Summary
It has been postulated that the differences in longevity observed between organisms of different sexes within a species can be attributed to differences in oxidative stress. It is generally accepted that differences are due to the higher female estrogen levels. However, in some species males live the same or longer despite their lower estrogen values. Therefore, in the present study, we analyze key parameters of mitochondrial bioenergetics, oxidative stress and apoptosis in the B6 (C57Bl/6J) mouse strain. There are no differences between males and females in this mouse strain, although estrogen levels are higher in females. We did not find any differences in heart, skeletal muscle and liver mitochondrial oxygen consumption (State 3 and State 4) and ATP content between male and female mice. Moreover, mitochondrial H2O2 generation and oxidative stress levels determined by cytosolic protein carbonyls and concentration of 8-hydroxy-2′-deoxyguanosine in mitochondrial DNA were similar in both sexes. In addition, markers of apoptosis (caspase-3, caspase-9 and mono- and oligonucleosomes: the apoptosis index) were not different between male and female mice. These data show that there are no differences in mitochondrial bioenergetics, oxidative stress and apoptosis due to gender in this mouse strain according with the lack of differences in longevity. These results support the Mitochondrial Free Radical Theory of Ageing, and indicate that oxidative stress generation independent of estrogen levels determines ageing rate.
Keywords: mitochondria, oxidative stress, apoptosis, mitochondrial DNA, males, females
Introduction
The free radical theory of aging suggests that the consequences of oxidative damage (Barja, 2004) are causal to the aging process. The rate of aging could be partly determined by the levels of reactive oxygen species (ROS) produced by mitochondria during normal metabolism. In the last decade, different studies have shown that species that live longer produce less ROS (Sohal et al., 1989) and have lower steady-state levels of damage in proteins and lipids (Pamplona et al., 2000). Furthermore, oxidative damage to mitochondrial DNA is lower in these species (Barja and Herrero, 2000). In addition, caloric, protein and methionine restriction increases mean and maximum life span and also decreases ROS production and oxidative damage (Sanz et al., 2004, 2005 a,b, 2006a; Seo et al.,2006).
Centenarian women outnumber male centenarian by a ratio of 5-to-1 in most countries, except Sicily (1-to-1) (Gurwitzh, 2005). In Europe, the average life span is 73.7 years for men and 83.8 years for women (Fernandez-Ballesteros et al., 1999). Similar differences have been described in several animal species (Smith, 1989), such as rats (Rollo et al., 2002), ungulates like the red deer (Carranza et al., 2004), marsupials like the northern quolls (Dasyurus hallucatus; Humphries and Stevens, 2001) and primates like the chimpanzee (Herndon et al., 1999). However, in most mouse strains there are no differences between genders, and the same occurs in some primates like Sumatran orangutan (Wich and et al., 2004). Furthermore, male syrian hamsters live longer than female (Asdell and Joshi, 1976), and the same is true for nematodes -as Caenorhabidittis elegans- with male having longer life span than hermaphrodite or female individuals (McCulloch and Gems, 2003). Therefore there is no evidence that higher female longevity is universal, and more research about this topic is necessary.
Recently, it has been shown that female Wistar rats (with a greater mean life-span compared to male rats) produce less ROS and have lower oxidative levels compared to male Wistar rats (Borras et al., 2003). Similar results in ROS production have been obtained by Jang et al., 2004 in Fischer 344 rats. In both of these strains females live longer than males. It was proposed by different authors that estrogens might have a protective effect by decreasing oxidative damage and increasing antioxidants defenses (Vina et al., 2005). However, it is still debated whether sex differences in mitochondrial bioenergetics, apoptosis and free radical biology are consistently present within all species. For instance, females do not have increased longevity in most mouse strains (Yunis et al., 1984; Tanaka et al., 2000, Rollo, 2002), although estrogens levels are higher than in males. In fact, in some of these strains, males live longer than females. In the present study, we have chosen one strain of rodents (C57Bl/6J) without differences in longevity between genders (Rowlatt et al., 1976; Tanaka et al., 2000) and we have investigated mitochondrial bioenergetics, oxidative stress and markers of apoptosis.
Material and Methods
Animals
Mixed background 129Sv/ICR/B6 mice that were backcrossed into C57BL/6J for four generations (~94% B6) were bred at the University of Wisconsin Department of Genetics and Medical Genetics (Madison, WI, USA). When the mice had reached 10 months of age, 11 males and 12 females were shipped to the University of Florida where they were acclimated for a minimum of one week prior to experimental procedures. All experimental procedures were approved by the University of Wisconsin and the University of Florida’s Institute on Animal Care and Use Committee.
Mitochondrial isolation
Immediately after sacrifice, heart, skeletal muscle (both muscle gastrocnemius and quadriceps were mixed) and liver were removed, rinsed in saline, blotted dry, and weighed. Methods for isolating functional mitochondria varied slightly, depending upon the tissue: see Mela and Seitz with modifications (1969) for heart mitochondria, Dirks and Leeuwenburgh (2002) for skeletal muscle mitochondria, and Sanz et al. 2004 for liver mitochondria. Briefly, dissected cardiac ventricles were minced and homogenized in heart isolation buffer (220 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM Tris, 0.2% BSA pH 7.4) (1:10 wt/vol) using a Potter-Elvehem homogenizer. The nuclei and cell debris were removed by centrifugation at 700 g for 10 min. Heart mitochondria were obtained by centrifugation of the supernatant 8,000 g for 10 min. The mitochondrial pellets were suspended in 350 μl of heart isolation buffer without BSA. Skeletal muscle samples were minced and homogenized in isolation buffer (225 mM mannitol, 70 mM sucrose, 5 mM Hepes, 1 mM EDTA, 0.2% BSA, pH 7.4) (1:5 wt/vol). The homogenate was centrifuged at 1,000 g for 10 min. The supernatant was centrifuged again at 14,000 g for 20 min. The final mitochondrial pellet was resuspended in 350 μl of isolation muscle buffer without BSA. Approximately one gram of liver was homogenized in liver isolation buffer (210 mM manitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA) and the homogenate was centrifuged at 1,000 for 10 min. The supernatant was centrifuged again at 10,000 g for 10 min. The mitochondrial pellet was resuspended in 10 ml of liver isolation buffer without EDTA, and mitochondria were centrifuged again at 10,000 g for 10 min. The final mitochondrial pellet was suspended in 400 μl of liver isolation buffer without EDTA. Mitochondrial fractions were analyzed immediately and cytosolic fractions were stored at −80ºC for future measurements.
Mitochondrial H2O2 generation & oxygen consumption
The final mitochondrial suspensions were kept on ice and were immediately used for the measurements of oxygen consumption, ATP content and H2O2 production. The rate of mitochondrial H2O2 production was assayed by fluorometry with the homovanilic-horseradish peroxidase method according to Sanz and Barja (2006) adapted to a microplate reader. Substrates were 2.5 mM pyruvate/2.5 mM malate (in heart and skeletal muscle), 2.5 mM glutamate/2.5 mM malate (in liver), 5 mM succinate (without or with + 2 μM rotenone; in the three tissues). For maximum rates of H2O2 production the following concentrations of respiratory chain inhibitors were used: 2 μM rotenone (pyruvate/malate or glutamate/malate) and 10 μM antimycin A (succinate + 2 μM rotenone). Pyruvate/malate (or glutamate/malate) was used to study complex I+III ROS production, and succinate was used to study complex III ROS production (for details see Sanz and Barja, 2006). Mitochondrial oxygen consumption was measured by polarography using an Oxytherm System with a Clark oxygen electrode (Hansatech, Instruments, Norfolk, UK) under similar conditions to H2O2 production measurements. The assays were performed in the absence (State 4-resting) and in the presence (State 3-phosphorylating) of 500 μM ADP. ADP:O ratio was calculated as the ADP converted to ATP to the amount of oxygen used during state 3 respiration. The mitochondrial protein concentration used for run was: 1–1.5 mg/ml in heart and muscle and 3–4 mg/ml in liver. Both for ROS production and oxygen consumption mitochondria were incubated in a respiratory buffer (145 mM KCl, 30 mM Hepes, 5 mM KH2PO4, 3 mM MgCl2, and 0.1 mM EGTA, 0.1% albumin).
Mitochondrial ATP content
Mitochondria isolated from heart and skeletal muscle were used immediately after isolation to determine mitochondrial ATP content (Drew and Leeuwenburgh, 2003). In order to determine ATP content, freshly isolated mitochondria were added to a cuvette plus 1.25 mM pyruvate, 1.25 mM malate, and a luciferin-luciferase ATP monitoring reagent (ATP Determination Kit A-22066, Molecular Probes, Eugene, OR). A blank cuvette containing no sample, only reaction mixture, was assayed to account for background luminescence, and known concentrations of ATP were used to establish a standard curve. All mitochondrial samples were assayed in duplicate, and an average of these results was used to determine ATP content.
Protein oxidation
Protein carbonyls were measured in cytosolic fractions from heart, skeletal muscle and liver using an enzyme immunoassay (Zentech PC Test, Zenith Technology Corp., Dunedin, NZ) with slight modifications. Cytosolic fractions was obtained after: one low speed centrifugation to remove nuclei and 2) one high-speed centrifugation to precipitate mitochondria. Supernatant of this last centrifugation was used as cytosolic fraction. Briefly, all samples were normalized to 1.8 mg/ml (protein concentration of the lowest sample). Next, 11 μl of each sample, standard, and control was incubated in 19 μl of dinitrophenylhydrazine (DNPH) for 45 minutes at room temperature. Following derivitization with DNPH, 7.5 μl of each sample (including standards and controls) was diluted into 1 ml of EIA buffer. The manufacture’s instructions were then followed starting with the section entitled ELISA procedure. All samples, standards, and controls were run in triplicate.
Measurement of oxidative damage to mtDNA
Oxidative damage to mtDNA was determined according to Sanz et al. 2005b with slight modification. Briefly, mitochondrial DNA, free of nDNA, was isolated by the method of Latorre et al. 1986 as adapted to mammals (Asuncion et al., 1996). This isolation method avoids phenol use and extracts mtDNA directly from tissue (not from isolated mitochondria), avoiding the two more dangerous processes that can cause artificial mitochondrial oxidation. After isolation, mtDNA was completely dissolved in 85 μL of 30 μM DFOM, and the DNA was digested with 10 μl (4 U of Nuclease P1, dissolved in 300 mM sodium acetate, 0.2 mM ZnCl2, pH 5.3), and 5 μl of alkaline phosphatase (5 U) during 60 min at 50ºC. After filtering, samples were placed into an autosampler vial for HPLC-EC-UV analysis. 8-oxodG and dG were analyzed by HPLC with online electrochemical and ultraviolet detection, respectively according to Hofer and Moller (1998), and Hofer et al. 2006. For analysis, the nucleoside mixture was injected into two Delta-Pak (150x3.9mm id, 5 μm) C-18 reversed-phase columns (Waters, Milford, MA); 8-oxodG was detected with an electrochemical detector (Coulochem III, ESA Inc, Chelmsford, MA, USA) with a PEEK filter protected 5011A analytical cell (ESA, 5 nA, screen electrode: +205 mV analytical electrode: +275), and dG was measured with a SpectraSYSTEM UV1000 detector (Thermo Electron Corp., San Jose, CA, USA) set at 290 nm. Chromatograms were recorded using EZChrome Elite (Scientific Software INC., Pleasanton, CA, USA). Calibration curves for dG and 8-oxodG were constructed by injection of each standard 3–4 times. The HPLC buffer consisted of 9% v/v methanol and 50 mM sodium acetate set to pH 5.3 with acetic acid filtered through a CN 0.2 μm filter from Nalgene Nunc (Rochester, NY, USA).
Caspase-3 and caspase-9 activities
Caspase-3 and -9 activities were measured in cytosolic fractions from heart, skeletal muscle and liver using a fluorometric Protease assay Kit: Caspase-3/CPP32 and Caspase-9/Mch6, (Biovision, Mountain View, CA, USA) according to the manufacturer’s instructions. Briefly, the assays are based on detection of cleavage of substrate DEVD-AFV (AFC:7-amino-4-trifluoromethyl coumarin) by caspase-3, and LEHD-AFC (AFC:7-amino-4-trifluoromethyl Coumarin) by caspase-9. DEVD-AFC and LEHD-AFC emit blue light (λmax = 400 nm); upon cleavage of the substrate by caspase-3 and -9, free AFC emits a yellow-green fluorescence (λmax = 505 nm), which can be quantified using a fluorescence microtiter plate reader. All samples were run in triplicate and the means expressed as arbitrary OD units normalized to milligram of cytosolic protein, with sample protein concentrations determined by the Bradford method.
Determination of cleaved caspase-3 content
The active form of caspase-3, cleaved caspase-3, was quantified by Western blotting from cytosolic fractions of heart, muscle and liver. Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated fragments. The specific antibody used (Cell Signaling, Beverly, MA, USA) detects endogenous levels of the large fragment (17/19 kDa) of active caspase-3 resulting from cleavage adjacent to Asp175. For quantification of cleaved caspase-3 by Western blot analysis, cytosolic proteins from the heart, skeletal muscle and liver were separated using 15% PAGEr® Gold pre-cast Tris-glycine gels (BioWittaker Molecular Applications, Rockland, Maine, USA) under denaturing conditions, and then transferred to PVDF membranes (0.2 μm, Trans-Blot® Transfer Medium, Bio-Rad Laboratories, CA, USA). Protein concentration was determined using the Bradford assay and subsequently normalized so that the protein content among samples was identical. Subsequently, 20 μl of sample was loaded to each well. A 15 μl sample of HL-60 cell extract induced with etoposide (EMD Biosciences, Inc., San Diego, CA, USA) was used as a positive control. Membranes were blocked for 1h using a blocking solution containing PBS and 5% milk. Membranes were then incubated overnight in the 5% blocking solution containing the rabbit monoclonal primary anti-cleaved caspase-3 antibody (Cell Signaling, Beverly, MA, USA) with an appropriate dilution (1:100). The following day membranes were incubated for 1 h at room temperature with anti-rabbit IgG horseradish peroxidase-linked whole secondary antibody (1:1000, Amersham Biosciences UK Ltd, Amersham, UK). Specific protein bands were visualized using ECL reagent (Amersham Pharmacia Biotech, UK). The resulting Western blots were exposed to film (Hyperfilm™ ECI™, Amersham Pharmacia Biotech, UK) and analyzed using the Alpha Innotech FluorChem SP imaging system. Values were expressed as arbitrary OD units/mm2 according to software manufacture’s intructions.
Determination of cytosolic mono- and oligonucleosomes
Endogenous endonucleases activated during apoptosis cleave double-stranded DNA in the linker region between nucleosomes to generate mono- and oligonucleosomes of 180 bp or multiples. Apoptotic DNA fragmentation was quantified in cytosolic fractions from heart, skeletal muscle, and liver by measuring the amount of cytosolic mono- and oligonucleosomes using a Cell Death ELISA kit (Roche Molecular Biochemicals, Germany) with the peroxidase substrate ABTS. All samples were run in triplicate and the means expressed as arbitrary OD units normalized to milligram of cytosolic protein, with sample protein concentrations determined by the Bradford method.
Statistical analyses
Results are expressed as means ± SEM and they were analysed using independent t tests. Statistical analyses were carried out using a Graph-pad Prism statistical analysis program (San Diego, CA, USA). Statistical significance was set at P<0.05.
Results
Morphological characteristics.
Body weights in the male mice were significantly greater (20%) as compared to the female animals (Table 1). Heart, liver, quadriceps and gastrocnemius tissue weights were also significantly greater in the male animals. When the tissue weights were normalized to gram of body weight no differences were observed between the male and female animals (Table 1).
Table 1.
Body and tissue weight in male and females mice.
| Males (11) | Females (12) | |
|---|---|---|
|
Body weight (g)
Weight of tissues |
30.1 ± 0.55 | 24.1 ± 1.04*** |
|
| ||
| Heart (mg) | 171 ± 0.01 | 135 ± 0.01*** |
| Liver (g) | 1.52+0.09 | 1.15 ± 0.09** |
| Quadriceps (mg) | 220 ± 0.00 | 174 ± 0.00*** |
| Gastrocnemius (mg) | 183 ± 0.00 | 144 ± 0.00*** |
|
Weight of tissues mg/mg BW
| ||
| Heart | 5.69 ± 0.23 | 5.69 ± 0.35 |
| Liver | 50.22 ± 2.42 | 48.75 ± 3.18 |
| Quadriceps | 7.33 ± 0.17 | 7.38 ± 0.39 |
| Gastrocnemius | 6.11 ± 0.11 | 6.08 ± 0.30 |
Data are shown as means ± SEM. The number of animals per group is indicated in parentheses.
Significant differences between males and females.
p <0.01;
p < 0.001. BW = body weight.
No differences in oxidative phosphorylation between male and female mice.
Previous studies suggest differences in mitochondrial metabolism between male and female rats (Rodriguez-Cuenca et al., 2002; Valle et al., 2005). We determined mitochondrial bioenergetics in mice by measuring oxygen consumption, ADP:O ratio and ATP content. Following the isolation procedure we immediately determined the respiratory control ratio (RCR) and found that all values were greater than 4 (independent on the substrate used) indicating that the isolated mitochondria were well coupled. We did not detect any differences in oxygen consumption (state 4 or state 3 respiration) between male and female animals (Table 2). We also quantified the mitochondrial ATP content (Table 3), and we did not detect any differences between sexes in mitochondrial ATP content neither in skeletal muscle nor in heart. Moreover, no differences in ADP:O ratio were found between male and female mice (Table 3).
Table 2.
Oxygen consumption determined in heart, skeletal muscle and liver mouse mitochondria.
| Hearta | Skeletal Musclea | Liverb | ||||
|---|---|---|---|---|---|---|
| Males (11) | Females (12) | Males (10) | Females (12) | Males (3) | Females (6) | |
| State 4a | 15.0 ± 1.3 | 14.8 ± 1.5 | 11.1 ± 1.4 | 11.8 ± 1.5 | 8.4 ± 1.7 | 6.9 ± 1.4 |
| State 3a | 77.8 ± 7.5 | 65.0 ± 7.3 | 61.4 ± 4.9 | 64.8 ± 5.5 | 36.1 ± 4.5 | 34.9 ± 2.5 |
| RCRa | 5.2 ± 0.3 | 4.5 ± 0.3 | 6.9 ± 0.9 | 5.9 ± 0.6 | 4.8 ± 0.8 | 5.9 ± 0.8 |
Results are shown as means ± SEM. The number of animals per group is indicated in parentheses. Oxygen consumption is expressed as nmol O2/min mg protein.
Pyruvate/malate was used as a substrate for heart and skeletal muscle.
Glutamate/malate was used as a substrate for liver.
Table 3.
ATP content and ADP:O ratio determined in heart and skeletal muscle .mouse mitochondria,
| Heart | Skeletal Muscle | |||
|---|---|---|---|---|
| Males (10) | Females (8) | Males (7) | Females (8) | |
| ATP content | 3.7±0.5 | 2.8±0.4 | 0.12±0.05 | 0.28±0.06 |
| ADP:O | 3.6±0.4 | 4.0±0.5 | 4.3±0.3 | 4.3±0.5 |
Results are shown as means ± SEM. The number of animals per group is indicated in parentheses. ATP content is expressed as nmol ATP/mg protein.
Complex I and complex III ROS production in male and female mice.
Differences in ROS production between male and female mice have been reported in Wistar (Borras et al., 2003) and Fischer 344 (Jang et al., 2004) rats. Specific inhibitors and substrates were used to study maximal ROS production by complex I (pyruvate/malate or glutamate/malate; see materials and methods) and complex III (succinate). We did not detect any differences in complex I or complex III ROS production in the liver and heart tissues using pyruvate/malate, glutamate/malate or succinate (Table 4), except in female skeletal muscle where greater levels of ROS were detected using succinate as the substrate.
Table 4.
ROS production from heart, skeletal muscle and liver mouse mitochondria.
| Heart | Skeletal Muscle | Liver | ||||
|---|---|---|---|---|---|---|
| Males (11) | Females (12) | Males (10) | Females (12) | Males (3) | Females (6) | |
| Pyr/mala | 0.7 ± 0.1 | 0.7 ± 0.05 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.13 ± 0.05 | 0.13 ± 0.05 |
| Pyr/mal+Rota | 3.3 ± 0.2 | 3.6 ± 0.3 | 2.7 ± 0.4 | 3.0 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.1 |
| Succ | 5.9 ± 0.7 | 6.0 ± 0.5 | 5.9 ± 0.5 | 7.5 ± 0.5* | 1.0 ± 0.4 | 1.9 ± 0.5 |
| Succ+Rot | 1.9 ± 0.2 | 1.9 ± 0.1 | 1.8 ± 0.2 | 1.6 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.2 |
| Succ+Rot+Ant | 22 ± 3 | 21 ± 2 | 13 ± 1 | 12 ± 1 | 2.2 ± 0.6 | 3.6 ± 0.5 |
Results are shown as means ± SEM. The number of animals per group is indicated in parentheses. ROS production is expressed as nmol H2O2/min mg protein
Glutamate/malate was used as substrate instead of pyruvate/malate in liver. Pyr/mal = pyruvate/malate; Succ = succinate; Rot = rotenone; Ant = antymicin A.
Significant differences between males and females (p<0.05).
No difference in oxidative stress markers between males and females.
We examined levels of oxidative stress in the cytosol by measuring protein carbonyls. No differences associated with sex were detected in any tissue (Fig. 1). Moreover, measurement of 8-oxodG in mitochondrial DNA is a good indicator of steady-state levels of oxidative stress in mtDNA. Consistent with our measurements of ROS production, no differences in the levels of 8-oxodG in mtDNA were detected in muscle or liver between male and female mice (Fig. 2).
Fig. 1.

Heart, skeletal muscle and liver mouse cytosolic protein carbonyls determined by ELISA (see Methods). Data are shown as means ± SEM. For heart & skeletal muscle, n=6; for liver, n=5.
Fig. 2.
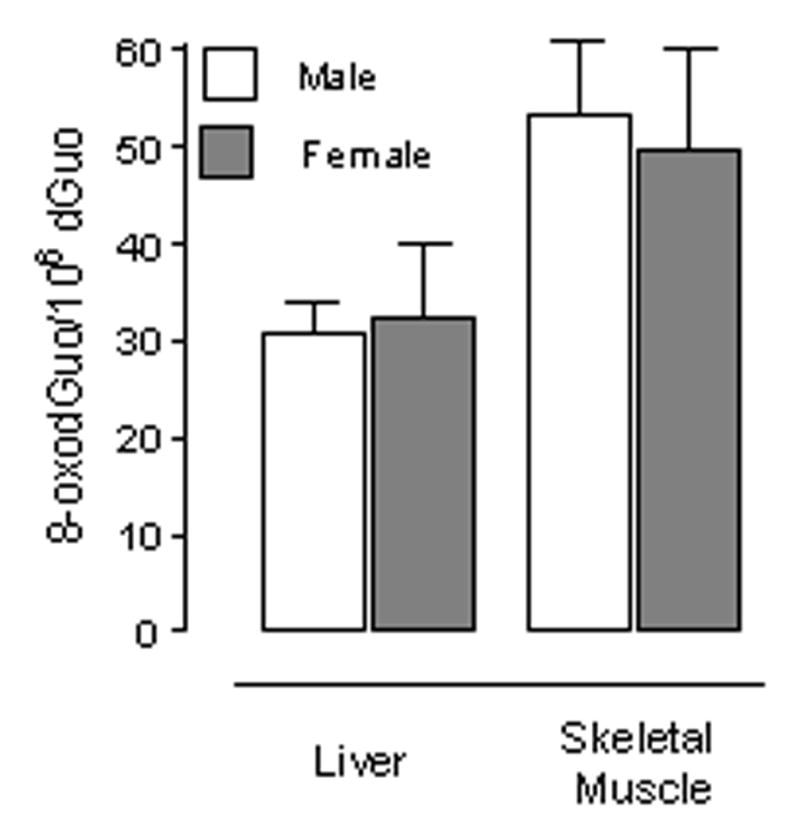
Levels of mitochondrial oxidized DNA (8-oxodGuo/106 dGuo) in liver and skeletal muscle. Data are shown as means ± SEM. For heart & skeletal muscle, n=6; for liver, n=5.
No sex differences in apoptotic markers.
A growing interest in the link between apoptosis and aging is supported by recent reports (Pollack et al., 2001; Shelke and Leeuwenburgh, 2004; Kujoth et al., 2005). Several methods are widely used to determine the extent of apoptosis in tissue. These include quantification of effector caspases as well as the measurement of DNA fragmentation indicated by the presence of mono- and oligonucleosomes in the cytosol. Moreover, pro-caspase 9 can be activated when present in a complex containing mitochondrial cytochrome c, Apaf-1, and ATP. Upon activation, caspase 9 can cleave pro-caspase 3. Activation of caspase 3 is considered a central marker of apoptosis. We measured cytosolic activity of caspase 9 and caspase 3 in heart, skeletal muscle and liver. No differences in caspase 9 and caspase-3 activities were detected between sexes in any of the tissues examined (Fig. 3). Similarly, we also found no differences in cleaved caspase 3 levels as measured by Western blotting between male and female mice (Fig. 4). We also did not observe any sex differences in the levels of mono- and oligonucleosomes in heart, skeletal muscle or liver cytosol (Fig. 5).
Fig. 3.

Caspase-3 and caspase-9 activity in heart, skeletal muscle and liver mouse cytosol. Results are reported as arbitrary fluorescence units. Data are shown as means ± SEM. For heart & skeletal muscle, n = 6; for liver, n=5.
Fig. 4.

Heart, skeletal muscle and liver cytosolic cleaved caspase-3 content. Results are reported as arbitrary optical density (OD) units/mm2. Data are shown as means ± SEM. For heart & muscle groups, n = 6; for liver groups, n = 5.
Fig. 5.

Quantification of mono- and oligonucleosomes (apoptotic index) in the heart, skeletal muscle and liver of males and females mice using a quantitative ELISA. Results are reported as arbitrary optical density (OD) units/mg protein. Data are shown as means ± SEM. For heart & muscle groups, n = 6; for liver groups, n = 5.
Discussion
In most countries there are differences in longevity between men and women (Fernandez-Ballesteros y col., 1999). This also happens in many animal species (see introduction for examples). Maximum life span is greater in females in most of rat strains. However maximum longevity does not vary significantly with gender in mice (Rollo, 2002). Furthermore, Syrian hamster (Mesocricetus auratus) males live longer than females (Asdell and Joshi, 1976).
According to the mitochondrial free radical theory of aging, the rate of mitochondrial ROS production contributes to determine the aging rate (Barja, 2004). Recently, it has been shown that mitochondria from female Wistar rats produce less ROS than those from males in liver and brain, and their mean life span is 16% longer (Borras et al., 2003). Leeuwenburgh’s laboratory has reported that heart mitochondria from female Fischer 344 rat heart also produce less ROS than those from males (Jang et al., 2004). Again, female Fisher 344 rats live longer than males (Tanaka et al., 2000). Borras et al. 2003 found that antioxidant defenses – Mn-superoxide dismutase (MnSOD) and glutathione peroxidase (Gpx)- were higher in the females, and estrogens were considered responsible for such an effect (Vina et al., 2005). However, Jang et al. (2004) found that Gpx was lower in female rats, although their estrogens levels were higher. This indicates that higher antioxidant content is not always present in longer-lived female compared to male rats. Furthermore, this last result (low ROS production and low antioxidant defenses) agrees with inter-species comparisons where long-lived animals show lower antioxidant levels than short-lived animals together with lower rates of mitochondrial ROS production (Barja, 2004).
Oxidative damage, rather than defense or repair of such kind of damage controls the aging rate (reviewed in Sanz et al., 2006). For this reason, in the present study, we have chosen to measure several parameters related to oxidative damage generation in males and females. We have studied ROS production in three different tissues, two post-mitotic (heart, skeletal muscle) and one mitotic (liver), and we have not found any significant difference between males and female mice. Consistent with these results, the maximum lifespan (MLSP) of the C57Bl/6 mouse strain and that of the mixed background strain do not differ between males and females (Rowlatt et al., 1976; Tanaka et al., 2000).
Borras et al. 2003 found lower levels of 8-oxodG in the mtDNA of female rats, consistently with the smaller ROS production of female liver mitochondria. We have not found any sex-related differences in the level of oxidative damage in the mtDNA of mice. This agrees with our results regarding ROS production as well as with the lack of differences in longevity between sexes in the studied mice. Furthermore, no differences in cytosolic oxidative damage to proteins (proteins carbonyls) were detected between sexes either, confirming that tissues from male mice are not subjected to a higher level of oxidative stress than those of females in this strain. Other studies also failed to find gender-related differences in lipid peroxidation in mouse liver (Sverko et al., 2002) supporting the idea that a lower oxidative stress in females is not universal in rodents.
The gap between male and female longevity, when present, has been attributed to estrogen action. It has been proposed (Borras et al., 2003, 2005) that estrogens increase antioxidant levels leading to a lower efflux of ROS from mitochondria. According to those authors, estrogens do not act directly as antioxidants; rather, they act by regulating the expression of antioxidant genes (Vina et al., 2005). Recently, it has been confirmed that estrogens increase SOD expression in cell culture protecting human cells against induced apoptosis (Pedram et al., 2006). Membrane estradiol receptor could activate the MAP kinase system, leading to activation of NF-kB. When this transcription factor is activated, it is imported to the nucleus, where it stimulates transcription of MnSOD and GPx genes (Borras et al., 2005). Although, some reports show that female rats have higher antioxidant concentration and activity than male rats (Borras et al., 2003; Baba et al., 2005), others did not found such difference (Jang et al., 2004; Sverko et al., 2002, 2004). This indicates that estrogen regulation may increase antioxidant defenses in some, but not in all strains. Moreover, it has been reported that menopause increases MnSOD activity in women skeletal muscle (Pansarasa et al., 2006) suggesting a role for additional, non-estrogen factors in the regulation of MnSOD activity. Furthermore, estrogen levels in our mouse strain are almost double in females at 9–10 months (Cousins et al., 2003), and we did not found any difference in oxidative stress. Anyway, antioxidants do not control aging rate (reviewed in Sanz et al. 2006b), so the possible increase in antioxidants levels -provoked by estrogens- is not relevant to determine longevity. According to that female Syrian hamsters have higher levels of antioxidants than males, but males live longer (Coto-Montes et al. 2001).
The role of estrogens on glutathione metabolism seems clearer since it has been described that women or female rodents (rats or mice) have higher GSH (the reduced glutathione form) concentration than their male counterparts (Chen et al., 1998; 2004: Pansarasa et al., 2000; Wang et al., 2003). This can be relevant since it has been found that the concentration of oxidized glutathione can modulate ROS production of isolated complex I (Taylor et al., 2003). Lower levels of oxidized glutathione in the female mitochondrial matrix could help to explain why mitochondrial oxidative stress is lower in some rodent strains. This could also explain the antiapoptotic properties of estrogen administration (Chen et al., 2000; Pedram et al., 2006). However, this explanation requires more experimental research.
Another possible alternative to explain the gender-specific modulation of ROS production is a direct regulation of mitochondrial genome by estrogens (Chen and Yager, 2004). In fact, the estrogen receptor is present in mitochondria (Grossman et al., 1989; Monje et al., 2001; Pedram et al., 2006). Moreover, estrogens induce transcription of mitochondrial genes (Bettini and Maggi, 1992) and of nuclear genes related to mitochondrial respiration (Weise et al., 2004), regulating mitochondria morphology and increasing oxygen consumption (Chen et al., 2005; Justo et al., 2005; Valle et al., 2005; Pedram et al., 2006). The possible effect of an increase in oxygen consumption on ROS production and longevity is discussed below.
Although estrogens can be responsible for the lower mitochondrial ROS production and longer life span of females in rat strains, why don’t they have the same effect in mice? One possible explanation is that the lower ROS production of female rats is not due to estrogens action. Instead, it can be linked to differences in body size. There is a negative correlation between body mass and longevity within species (Rollo 2002; Speakman, 2005). The general difference in body mass between genders is only 11% in mice, while in rats it reaches 70% (reviewed in Rollo, 2002). This could explain why rats but not mice of different sexes have different longevities. In agreement with that, in our studies the differences in body weight between males and females were 20% in mice, but about 45% in Fisher 344 and Wistar rats (Jang et al., 2005; Valle et al., 2005). Moreover, it has been reported that estrogen administration increases superoxide production in male Fischer 344 rats (Chen et al., 1999), which is opposite to a lowering effect of estrogens on ROS production.
The differences in ROS production between male and female Wistar rats could also be explained by differences in mitochondrial oxygen consumption because small animals (females) can consume more oxygen per gram than larger animals (males). Valle et al. (2005) have found that Wistar female liver mitochondria consume more oxygen than male mitochondria. In fact, it seems that female mitochondria show a greater tendency to respiratory chain uncoupling (Justo et al., 2005). Recently, it has been proposed that increased uncoupling can increase survival (Brand et al., 2004). When mitochondrial oxygen consumption increases within a species, the degree of electronic reduction of the electron transport chain (ETC) decreases provoking a lower mitochondrial ROS production (Barja, 1999). The higher oxygen consumption of female Wistar rats mitochondria could thus explain their lower rates of ROS production. That explanation would be also consistent with our finding of no changes in mitochondrial oxygen consumption and ROS production in mice. Although no differences between genders were detected in mitochondrial oxygen consumption in Fischer 344 rats, female mitochondria produced less ATP suggesting a mild uncoupling state (Jang et al., 2004). In our present study, neither mitochondrial oxygen consumption or ATP production were different in males and females, and we did not detect any differences in ROS production either. The lack of differences in mitochondrial ROS generation can be responsible for the lack of differences in longevity.
Recently Prolla and Leeuwenburghs’s laboratories have shown that mice expressesing a proofreading-deficient version of the mitochondrial DNA polymerase gamma (PolgD257A), develop accelerated aging without increases in oxidative stress, but have increased levels of caspase-3, the final effector caspase involved in the induction of apoptosis (Kujoth et al., 2005). This is consistent with previous reports showing increases in apoptotic markers in old animals (Dirks and Leeuwenburgh, 2002; Shelke and Leeuwenburgh, 2003). Moreover, it has been described that estrogens have antiapoptotic properties (Chen et al., 2000). We studied different parameters related with apoptosis to determine whether there are differences between males and females. No differences were detected in the activity of the pro-apoptotic enzymes caspase 3 and 9 or in the content of cleaved caspase 3. Accordingly, no differences in DNA fragmentation, an additional marker of apoptotic cell death, were detected in any tissue, either post-mitotic or mitotic. Studies in human coronary arterial wall have not found differences in apoptosis between women and men (Boddaert et al., 2005), although men cardiomyocytes suffer more from apoptosis than women cardiomyocytes (Mallat et al., 2001). Leeuwenburgh’s laboratory did not detected differences in apoptotic markers between males and females in Fisher 344 rats (Jang et al., 2004). However, this could due to the use of young animals which have low levels of apoptosis (Pollack et al.,2002). In the present study, we use older animals, and we did not detect differences either. However, it is possible that these differences appear later in life, in older animals. Therefore, further investigations are necessary in order to elucidate this point.
In summary, we conclude that there are no differences in the rate of generation of damage (mitochondrial ROS production) and in apoptosis markers between males and females in the mouse strain used in our investigation. These results agree with the lack of differences in longevity in this mouse strain. Furthermore, our results indicate that differences in mitochondrial ROS production and oxidative stress could not be caused by differences in estrogen levels. Instead other possibilities like differences in body size could be responsible of such differences. However, more studies -especially in models where males live longer than females (e.g. Syrian hamster)- are necessary before reaching more strong conclusions.
Acknowledgments
This research was supported by NIH grants AG021905 (T.A.P.), AG17994 (C.L.), and AG21042 (C.L.). Alberto Sanz received a predoctoral fellowship from UCM.
Abbreviations
- ROS
Reactive Oxygen Species
- mtDNA
mitochondrial DNA
- RCR
Respiratory Control Ratio
- Pyr/mal
Pyruvate/malate
- MnSOD
Mn-Superoxide dismutase
- Gpx
Glutathione peroxidase
- 8-oxodG
8-oxo,7,8-dihydro-2′-deoxyguanosine
- B6
C57Bl/6J
- ETC
Electron Transport Chain
- GSH
reduced glutathione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asdell SA, Joshi SR. Reproduction and longevity in the hamster and rat. Biol Rep. 1976;14:478–480. doi: 10.1095/biolreprod14.4.478. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion JG, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, Sastre J, Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 3.Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, Kurosawa H, Shirasawa T. Estrogen, insulin, and dietary signals to enhance resistance to oxidative stress in mice. J Biol Chem. 2005;280:16417–16426. doi: 10.1074/jbc.M500924200. [DOI] [PubMed] [Google Scholar]
- 4.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3 organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 5.Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 6.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Bettini E, Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J Neurochem. 1992;58:1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- 8.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 9.Borras C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the EKR1 and EKR2[MAPK]NFkappa B cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 10.Brand MD, Buckhinham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP Pakay JL, Talbot DA, Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004;71:203–213. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- 11.Boddaert J, Mallat Z, Fornes P, Esposito B, Lecomte D, Verny M, Tedgui A, Belmin J. Age and gender effects on apoptosis in the human coronary arterial wall. Mech Ageing Dev. 2005;126:678–684. doi: 10.1016/j.mad.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Carranza J, Alarcos S, Sánchez-Prieto CB, Valencia J, Mateos C. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature. 2004;432:215–218. doi: 10.1038/nature03004. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Delannoy M, Odwin S, He P, Trush MA, Yager JD. Enhanced mitochondrial gene transcript, ATP, bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes. Toxicol Sci. 1998;75:271–278. doi: 10.1093/toxsci/kfg183. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Li Y, Lavigne JA, Trush MA, Yager JD. Increased mitochondrial superoxide production in rat liver mitochondria, rat hepatocytes, and HepG2 cells following ethynyl estradiol treatment. Toxicol Sci. 1999;51:224–235. doi: 10.1093/toxsci/51.2.224. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Gokhale M, Schofield B, Odwin S, Yager JD. Inhibition of TGF-beta-induced apoptosis by ethinyl estradiol in cultured, precision cut rat liver slices and hepatocytes. Carcinogenesis. 2000;21:1205–1211. [PubMed] [Google Scholar]
- 16.Chen JQ, Yager JD. Estrogen’s effects on mitochondrial gene expression: mechanisms and potential contributions to estrogens carcinogenesis. Ann NY Acad Sci. 2004;1028:258–272. doi: 10.1196/annals.1322.030. [DOI] [PubMed] [Google Scholar]
- 17.Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Bioch Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Cousins SW, Marin-Castaño ME, Espinosa-Heidmann DG, Alexandridou A, Striker L, Elliot S. Female gender, estrogen loss, and sub-RPE deposit formation in aged mice. IOVS. 2003;44:1221–1229. doi: 10.1167/iovs.02-0285. [DOI] [PubMed] [Google Scholar]
- 19.Drew B, Leeuwenburgh C. Method for measuring ATP production in brain and liver mitochondria of Fischer-344 rats with age and caloric restriction. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1259–R1267. doi: 10.1152/ajpregu.00264.2003. [DOI] [PubMed] [Google Scholar]
- 20.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Ballesteros R, Diez-Nicolas J, Ruiz-Torres A. Spain. In: Schroots J, Fernandez-Ballesteros R, Rudigner E, editors. Aging in Europe. Amsterdam: IOS Press; 1999. pp. 107–121. [Google Scholar]
- 22.Grossman A, Oppenheim J, Grondin G, Jean PS, Beaudoin AR. Immunocytochemical localization of the [3h]estradiol-binding protein in rat pancreatic acinar cell. Endocrinology. 1989;124:2857–2866. doi: 10.1210/endo-124-6-2857. [DOI] [PubMed] [Google Scholar]
- 23.Gurwitz JH. The age/gender interface in geriatric pharmacotherapy. J Womens Healts (Larchmt) 2005;14:68–72. doi: 10.1089/jwh.2005.14.68. [DOI] [PubMed] [Google Scholar]
- 24.Humphries S, Stevens DJ. Out with a bang. Nature. 2001;410:758–759. doi: 10.1038/35071202. [DOI] [PubMed] [Google Scholar]
- 25.Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. J Comp Neurol. 1999;409:567–572. [PubMed] [Google Scholar]
- 26.Hofer T, Moller L. Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2′-deoxyguanosine. Chem Res Toxicol. 1998;11:882–887. doi: 10.1021/tx980041x. [DOI] [PubMed] [Google Scholar]
- 27.Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. Method to determine RNA and DNA oxidation by HPLC-ECD; greater RNA than DNA oxidation in rat liver from doxorubicin administration. Biol Chem. 2006;378:103–111. doi: 10.1515/BC.2006.014. [DOI] [PubMed] [Google Scholar]
- 28.Justo R, Fronter M, Pujol E, Rodriguez-Cuenca S, Llado FJ, Garcia-Palmer P, Roca M, Gianotti M. Gender –related differences in morphology and thermogenic capacity of brown adipose tissue mitochondrial subpopulations. Life Sci. 2005;76:1147–1158. doi: 10.1016/j.lfs.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Jang YM, Kendaia S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlegemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla T. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;15:309, 481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 31.Latorre A, Moya A, Ayala A. Evolution of mitochondrial DNA in Drosophila suboscura. Proc Natl Acad Sci USA. 1986;83:8649–8653. doi: 10.1073/pnas.83.22.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, Tedgui A. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56:M719–M723. doi: 10.1093/gerona/56.11.m719. [DOI] [PubMed] [Google Scholar]
- 33.McCulloch D, Gems D. Evolution of male longevity bias in nematodes. Aging Cell. 2003;2:165–173. doi: 10.1046/j.1474-9728.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 34.Mela L, Seitz S. Isolation of mitochondria with emphasis on heart mitochondria from small amounts of tissue. Methods Enzymol. 1979;55:39–46. doi: 10.1016/0076-6879(79)55006-x. [DOI] [PubMed] [Google Scholar]
- 35.Monje P, Zanello S, Holick M, Boland R. Differential cellular localization of estrogen receptor alpha in uterine and mammary cells. Mol Cel Endocrinol. 2001;181:117–129. doi: 10.1016/s0303-7207(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 36.Pamplona R, Portero-Otin M, Riba D, Requena JR, Thorpe SR, Lopez-Torres M, Barja G. Low fatty acid unsaturation: a mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life span. J Gerontol A Biol Sci Med Sci. 2000;55:B286–291. doi: 10.1093/gerona/55.6.b286. [DOI] [PubMed] [Google Scholar]
- 37.Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- 38.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Moll Biol Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci. 2001;56:B475–482. doi: 10.1093/gerona/56.11.b475. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P. Sex-dependent thermogenesis differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;45:42958–42963. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- 41.Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 42.Rowlatt C, Chesterman FC, Sheriff MU. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim. 1976;10:419–442. doi: 10.1258/002367776780956917. [DOI] [PubMed] [Google Scholar]
- 43.Sanz A, Caro P, Barja G. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J Bioenerg Biomembr. 2004;36:542–552. doi: 10.1007/s10863-004-9001-7. [DOI] [PubMed] [Google Scholar]
- 44.Sanz A, Caro P, Ibanez J, Gomez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr. 2005a;37:83–90. doi: 10.1007/s10863-005-4131-0. [DOI] [PubMed] [Google Scholar]
- 45.Sanz A, Gredilla R, Pamplona R, Portero-Otin M, Vara E, Tresguerres JA, Barja G. Effect of insulin and growth hormone on rat heart and liver oxidative stress in control and caloric restricted animals. Biogerontology. 2005b;6:15–26. doi: 10.1007/s10522-004-7380-0. [DOI] [PubMed] [Google Scholar]
- 46.Sanz A, Caro P, Ayala V, Portero-Otín M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak and oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006a;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 47.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antiox Redox Sign. 2006b;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 48.Sanz A, Barja G. Estimation of the rate of production of oxygen radicals at mitochondria. In: Conn M, editor. Handbook of Models for the study of Human Aging. Vol. 16. Academic Press; 2006. pp. 183–189. [Google Scholar]
- 49.Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- 50.Shelke R, Leeuwenburgh C. Life-long calorie restriction (CR) increases expression of apoptosis repressor with a caspase recruitment domain (ARC) in the brain. FASEB J. 2003;17:494–496. doi: 10.1096/fj.02-0803fje. [DOI] [PubMed] [Google Scholar]
- 51.Smith DW. Is greater female’s longevity a general finding among animals? Biol Rev Camb Physiol Soc. 1989;64:1–12. doi: 10.1111/j.1469-185x.1989.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 52.Sohal RS, Svensson I, Sohal BH, Brunk UT. Superoxide anion radical production in different animal species. Mech Ageing Dev. 1989;49:129–135. doi: 10.1016/0047-6374(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 53.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 54.Sverko V, Balog T, Sovocanec S, Gavella M, Marotti T. Age-associated alteration of lipid peroxidation and superoxide dismutase activity in CBA and AKR mice. Exp Gerontol. 2002;37:1031–1039. doi: 10.1016/s0531-5565(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 55.Sverko V, Sobocanec S, Balog T, Marotti T. Age and gender differences in antioxidant enzyme activity: potential relationship to liver carcinogenesis in mice. Biogerontology. 2004;5:235–242. doi: 10.1023/B:BGEN.0000038024.58911.6e. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka S, Segawa T, Tamaya N, Ohno T. Establishment of an aging farm of F344:N rats and C57BL/6 mice at the national institute for longevity sciences (NILS) Arch Gerontol Geriatr. 2000;30:215–223. doi: 10.1016/s0167-4943(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 57.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 58.Valle A, Catala-Niell B, Colom FJ, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289:E15–E22. doi: 10.1152/ajpendo.00553.2004. [DOI] [PubMed] [Google Scholar]
- 59.Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005;579:2541–2545. doi: 10.1016/j.febslet.2005.03.090. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Liu H, Liu R. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 61.Weisz A, Basile W, Scafoglio L, Altucci F, Bresciani A, Facchiano A, Sismondi L, Cicatiello M, De Bortoli M. Molecular identification of eralpha-positive breast cancer cells by the expression of profile of an intrinsic set of estrogen regulated genes. J Cell Physiol. 2004;200:440–450. doi: 10.1002/jcp.20039. [DOI] [PubMed] [Google Scholar]
- 62.Which SA, Utami-Atmoko SS, Setia TM, Rijksen HD, Schurmann C, van Hooff JARAM, van Schaik CP. Life story of wild Sumatran orangutans (Pongo abelii) J Human Evol. 2004;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Yunis EJ, Watson ALM, Gelman R, Sylvia SJ, Bronson R, Dorf ME. Traits that influence longevity in mice. Genetics. 1984;108:999–1011. doi: 10.1093/genetics/108.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]


