Abstract
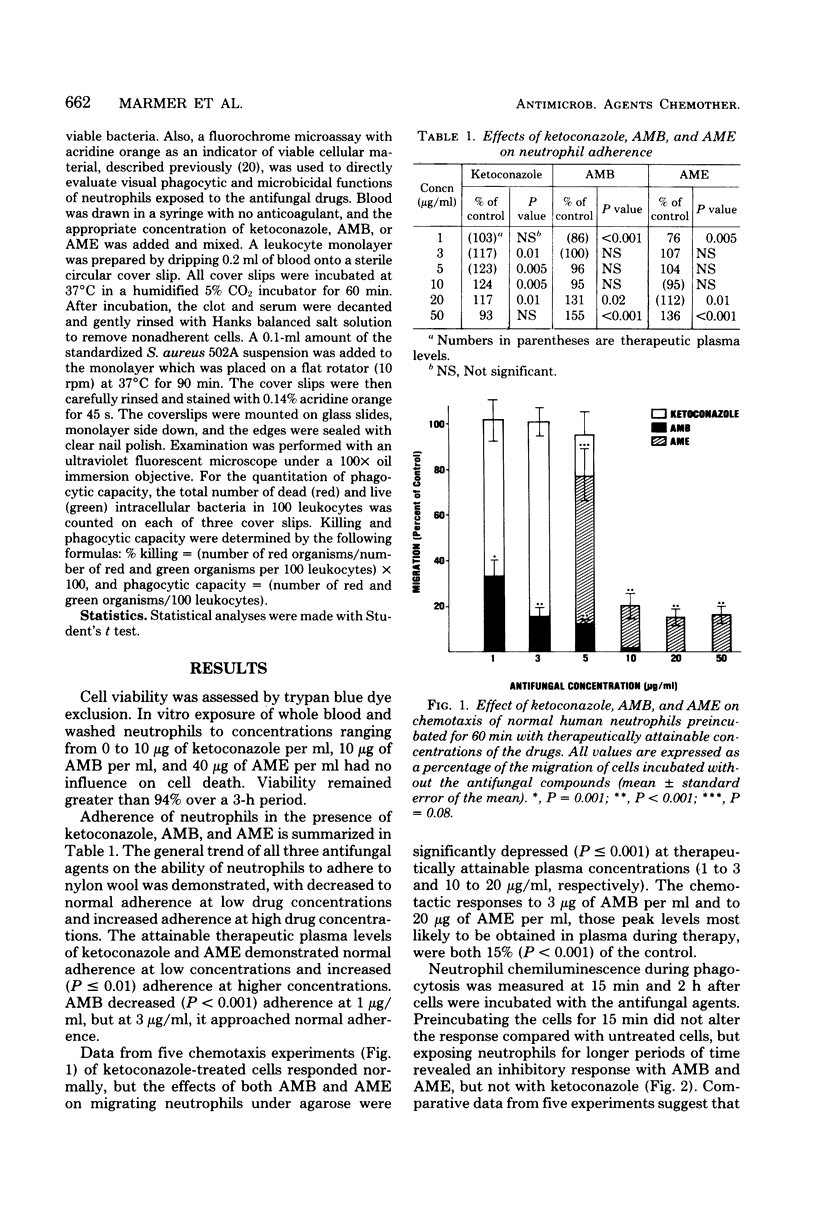
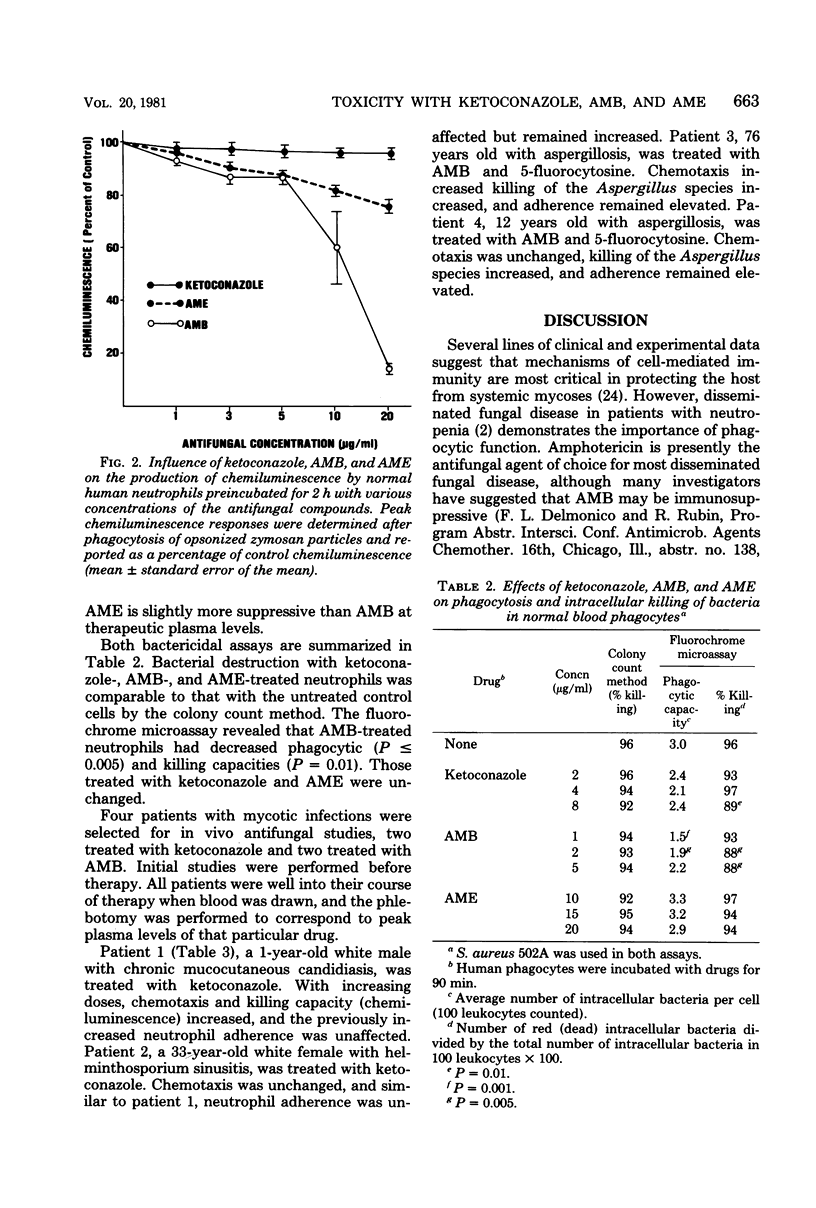
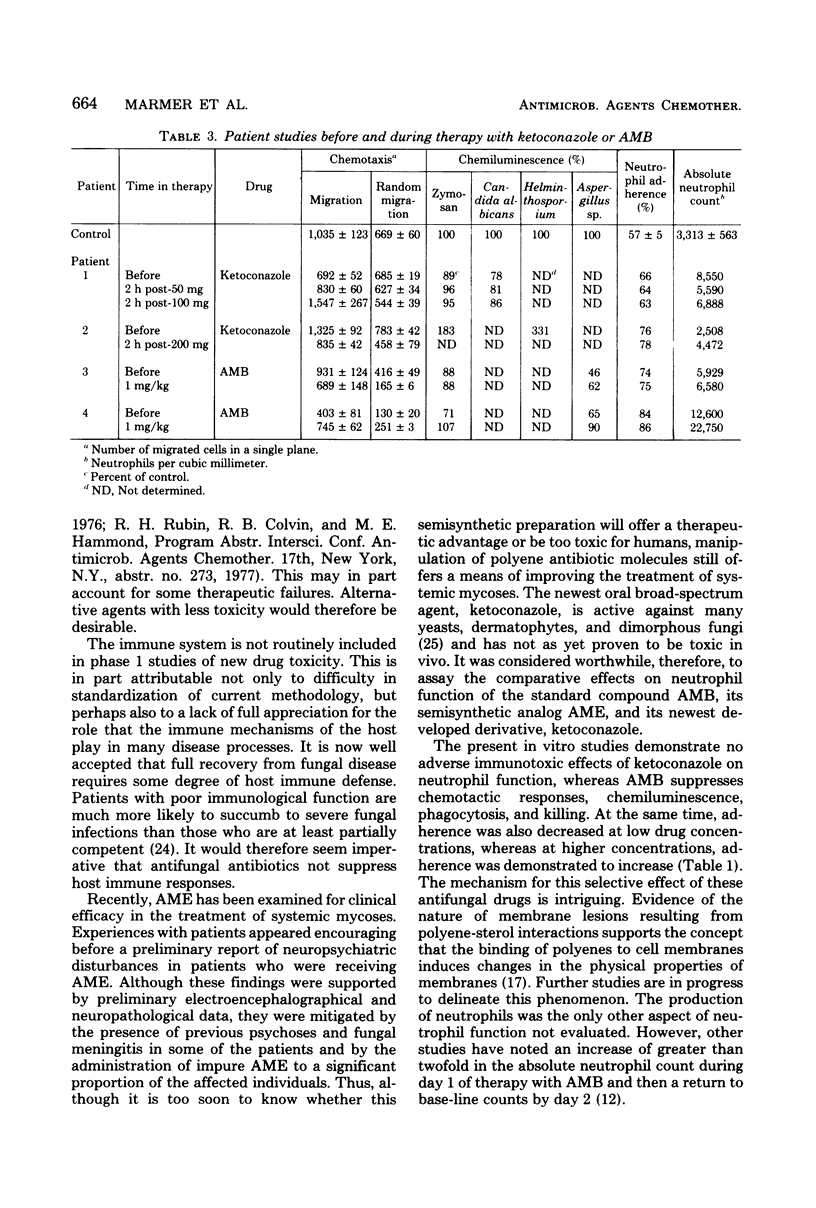
We investigated a number of parameters for host defense after the in vitro addition of the antifungal agents ketoconazole, amphotericin B (AMB), and amphotericin B methyl ester (AME). Similar assays were repeated before and after patients received the former two drugs. Viability by trypan blue exclusion, adherence by nylon wool columns, chemotaxis by the under-agarose technique, phagocytosis and killing by chemiluminescence, colony counts, and acridine orange direct visualization were assayed. In striking contrast to AMB and AME, ketoconazole demonstrated no significant effect on neutrophils. Adherence in the presence of therapeutic plasma levels of AMB and AME was decreased (P less than or equal to 0.005) at low drug concentrations, whereas at higher concentrations, adherence was increased (P less than 0.001). The chemotactic responses of cells incubated with AMB and AME demonstrated marked suppression. Phagocytic capacity and killing were decreased (P less than or equal to 0.005) with AMB as compared with control assays and assays performed in the presence of ketoconazole and AME. However, no difference were observed between two patients who received AMB and two other treated with ketoconazole.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P. Fungal infections complicating acute leukemia. J Chronic Dis. 1966 Jun;19(6):667–687. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Chunn C. J., Starr P. R., Gilbert D. N. Neutrophil toxicity of amphotericin B. Antimicrob Agents Chemother. 1977 Aug;12(2):226–230. doi: 10.1128/aac.12.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Root R. K., Kimball H. R., Kirkpatrick C. H. Defective neutrophil chemotaxis and cellular immunity in a child with recurrent infections. Ann Intern Med. 1973 Apr;78(4):515–519. doi: 10.7326/0003-4819-78-4-515. [DOI] [PubMed] [Google Scholar]
- Fields B. T., Jr, Bates J. H., Abernathy R. S. Amphotericin B serum concentrations during therapy. Appl Microbiol. 1970 Jun;19(6):955–959. doi: 10.1128/am.19.6.955-959.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Schmeling D. Effect of antibiotics of chemotaxis of human leukocytes. Antimicrob Agents Chemother. 1977 Apr;11(4):580–584. doi: 10.1128/aac.11.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Schmeling D., Quie P. G. Effect of tetracycline on the phagocytic function of human leukocytes. J Infect Dis. 1974 Oct;130(4):412–415. doi: 10.1093/infdis/130.4.412. [DOI] [PubMed] [Google Scholar]
- Gadebusch H. H., Pansy F., Klepner C., Schwind R. Amphotericin B and amphotericin B methyl ester ascorbate. I. Chemotherapeutic activity against Candida albicans, Cryptococcus neoformans, and Blastomyces dermatitidis in mice. J Infect Dis. 1976 Nov;134(5):423–427. doi: 10.1093/infdis/134.5.423. [DOI] [PubMed] [Google Scholar]
- Keim G. R., Jr, Poutsiaka J. W., Kirpan J., Keysser C. H. Amphotericin B methyl ester hydrochloride and amphotericin B: comparative acute toxicity. Science. 1973 Feb 9;179(4073):584–585. doi: 10.1126/science.179.4073.584. [DOI] [PubMed] [Google Scholar]
- Lawrence R. M., Hoeprich P. D. Comparison of amphotericin B and amphotericin B methyl ester: efficacy in murine coccidioidomycosis and toxicity. J Infect Dis. 1976 Feb;133(2):168–174. doi: 10.1093/infdis/133.2.168. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Medoff G., Kobayashi G. S. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977 Jan;11(1):154–160. doi: 10.1128/aac.11.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Majeski J. A., McClellan M. A., Alexander J. W. Effect of antibiotics on the in vitro neutrophil chemotactic response. Am Surg. 1976 Oct;42(10):785–788. [PubMed] [Google Scholar]
- Martin R. R., Warr G. A., Couch R. B., Yeager H., Knight V. Effects of tetracycline on leukotaxis. J Infect Dis. 1974 Feb;129(2):110–116. doi: 10.1093/infdis/129.2.110. [DOI] [PubMed] [Google Scholar]
- Mechlinski W., Schaffner C. P. Polyene macrolide derivatives. I. N-acylation and esterification reactions with amphotericin B. J Antibiot (Tokyo) 1972 Apr;25(4):256–258. [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S. Strategies in the treatment of systemic fungal infections. N Engl J Med. 1980 Jan 17;302(3):145–155. doi: 10.1056/NEJM198001173020304. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Orr W., Ward P. A. Quantitation of leukotaxis in agarose by three different methods. J Immunol Methods. 1978;20:95–107. doi: 10.1016/0022-1759(78)90248-x. [DOI] [PubMed] [Google Scholar]
- Pantazis C. G., Kniker W. T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979 Aug;26(2):155–170. [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner C. P., Mechlinski W. Polyene macrolide derivatives. II. Physical-chemical properties of polyene macrolide esters and their water soluble salts. J Antibiot (Tokyo) 1972 Apr;25(4):259–260. [PubMed] [Google Scholar]
- Steele R. W., Cannady P. B., Jr, Moore W. L., Jr, Gentry L. O. Skin test and blastogenic responses to Sporotrichun schenckii. J Clin Invest. 1976 Jan;57(1):156–160. doi: 10.1172/JCI108255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Cornelissen F., Lauwers W. F., van Cutsem J. M. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980 Jun;17(6):922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]



