscles by brain stem respiratory neurons in the cat. J.Neurophysiol. 1985;54:155–167. doi: 10.1152/jn.1985.54.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Miller AD, Nonaka S, Siniaia MS, Jakus J. Multifunctional ventral respiratory group: bulbospinal expiratory neurons play a role in pudendal discharge during vomiting. J Autonom Nerv Syst. 1995;54:253–260. doi: 10.1016/0165-1838(95)00018-s. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 33.Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J Appl Physiol. 1989;66:2642–2646. doi: 10.1152/jappl.1989.66.6.2642. [DOI] [PubMed] [Google Scholar]
- 34.Poliacek I, Stransky A, Jakus J, Barani H, Tomori Z, Halasova E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res. 2003;52(6):749–762. [PubMed] [Google Scholar]
- 35.Poliacek I, Jakus J, Stransky A, Barani H, Halasova E, Tomori Z. Cough, expiration and aspiration reflexes following kainic acid lesions to the pontine respiratory group in anesthetized cats. Physiol Res. 2004;53(2):155–163. [PubMed] [Google Scholar]
- 36.Poliacek I, Stransky A, Szereda-Przestaszewska M, Jakus J, Barani H, Tomori Z, Halasova E. Cough and laryngeal muscle discharges in brainstem lesioned anaesthetized cat. Physiol Res. 2005;54(6):645–654. [PubMed] [Google Scholar]
- 37.Sant'Ambrogio G, Kuna ST, Vanoye CR, Sant'Ambrigio FB. Activation of intrinsic laryngeal muscles during cough. Am J Respir Crit Care Med. 1997;155(2):637–641. doi: 10.1164/ajrccm.155.2.9032206. [DOI] [PubMed] [Google Scholar]
- 38.Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm Pharmacol. 1996;9(56):343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 39.Shannon R, Bolser DC, Lindsey BG. Neural control of coughing and sneezing. In: Miller AD, Bianchi AL, Bishop BP, editors. Neural Control of the Respiratory Muscles. CRC Press; Boca Raton: 1997. pp. 213–222. [Google Scholar]
- 40.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84(6):2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 41.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525(1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiba K, Umezaki T, Zheng Y, Miller AD. The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp Brain Res. 1997;115(3):513–519. doi: 10.1007/pl00005721. [DOI] [PubMed] [Google Scholar]
- 43.Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J Neurosci. 1999;19(7):2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281(1):69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- 45.Speck DF, Beck ER. Respiratory rhythmicity after extensive lesions of the dorsal and ventral respiratory groups in the decerebrate cat. Brain Res. 1989;482(2):387–392. doi: 10.1016/0006-8993(89)91206-7. [DOI] [PubMed] [Google Scholar]
- 46.Tatar M, Korpas J, Polacek H, Zahradny V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration. 1986;49:114–121. doi: 10.1159/000194868. [DOI] [PubMed] [Google Scholar]
- 47.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411–420. doi: 10.1113/jphysiol.1988.sp017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonkovic-Capin V, Stucke AG, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential processing of excitation by GABAergic gain modulation in canine caudal ventral respiratory group neurons. J Neurophysiol. 2003;89:862–870. doi: 10.1152/jn.00761.2002. [DOI] [PubMed] [Google Scholar]
- 49.Umezaki T, Zheng Y, Shiba K, Miller AD. Role of nucleus retroambigualis in respiratory reflexes evoked by superior laryngeal and vestibular nerve afferents and in emesis. Brain Res. 1997;769(2):347–356. doi: 10.1016/s0006-8993(97)00756-7. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Boyarski LL, Frazier DT. The effect of transmitter antagonists on phasic respiratory neurons. J Neurosci Res. 1982;8:657–664. doi: 10.1002/jnr.490080410. [DOI] [PubMed] [Google Scholar]
- 51.Zhang SP, Davis PJ, Carrive P, Bandler R. Vocalization and marked pressor effect evoked from the region of the nucleus retroambigualis in the caudal ventrolateral medulla of the cat. Neurosci Lett. 1992;140(1):103–107. doi: 10.1016/0304-3940(92)90692-z. [DOI] [PubMed] [Google Scholar]