Abstract
Background
The purpose of this case series was to quantify different strategies used to compensate in gait for hip muscle weakness.
Methods
An instrumented gait analysis was performed of 3 females diagnosed with idiopathic inflammatory myopathies and compared to a healthy unimpaired subject. Lower extremity joint moments obtained from the gait analysis were used to drive an induced acceleration model which determined each moment’s contribution to upright support, forward progression, and hip joint acceleration.
Findings
Results showed that after midstance, the ankle plantar flexors normally provide upright support and forward progression while producing hip extension acceleration. In normal gait, the hip flexors eccentrically resist hip extension, but the hip flexor muscles of the impaired subjects (S1–3) were too weak to control extension. Instead S1–3 altered joint positions and muscle function to produce forward progression while minimizing hip extension acceleration. S1 increased knee flexion angle to decrease the hip extension effect of the ankle plantar flexors. S2 & S3 used either a knee flexor moment or gravity to produce forward progression, which had the advantage of accelerating the hip into flexion rather than extension, and decreased the demand on the hip flexors.
Interpretation
Results showed how gait compensations for hip muscle weakness can produce independent (i.e. successful) ambulation, although at a reduced speed as compared to normal gait. Knowledge of these successful strategies can assist the rehabilitation of patients with hip muscle weakness who are unable to ambulate and potentially be used to reduce their disability.
INTRODUCTION
The hip joint serves as the junction between the lower limb and the trunk, and thus its role in locomotion is critical (Perry 1992). During the stance phase of gait, the primary role of the hip musculature is to provide stabilization of the superimposed trunk (Perry 1992; Winter 1991). In the sagittal plane, the hip is flexed at initial contact and then progressively extends throughout stance as the body progresses forward over the fixed foot (Perry 1992). Initially this motion is controlled concentrically by a hip extensor moment, but for most of the interval it is controlled eccentrically by a hip flexor moment (Winter 1991). In very late stance, the hip flexor moment persists (Winter 1991), but the motion reverses direction, so the hip flexor concentrically flexes the hip in preparation for limb advancement in swing phase (Perry 1992).
While a Trendelenburg gait secondary to hip abductor muscle weakness is quite familiar to most rehabilitation clinicians, the impact of hip muscle weakness in the flexor and extensor groups on gait has been less thoroughly documented (Perry 1992). Traditional gait analysis techniques using kinematic and kinetic variables have not been widely used to describe the effects of isolated muscle weakness at the hip (Armand et al. 2005; Perry & Clark 1997) if the weakness is not associated with other confounding neurological or orthopaedic diagnoses. However, the hip flexor (Siegel et al. 2004a) and extensor (Slavin et al. 1998) muscle groups both have been shown to be predictive of overall gait performance in diagnoses causing muscle weakness. When muscle weakness becomes moderate to severe, some individuals continue to ambulate independently, while others do not, resulting in substantial disability.
The even more powerful technique of induced acceleration analysis can identify a causal relationship between muscle function and the resulting gait pattern (Zajac et al. 2003). This technique has improved our understanding of how muscle weakness can lead to gait limitations, and this knowledge can potentially allow rehabilitation specialists to exploit this relationship to reduce disability. In induced acceleration analysis, dynamical models driven by net joint moments obtained from traditional gait analysis or by muscle forces obtained from dynamical simulations allow the effect of a joint moment or muscle force on the acceleration of all body segments to be quantified directly (Zajac et al. 2003). Research using this approach has expanded our understanding of how individual muscles or muscle groups control forward progression, vertical support, joint acceleration, and segmental power during normal gait (Anderson & Pandy 2003; Kepple et al. 1997; Neptune et al. 2001; Riley et al. 2001; Siegel et al. 2004b). However, the technique also is appropriate for the study of pathological gait because subjects with impaired motor control or muscle strength often must find alternative strategies to control limb position during ambulation if they are to successfully minimize disability. Clinical research using induced acceleration analysis techniques have studied the causes of stiff-knee gait in patients with upper motor neuron impairments such as stroke (Riley & Kerrigan 1999) or cerebral palsy (Goldberg et al. 2003) and identified compensatory gait strategies to achieve knee stability in stance phase used by patients with knee extensor weakness (Siegel et al. 2006). However, the technique should be of benefit in analyzing an even wider variety of patients with gait limitations including muscle weakness secondary to myopathy, the focus of the present study.
Idiopathic inflammatory myopathies (IIM) are disorders of chronic skeletal muscle inflammation that result in symmetrical weakness affecting axial muscles and proximal arm and leg muscles (Amato & Barohn 1997). Despite moderate to severe weakness, some individuals with IIM continue to ambulate independently, while others do not. Past studies that have correlated muscle weakness with walking speed have found that muscle strength only explains some of the variability in ambulatory status (Siegel et al. 2004a). If muscle strength cannot completely predict locomotor function, then the adaptive gait strategy selected by an individual or trained by their rehabilitation specialist to compensate for muscle weakness may account for some of the unexplained variability in the relationship between muscle weakness and gait performance. Due to the distribution of muscle weakness caused by IIM, this diagnosis provides an opportunity to study how hip muscle weakness and its compensations in the sagittal plane can impact gait. The purpose of this report is to present a case series of three patients diagnosed with IIM and hip muscle weakness who each used a different strategy to compensate in gait.
METHODS
Data were reviewed from patients with hip muscle weakness who had been referred for gait analyses as part of their participation in institution review board approved research studies for which they or their parents had provided informed consent. From this group, 3 subjects who each used a different compensatory strategy during gait were selected for presentation (S1–3) and compared to a healthy unimpaired subject (normal or NL). All subjects with impairment were female, diagnosed with probable or definite IIM (Bohan et al. 1977), either polymyositis (S1 and S3) or dermatomyositis (S2), and had less than antigravity strength in their hip muscles (Table 1). Other lower extremity muscles were able to move against gravity with at least moderate resistance. Analyses were performed on the side of the weaker hip of the subjects with impairment. The exception was S3 who had more severe distal weakness than the other two subjects. She had suffered nerve injuries in her left leg after being struck as a pedestrian by an automobile 20 years previously. In her case, the right leg was analyzed because it was weak only proximally and only due to the myositis.
Table 1.
Subject Characteristics
| S1 | S2 | S3 | NL | |
|---|---|---|---|---|
| Subject Characteristics | ||||
| Age (yr) | 43 | 12 | 38 | 37 |
| Height (m) | 1.55 | 1.60 | 1.59 | 1.77 |
| Weight (kg) | 60.6 | 53.5 | 64.3 | 69.3 |
| BMI (kg/m2) | 25.2 | 20.8 | 25.1 | 22.1 |
| Muscle Strength (on 5 point scale) (Hislop & Montgomery 2002) | ||||
| Hip Flexors | 3− | 2+ | 3+ | 5 |
| Hip Extensors | 3− | 2 | 2+ | 5 |
| Hip Abductors | 3− | 2 | 5 | 5 |
| Muscles Distal to Hip | 4 | 4+ | 4* | 5 |
| Gait Variables | ||||
| Stride length (statures) | 0.71 | 0.60 | 0.58 | 0.77 |
| Cycle time (s) | 1.36 | 1.25 | 1.31 | 1.03 |
| Speed (statures/s) | 0.52 | 0.48 | 0.44 | 0.75 |
| DLS (% gait cycle) | 27 | 26 | 19 | 15 |
| analyzed frame (% stance) | 65 | 71 | 57 | 71 |
| analyzed side | left | left | right | left |
More severe distal weakness present on side not analyzed secondary to nerve injury not IIM. See methods for additional information.
Gait analyses were performed on all subjects walking barefoot independently without assistive devices at a self-selected speed. Motion of reflective target clusters attached to the pelvis and bilateral thighs, shanks, and feet was sampled at 60 Hz with a 6-camera motion capture system (Vicon Motion Systems, Lake Forest, CA, USA) and low pass filtered at 6 Hz. Ground reaction forces were sampled at 360 Hz from 2 force platforms (AMTI, Watertown, MA, USA) mounted in series along the middle of a 6 m walkway and low pass filtered at 25 Hz. Approximately 10 repeated gait trials were collected per subject, yielding 4 trials with adequate force plate contacts for kinetic analysis (only 3 trials on the right for S3). Computed gait variables (Visual 3D, C-Motion, Inc., Rockville, MD, USA) included temporal-spatial variables, joint angular displacements, and net internal joint moments. Means and 1 standard deviation were plotted for each variable to assess intrasubject repeatability.
Induced acceleration analysis assessed the relative ability of each lower extremity joint moment and gravity to produce either hip joint angular acceleration or the ground reaction force (GRF) (Kepple et al. 1997). The GRF vertical component represented the vertical acceleration of the body center of mass or upright support. The anterior component of the anterior/posterior (A/P) GRF represented forward acceleration of the body center of mass or forward progression. Individually customized models were created for each subject and previously have been described and validated (Kepple et al. 1997; Kepple et al. 2002).
The 7 segment model included bilateral thighs, shanks, and feet and a combined head, arms, and trunk segment (HAT). The ankle was designed as a universal joint (dorsi/plantar flexion and inversion/eversion only), the knee as a revolute joint (flexion/extension only), and the hip as a spherical joint (flexion/extension, ab/adduction, and internal/external rotation). Each subject’s joint and segment positions were obtained from the gait analysis and served as input to the model. After the model was configured, gravity and all joint moments were set to zero. One joint moment (as calculated from the gait data) then was entered into the model and the resultant hip angular acceleration or GRF was computed (Kepple et al. 1997). The input joint moment was then set back to zero, and another joint moment or gravity (from the same frame of the gait data) was sequentially entered into the model. The model output provided the portion of the hip joint angular acceleration or GRF that was generated individually by each input joint moment or gravity (passive source). This process identified the compensatory strategy used by each subject to control hip joint motion, generate upright support, and produce forward progression.
The induced acceleration analysis was performed at one critical frame during late single limb support of one representative trial (Table 1). This time was selected to minimize contributions from the contralateral limb which increase in double limb support, but after the A/P GRF crossed zero and forward progression had begun. Specifically, the analyzed frame was selected midway during the interval between ipsilateral heel rise and contralateral initial contact. The exception was S3 where ipsilateral heel rise did not occur prior to contralateral initial contact, but a similarly timed frame was selected. The primary rationale for including data from only one frame was to facilitate reporting of results, but data from other frames of the interval yielded similar conclusions. On average, the analyzed frame corresponded with 66% of the stance phase of gait.
A sensitivity analysis also was performed to examine the ability of the ankle plantar flexor moment to produce hip joint acceleration over a range of knee flexion angles. In this analysis, the knee flexion angle in the model was increased by 1, 5 and 10 degrees. The induced acceleration analysis was repeated, and the effect of the ankle plantar flexor moment on producing hip joint accelerations was recomputed for each new knee joint angle.
RESULTS
All 3 subjects with weakness walked at a reduced speed; 57–68% of the speed of the unimpaired subject (Table 1). This was due to a combination of prolonged gait cycle times and reduced stride lengths for S2 and S3, but primarily prolonged gait cycle times for S1. Double limb support duration was prolonged for S1 and S2, but not S3.
Joint flexion/extension angles, net internal flexor/extensor moments, and GRF components in the A/P and vertical directions are shown in Figure 1. S1 generated the largest hip flexor moment of the three subjects with weakness, and it was larger than expected based on manual muscle test scores. S1 also had reduced extension range of motion available at her hip and she extended the joint to its end range of motion in gait. The hip flexor moment was not observed until the hip neared the end range of extension, so it is possible that this moment was generated through passive soft tissue stretch rather than active force generation. S1 also displayed increased knee flexion angles throughout stance phase, which were associated with small prolonged knee extensor moments for the duration of stance. With the exception of increased peak ankle dorsiflexion in late stance secondary to increased knee flexion angles, findings at the ankle were unremarkable.
Figure 1.
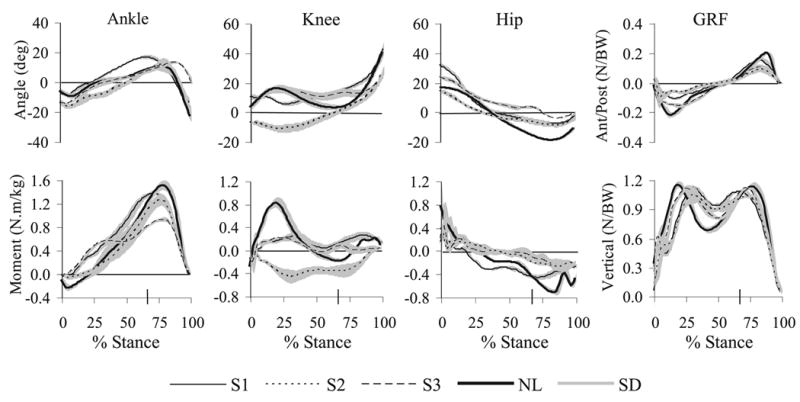
Results from the gait analysis as a percent of stance phase for the 3 subjects with weakness (S1–S3) and the unimpaired subject (NL). Joint angle is in the top row and flexion angles are positive. Joint moments are in the bottom row and internal extensor moments are positive. Ground reaction forces are shown on the far right, with anterior on the top, and vertical (upward) on the bottom being positive. Results represent the mean (and standard deviation, gray lines) of 4 repeated trials per subject, except S3 contributed only 3 trials. The large tick mark on the horizontal axis is at 66% of stance phase and corresponds to the average time at which the induced acceleration analysis was performed.
Despite similar hip muscle strength (Table 1), S2 demonstrated a different gait pattern from S1 (Figure 1). Her total hip excursion was the smallest of any of the subjects, and hip flexor moments were quite small. She demonstrated the greatest gait deviations at the knee of any of the subjects. Throughout the first half of stance phase, she positioned her knee in hyperextension. This position was associated with an internal knee flexor moment throughout the entire stance phase. The manual muscle test revealed 4+/5 strength in the quadriceps femoris (Table 1) so muscle weakness did not explain the absent knee extensor moment. Results at the ankle were consistent with normal gait, except the onset of heel rise in mid to late stance was delayed slightly.
S3 showed yet a third gait pattern despite a pattern of hip muscle strength similar to the other two subjects (Table 1). She showed increased flexion angles and decreased total joint excursions at both the hip and knee joints (Figure 1). Her hip flexor and knee extensor moments were near zero during most of stance. Although her ankle plantar flexor moments increased earlier in stance than did the other subjects, her peak moments in later stance were reduced. She also failed to move her ankle into a plantar flexed position prior to toe-off which was unique among the impaired subjects.
The results of the induced acceleration analysis are presented in Figure 2. All subjects except S3 generated nearly all of their vertical support with their ankle plantar flexor moments. This was because the ankle plantar flexors were responsible for generating heel rise which raised the body against gravity and maintained vertical support. For S3, vertical support was evenly divided between the ankle plantar flexor moment and passive support strategies. This was because ankle plantar flexion motion was limited in late stance of S3 (Figure 2).
Figure 2.
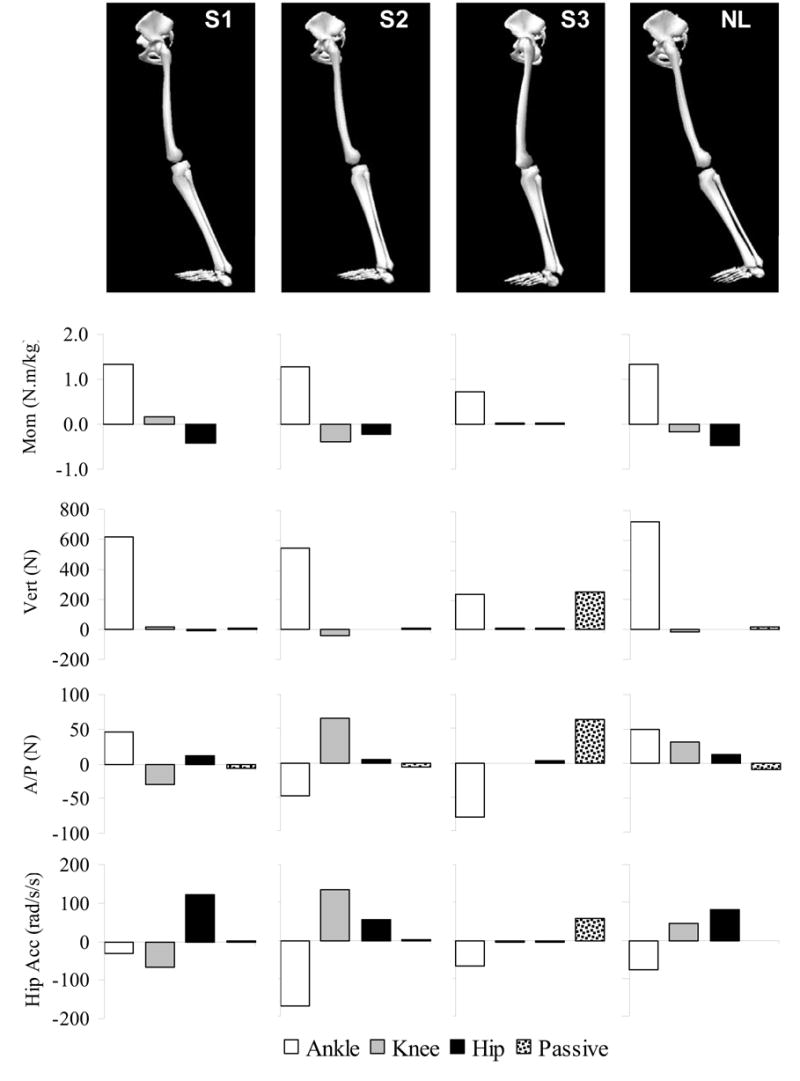
Input to and results from the induced acceleration analysis for the 3 subjects with weakness (S1–S3) and the unimpaired subject (NL). From top to bottom, graphic showing input joint positions, input joint moments (internal extensor moments are positive), output vertical GRF (up is positive), A/P GRF (anterior is positive), and hip acceleration (flexion is positive). Output bar graphs show how much each input joint moment or gravity (passive source) contributed to producing upright support, forward progression, or hip joint acceleration.
There was much more variability across subjects in the strategy used to generate forward acceleration of the body center of mass (Figure 2) than that used to generate vertical support. The normal subject generated the anterior GRF primarily with the ankle plantar flexors, and S1 used a reduced version of this same strategy. In contrast, S2 generated the anterior GRF with the knee flexor moment. The ankle plantar flexors produced a posterior GRF, probably due to delayed heel rise. Finally, S3 used a passive strategy, and gravity accelerated the center of mass forward. This was resisted by the ankle plantar flexors, suggesting use of a controlled fall to move forward.
In addition to providing vertical support, the ankle plantar flexor moment also accelerated the hip into extension (Figure 2). In normal gait, the hip flexors worked eccentrically to control the rate of hip extension during this interval of gait. The sensitivity analysis showed that the magnitude of the ankle plantar flexor moment affect at the hip was influenced by knee flexion angle. For each additional degree of knee flexion, the ankle plantar flexor moment produced 10 rad/s2 less hip extension acceleration. For example, the strategies used by S1 and NL were fairly similar apart from differences in knee flexion angle. In the analyzed frame, NL demonstrated only 4 deg of knee flexion, while S1 showed 15 deg of knee flexion. A 5 deg increase in knee flexion angle could be expected to produce nearly a 60% decrease in the hip extension acceleration produced by the ankle plantar flexor moment.
While there was consistency across subjects concerning the source of hip extension acceleration, each subject used a different strategy to generate hip flexion acceleration to oppose the hip extension effect of the ankle plantar flexors (Figure 2). S1 generated a hip flexor moment, probably passively, by positioning the hip at the end range of extension. S2 hyperextended the knee to generate a knee flexor moment which produced a hip flexion acceleration. Finally, S3 used gravity to produce a hip flexion acceleration.
DISCUSSION
This study has several limitations and assumptions stemming from the analyses performed and the models employed. Induced acceleration analysis can be performed throughout the gait cycle, although the results reported included only one representative frame of data for simplicity and clarity of presentation. Late single limb support was the focus of the analysis because this is when the ground reaction force is directed anteriorly and the hip flexor moment peaks in controlling the rate of hip extension in normal gait. It also allows focus on within limb compensations for hip muscle weakness, because cross limb compensations could be possible during double limb support. The musculoskeletal model allowed joint motion to occur in additional planes of movement at the ankle and hip, but results were presented in only one plane because the focus of the analysis was to study how subjects with hip muscle weakness functioned in the sagittal plane, the plane of progression during gait.
The three subjects of this case series were specifically selected for analysis because each used a different strategy to compensate in gait. However, additional adaptive strategies for hip muscle weakness other than those presented certainly are possible and the relative prevalence of each observed strategy is unknown. While muscle weakness is the hallmark feature of IIMs, they also are known to be associated with other conditions including cardiopulmonary complications, fatigue, arthralgias, arthritis, and contractures that limit joint motions (Amato & Barohn 1997). These were not substantial confounding issues for the subjects participating in this study, but could limit the generalizability of the results to other individuals with IIMs. Also, one of the subjects of this study was an adolescent, not an adult. While longstanding juvenile dermatomyositis of the chronic unremittive type in a skeletally immature child theoretically could affect skeletal development and have additional affects on gait apart from weakness, skeletal deformities were not present in S2. As for her age alone, studies have shown that children develop mature gait patterns by ages 5–7, or at the ankle as late as age 9 (Chester et al. 2006), all well below 12 years, the age of S2.
The results of the study showed that late in the single limb support phase of normal gait, the ankle plantar flexors produce vertical support, forward progression, and hip extension which must be eccentrically resisted by the hip flexors. In the presence of hip flexor weakness, the ankle plantar flexors continue to produce vertical support and hip extension, but an alternative strategy must be found to produce hip flexion acceleration to balance the extension effect of the ankle plantar flexors. One compensatory strategy is to decrease the demand on the hip flexors by increasing knee flexion angle, which decreases the hip extension effect of the ankle plantar flexors. Additional strategies to produce hip flexion acceleration can come from knee flexor moments or gravity. These alternate strategies can provide forward progression while simultaneously producing hip flexion acceleration which can balance the extension effect of the ankle plantar flexors.
These complex and interesting relationships that appear to govern the ways in which an individual with impairment can compensate for hip flexor weakness were not identified with the traditional gait analysis and have not been described previously (Perry 1992). Authors of an earlier study did notice an association between the magnitude of the ankle plantar flexor moment and the hip flexor moment during gait of subjects with hip muscle weakness caused by Duchenne muscular dystrophy, but they proposed a different mechanism to explain this association (Armand et al. 2005). Also, they did not appreciate the potential benefit the knee flexor moment could have played in controlling hip joint extension. The induced acceleration analysis performed in this study quantified the effect of the ankle and knee moments on hip joint acceleration. Induced acceleration analysis additionally quantified the effect of lower extremity joint moments on upright support and forward progression which was helpful in determining the goals of the various compensatory gait strategies selected by each subject. While each of the different strategies allowed the subjects to remain ambulatory despite their weakness and therefore could be considered successful, no one strategy appeared to have a clear functional advantage over another.
Examples of similar success among the strategies include results such as all subjects walking at similar, yet reduced, speeds ranging from 57–68% of normal, and none requiring the use of an assistive gait device. However, each strategy also had a potential disadvantage and therefore might not be considered an optimal or best possible outcome. Two subjects relied on positioning at least one joint at the end of its available range of motion during at least part of stance phase. This could lead to damage of joints and associated structures if the muscle weakness is long standing. This could be especially troubling for patients diagnosed with IIMs who may also suffer from arthralgias and arthritis in addition to muscle weakness (Amato & Barohn 1997). S2’s compensation relied on knee hyperextension so the knee flexors could generate forward progression and hip flexion acceleration. Knee hyperextension can result in quadriceps disuse, or excessive stress on the anterior cruciate ligament, the anterior joint, or the posterolateral corner of the knee (Loudon et al. 1998). S1 increased her knee flexion angle to decrease the effect of the ankle plantar flexors at the hip, and decrease the demand for the hip flexors. She then used a scaled down version of the normal gait strategy, but generated the hip flexor moment by positioning her hip at the end of its available range of motion. An increased hip extension strategy in gait increases anterior hip joint reaction forces and may lead to pain and increased risk of acetabular labral tears (Lewis et al. 2005). S3’s compensation consisted of a controlled fall where gravity provided forward progression and hip flexion acceleration. This passive approach resulted in knee and hip moments that were near zero for most of stance. This strategy likely was less stressful to the joints than the patterns used by the other two subjects. However, it may place S3 at greater risk for fall because reduced muscle activation associated with such small joint moments may leave her less able to respond to perturbations to her gait or to uneven walking surfaces. Another advantage of this passive strategy is that it may be associated with less metabolic energy demands in gait. This could be beneficial because IIMs can be associated with several cardiopulmonary conditions and fatigue (Amato & Barohn 1997) . Additional information such as metabolic testing, measures of joint function, and long-term outcome studies may be helpful in identifying which of the observed compensatory strategies are best or optimal for any one individual.
The results from this case series have clinical and research implications. It is valuable to know that there are several options to compensate for hip flexor weakness that produce similar functional outcomes when rehabilitating patients with these impairments. This would be especially important if hip muscle weakness existed in combination with other impairments that would make one of the described gait strategies impractical (e.g. knee flexors too weak to utilize S2’s strategy). These results also may offer insight into the variability in functional outcomes for patients with similar strength patterns. Correlational studies of muscle strength and function have only predicted a portion of the variability in walking speed among various patient groups (Bohannon 1986; Bohannon et al. 1994; Perry et al. 1995; Powers et al. 1996; Siegel et al. 2004a). Induced acceleration analysis can identify the compensatory strategies adopted by individuals with muscle weakness and has the potential to elucidate the source of the unexplained variability in these previous studies.
CONCLUSIONS
After midstance, the ankle plantar flexor moment normally provides upright support and forward progression while accelerating the hip into extension. When the hip flexors are too weak to control this hip extension, individuals can alter lower extremity joint positions and moments to produce forward progression while minimizing hip extension acceleration. These compensatory strategies permit independent ambulation, although at a reduced speed as compared to normal gait. These strategies were identified through an induced acceleration analysis, but not with traditional gait analysis techniques. Knowledge of these successful strategies can assist the rehabilitation of patients with hip muscle weakness who have functional gait limitations. Additional study is needed to determine the prevalence of these and other possible compensatory gait strategies in all subjects with weakness and which among them might be optimal for any one individual.
Acknowledgments
We gratefully acknowledge the contributions of Paul H. Plotz, MD, Chief, and the staff of the Arthritis and Rheumatism Branch of the National Institute of Arthritis Musculoskeletal and Skin Diseases, and Lisa G. Rider, MD, Deputy Chief, and the staff of the Environmental Autoimmunity Group of the National Institutes of Environmental Health Sciences, for their work on the clinical research studies in which our subjects were participating at the time of their gait analyses.
Footnotes
The opinions presented in this report reflect the views of the authors and not necessarily those of the National Institutes of Health or the US Public Health Service.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato AA, Barohn J. Idiopathic inflammatory myopathies. Neurologic Clinics. 1997;15:615–648. doi: 10.1016/s0733-8619(05)70337-6. [DOI] [PubMed] [Google Scholar]
- Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait & Posture. 2003;17:159–169. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- Armand S, Mercier M, Watelain E, Patte K, Pelissier J, Rivier F. A comparison of gait in spinal muscular atrophy, type II and Duchenne muscular dystrophy. Gait & Posture. 2005;21:369–378. doi: 10.1016/j.gaitpost.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine. 1977;56:255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Strength of lower limb related to gait velocity and cadence in stroke patients. Physiotherapy Canada. 1986;38:204–206. [Google Scholar]
- Bohannon RW, Hull D, Palmeri D. Muscle strength impairments and gait performance deficits in kidney-transplantation candidates. American Journal of Kidney Diseases. 1994;24:480–485. doi: 10.1016/s0272-6386(12)80905-x. [DOI] [PubMed] [Google Scholar]
- Chester VL, Tingley M, Biden EN. A comparison of kinetic gait parameters for 3–13 year olds. Clinical Biomechanics. 2006;21:726–732. doi: 10.1016/j.clinbiomech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Ounpuu S, Delp SL. The importance of swing-phase initial conditions in stiff-knee gait. Journal of Biomechanics. 2003;36:1111–1116. doi: 10.1016/s0021-9290(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Hislop H, Montgomery J. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination. 7. WB Saunders; Philadelphia: 2002. [Google Scholar]
- Kepple TM, Siegel KL, Stanhope SJ. Relative contributions of the lower extremity joint moments to forward progression and support during gait. Gait & Posture. 1997;6:1–8. [Google Scholar]
- Kepple TM, Siegel KL, Stanhope SJ. Comparison of two foot-floor interfaces in induced acceleration analysis. Gait and Posture. 2002;16:S67–S68. [Google Scholar]
- Lewis CL, Sahrmann SA, Moran DW. Walking in greater hip extension increases predicted anterior hip joint reaction forces. Proceedings ISB XXth Congress - ASB 29th Annual Meeting; 2005. p. 656. [Google Scholar]
- Loudon JK, Goist HL, Loudon KL. Genu recurvatum syndrome. Journal of Orthopaedic & Sports Physical Therapy. 1998;27:361–367. doi: 10.2519/jospt.1998.27.5.361. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. SLACK Incorporated; Thorofare, NJ: 1992. [Google Scholar]
- Perry J, Clark D. Biomechanical abnormalities of post-polio patients and the implications for orthotic management. Neurorehabilitation. 1997;8:119–138. doi: 10.3233/NRE-1997-8206. [DOI] [PubMed] [Google Scholar]
- Perry J, Fontaine JD, Mulroy S. Findings in post-poliomyelitis syndrome. Weakness of muscles of the calf as a source of late pain and fatigue of muscles of the thigh after poliomyelitis. Journal of Bone and Joint Surgery-American. 1995;77:1148–1153. doi: 10.2106/00004623-199508000-00002. [DOI] [PubMed] [Google Scholar]
- Powers CM, Boyd LA, Fontaine CA, Perry J. The influence of lower-extremity muscle force on gait characteristics in individuals with below-knee amputations secondary to vascular disease. Physical Therapy. 1996;76:369–377. doi: 10.1093/ptj/76.4.369. [DOI] [PubMed] [Google Scholar]
- Riley PO, Della Croce U, Kerrigan DC. Propulsive adaptation to changing gait speed. Journal of Biomechanics. 2001;34:197–202. doi: 10.1016/s0021-9290(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Riley PO, Kerrigan DC. Kinetics of stiff-legged gait: induced acceleration analysis. IEEE Transactions on Rehabilitation Engineering. 1999;7:420–426. doi: 10.1109/86.808945. [DOI] [PubMed] [Google Scholar]
- Siegel KL, Hicks JE, Koziol DE, Gerber LH, Rider LG. Walking ability and its relationship to lower-extremity muscle strength in children with idiopathic inflammatory myopathies. Archives of Physical Medicine and Rehabilitation. 2004a;85:767–771. doi: 10.1016/j.apmr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Siegel KL, Kepple TM, Stanhope SJ. Joint moment control of mechanical energy flow during normal gait. Gait & Posture. 2004b;19:69–75. doi: 10.1016/s0966-6362(03)00010-9. [DOI] [PubMed] [Google Scholar]
- Siegel KL, Kepple TM, Stanhope SJ. Using induced acceleration analysis to understand knee stability during gait of individuals with muscle weakness. Gait & Posture. 2006;23:435–440. doi: 10.1016/j.gaitpost.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Slavin MD, Jette DU, Andres PL, Munsat TL. Lower extremity muscle force measures and functional ambulation in patients with amyotrophic lateral sclerosis. Archives of Physical Medicine and Rehabilitation. 1998;79:950–954. doi: 10.1016/s0003-9993(98)90093-4. [DOI] [PubMed] [Google Scholar]
- Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly, and Pathological. 2. University of Waterloo Press; 1991. [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking Part II: Lessons from dynamical simulations and clinical implications. Gait & Posture. 2003;17:1–17. doi: 10.1016/s0966-6362(02)00069-3. [DOI] [PubMed] [Google Scholar]


