Abstract
The coexistence of many plant species competing for a few resources is one of the central puzzles of community ecology. One explanation is that different species may be competitively superior in different microhabitats. Many species could then coexist within each piece of a mosaic landscape by what has been termed “mass effects,” because subpopulations in areas with negative growth rates would be supplemented by propagules from areas with reproductive surpluses. If mass effects are important, plant species diversity should increase near habitat boundaries, especially where habitat differences are moderate. In the first experimental test of this prediction, plants were censused on 54 transects within the long-established Rothamsted Park Grass plots. Very few showed significant declines in species richness with distance from subplot boundaries. Nonetheless, the regression coefficients were negative much more often than expected by chance, suggesting that weak mass effects operated. The effect was strongest where neighboring subplots differed greatly, with no evidence of the predicted decline where differences were extreme. Detailed analyses of transects with apparent mass effects revealed few species that behaved as predicted. This study serves both to provide evidence of the existence of mass effects and to question their importance in the maintenance of local plant diversity in this system.
Keywords: coexistence, source–sink dynamics, dispersal, environmental heterogeneity, species diversity
The maintenance of diversity in plant communities has been an issue of interest to ecologists for many years. How is it that so many species of plants can coexist when all of them need essentially the same array of resources; light, water, carbon dioxide, and a few minerals (1)? One possibility that has gained a certain amount of currency in the last decade has been variously termed “spatial mass effects,” “source and sink dynamics,” or “spillover effects” (the former term is generally employed by plant community ecologists, the second by animal population ecologists, and the last by animal community ecologists; see refs. 2–7). Where different habitat types potentially supporting different equilibrium communities occur as interspersed patches within a mosaic landscape, the local diversity within any one patch may be augmented by immigration from nearby patches. Even if the local population growth rates of these immigrant species were always negative (constituting a demographic “sink”), their populations could be supported by a constant rain of propagules from nearby populations with positive growth rates (“source” populations). Mass effects create α-diversity (within-site diversity) from β-diversity (differences between sites). Applied at a coarse spatial scale, where the scale of habitat patches greatly exceeds plant dispersal distances, this process could explain the existence of fugitive plant individuals in grossly inappropriate environments, a potentially significant but not particularly interesting component of local species richness. Applied at a microenvironmental scale, however, with patches at or below the scale of dispersal, the mass effects model promises more. Within a mosaic of such microhabitat patches, each patch could have its local species richness augmented by dispersal from its various neighbors, blurring or obscurring completely the boundaries between them. Dispersal between sources and sinks within such a mosaic landscape would allow the local coexistence (within each microhabitat) of many more species than could otherwise share a uniform site of the same characteristics.
The argument is conceptually sound and intuitively appealing. There is a growing appreciation that spatial structure, acting at a wide range of spatial scales, may be crucial to understanding many ecological problems, including species coexistence (8). The assumption of spatial uniformity implicit in most simple ecological models is almost certainly unrealistic, and the lack of such uniformity is precisely what should create the potential for mass effects. Indeed, there is growing evidence that some species exhibit source and sink populations in nature (refs. 9–11; for reviews see refs. 12 and 13). Nonetheless, the importance of mass effects at the community level for maintaining diversity in the field remains to be demonstrated. The very nature of the process involved makes such demonstrations difficult. Mass effects are the equilibrium result of spatial (or, by analogy, temporal) variability, and both the nature of variability and the requirement of equilibrium can create methodological difficulties for research. In nature, habitat mosaics are seldom as well defined as those posited by theoreticians—their borders are generally gradual, irregularly shaped, and difficult to observe. Experimentally produced mosaics, on the other hand, allow researchers to study much more highly controlled transitions, but it is rare for experimental treatments to be maintained long enough for communities of even short-lived plants to settle into equilibrium conditions.
There is at least one place, however, where experimental habitat mosaics have been established and maintained long enough for mass effects to be studied with some confidence. The Park Grass experiments at the Rothamsted Experimental Station are a series of closely abutting plots that have been subjected continually to different fertilization treatments for well over a century. If mass effects are important, we should expect plant species diversity to be higher near the boundaries between these areas than in their centers. If significant mass effects cannot be detected at Rothamsted, they are unlikely to be important in other, similar systems. If they can be detected there, it may be possible to study their properties in greater detail, so as to clarify the conditions under which they are most likely to be of interest.
There are reasons to expect that both the nature of the boundary and the nature of the plant species involved should affect the strength of mass effects. If two neighboring habitats are extremely similar, there is little reason to expect a strong effect at their border, because mass effects are powered by the differences between sites in species composition (analogous to a diffusion gradient across a membrane). The more similar two plots are, the fewer species differences they are likely to exhibit, and the less potential there will be for increased diversity at their boundary. On the other hand, extreme habitat differences between neighboring plots may prevent successful establishment of immigrants from across the border (analogous to an impermeable membrane). To take an extreme example, if a limestone cliff face closely abuts a peat bog, it is unlikely that there will be significant colonization of either habitat by plants living in the other; the differences between the sites in moisture and pH are so extreme that no bog species should be able to survive in crevices on the cliff, and cliff-dwelling species will be equally unsuited to life in the bog. The strongest mass effects should occur where the differences between neighboring plots are intermediate (Fig. 1). In the grand tradition of ecological nomenclature, I will term this the Intermediate Difference Hypothesis.
Figure 1.
A graphical representation of the intermediate difference hypothesis described in the text. The broken line represents the rate of species invasions and the dotted line the success rate of those invaders, both expressed as functions of the similarity between adjacent habitat patches. The precise shapes of the curves are arbitrary, but should not matter so long as both are monotonic in the indicated directions. The strength of mass effects (represented by the solid line) is a product of the two. Where neighboring plots are very similar, there are likely to be few species that are not present in both, and thus, little potential for mass effects. Where they are extremely dissimilar, species moving between plots should have little chance of survival. The effects of mass effects should therefore be most pronounced where plot differences are of intermediate strength.
The properties of the plant species and their populations may also affect the strength of mass effects. The probability of a plant species successfully invading a neighboring plot should depend, to some extent, on the number of its propagules that arrive there. This suggests that common species and species with high per capita seed production should be more capable of supporting sink populations in otherwise inhospitable plots than their rarer or less fecund counterparts. Interspecific differences in seed dispersal properties should also be important, because widely dispersed propagules are more likely to cross environmental boundaries than are poorly dispersed ones. These three predictions, all of which concern properties affecting the rain of propagules into sink habitats, will be referred to collectively as the Propagule Density Hypothesis.
In this paper, I report the results of surveys along transects across the treatment borders separating the Rothamsted Park Grass experimental subplots. If mass effects are important in maintaining grassland plant diversity, the species richness of those samples should be inversely related to their distance from interplot boundaries. The strength of these effects will then be discussed relative to the nature of the plots involved and the species they contain.
METHODS
Study Area.
The Park Grass experiments at the Rothamsted Experimental Station (near Harpenden, Hertfordshire, United Kingdom) were started in 1856 by Sir John Lawes and have been maintained with little change since then because of continuing support first from the Lawes Agricultural Trust and subsequently by the Agricultural and Food Research Council of Great Britain (14–16). The 3.24 ha site is level and well drained, with a silt loam soil overlying yellow-red clay upon chalk (17), and initially supported a fairly uniform plant community dominated by grasses and legumes. Various combinations of fertilizers are applied annually to longitudinal (roughly east-west) sections of the field (“plots”), and are crossed with 2–4 liming treatments applied to transverse (roughly north-south) sections (thus delimiting “subplots”). Liming treatments are separated by mowed paths, but most adjoining fertilizer treatments directly abut one another. Consequently, I considered only fertilizer treatment boundaries in this study. The vegetation on all plots is cut twice each year and is sampled to determine the species composition. Slight changes have been made to the experimental protocols intermittently over the duration of the experiment but, with the exception of two plots (which were excluded from this study), treatments at the time of this study had remained largely unchanged at least since 1965, and generally much longer.
Soil Analyses.
To determine the sharpness of Rothamsted’s treatment boundaries, soil cores were collected along transects across four interplot boundaries. Two parallel transects were surveyed at each site, 0.5 and 1 m from the edge of the plot, and on each, samples were taken at distances 0, 10, 20, 30, 40, 50, 65, 80, 100, 125, 150, 175, 200, 250, 300, 350, 400, 450, and 500 cm from the treatment boundary on each side. The four boundaries used (those between subplots 14/2d and 1d, 1d and 2d, 4/1d and 4/2d, and 11/2d and 12d) were chosen because treatment differences had resulted in particularly sharp pH differences across them. More detailed analysis of soil chemistry in a subset of the samples strongly supported the notion that these pH differences could be used as a proxy for differences in nutrient levels (P. Poulton, personal communication). This allowed me to investigate the degree to which boundaries had been blurred by imprecise application, leaching, the movements of soil invertebrates, or other factors.
Survey Transects.
The configuration of the Park Grass experiment and the excluded plots leaves 13 well-defined fertilizer treatment boundaries, plus 1 boundary that has been maintained on only the north (high lime) side of the field. I chose to examine each of them at the two extremes of the liming treatments, no lime (pH 3.5–5.8) and full lime (pH 6.1–6.9) for a total of 27 boundaries (the intermediate liming treatments are of more recent vintage). Because I surveyed transects on both sides of each of these boundaries, a total of 54 subplot boundaries are considered in this study. Twenty-four of these transects were examined in May and June of 1993 and the rest in May 1994, before the first harvest of the year in each case. Use restrictions prohibited entry into the plots themselves, so transects were restricted to those portions of the experimental plots that were accessible from the surrounding mowed areas. This allowed two replicate transects to be surveyed across each boundary; one sited 0.5 m from the outer edge of the plot and the other 0.5 m from the access path that separates liming treatments. Each transect consisted of a continuous strip of 0.25 m2 (0.5 × 0.5 m) quadrats arranged from 0–5 m on each side of a subplot boundary. The 5-m transect length was constrained by the geometry of the site; the narrowest of the plots are approximately 11 m wide, and so a transect of this length reached nearly to the center of a treatment without overlapping with transects from the opposite boundary. In any case, this distance was significantly greater than the likely dispersal distances of most of the plant species involved (18), and so should be adequate for documenting mass effects in this system. In each surveyed quadrat, the number of reproductive plants of each species was recorded. Only plants with buds, flowers, or fruits were counted, both to assist correct identification and as a way of excluding species incapable of breeding at a given site. This results in a somewhat conservative estimate of mass effect strength.
Statistical Methods.
For each quadrat surveyed, I calculated the total number of species observed, and averaged these figures for the two replicate transects at a given subplot boundary. I then plotted this mean species richness value as a function of the distance from the boundary and calculated a least-squares linear-regression coefficient. Because of the inherently nonlinear nature of the dispersal processes that could fuel mass effects, an exponential regression model [n = c(1 + r)d, where c is a constant, d is the distance from plot boundary to quadrat center, and r is the fitted rate parameter] was also applied to each transect dataset. A species accumulation curve was calculated for the outer (0.5–2.5 m) and inner (3–5 m) segment of each transect, to test the effect of sample size on species richness differences. Using these data, local species richness was estimated for each transect segment using both total species counts and jackknife estimates (19); the former an underestimate and the latter generally an overestimate. Values for inner and outer transect segments were then compared by using paired t tests. In transects with significant negative species richness × distance regressions, the abundance of each species was also analyzed as a function of distance from the boundary, to identify species contributing to the community level pattern. To measure the degree of similarity between adjoining subplots, I analyzed similarities in plant communities rather than in fertilizer application regimes, per se, as some nutrients (e.g., nitrogen) have much greater impacts than others (e.g., sodium silicate). All quadrats more than 2.5 m from plot boundaries on the two replicates of each transect were pooled, and the relationship between neighboring plots was then computed by using Renkonen’s “Percent Similarity” index (19). Finally, the regression coefficients of species richness as a function of distance from boundaries was plotted as a function of this similarity index.
RESULTS
Soil Surveys.
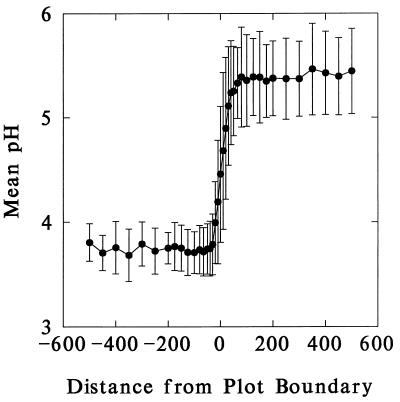
Results of soil pH surveys indicate that the boundaries of abutting treatment plots are quite sharp, with almost all of the variation occurring in the first 50 cm on either side of the border line (Fig. 2). Consequently, the first quadrat of each transect (from 0 to 0.5 m) was eliminated from the subsequent analyses of species richness reported below. This eliminates the possibility that any observed increases in species diversity near plot boundaries could be caused by the increased internal heterogeneity of quadrats sampling steep gradients, or of more equitable conditions in mid-gradient, than found in either plot (see Discussion).
Figure 2.
Mean pH of soil samples taken on transects across four interplot boundaries at Rothamsted. The data have been rearranged so that, in each case, the higher pH plot is on the right. Note that the transitions in soil chemistry are restricted almost entirely to 50 cm on either side of the boundary.
Species Richness.
Species richness scores varied greatly between and within study plots. The most depauperate subplots were near monocultures, dominated by a single species of grass (e.g., Holcus lanatus in plot 10d, Anthoxanthum oderatum in plot 1d), whereas as many as 17 species of plants could be found in a single 0.25 m2 quadrat in more diverse plots. Within a subplot, species richness scores commonly differed by as much as 2-fold, even between contiguous quadrats. Much of the variation was stochastic, but in some of the plots clear patterns emerged as a function of distance from neighboring plots. Of the 54 transects, 7 showed significant trends for lowered diversity with increasing distance from the boundary (as predicted with mass effects), whereas 1 showed a significant tendency in the opposite direction. These results are identical whether linear or exponential regression models are used. By chance alone, one would expect roughly one positive and one negative association to have been significant at the P < 0.05 level. Even though very few of the regressions were individually significant, taken as a group there is a significant tendency for species diversity to fall with distance. Of the 51 nonzero regressions (in 3 depauperate subplot transects, no variation was found in species richness values), 34 had negative linear regression coefficients—precisely twice the number of positive values. The binomial probability of finding 17 or fewer positive regressions out of 51 trials is 0.012, suggesting that these results would not be expected from chance alone. Precisely the same pattern is revealed in the exponential regressions.
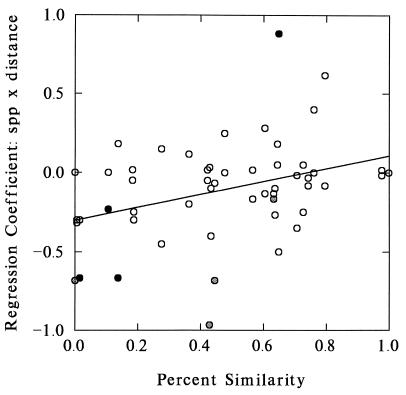
The observed trends in species richness were not randomly assigned. Fig. 3 displays the linear regression coefficients of the 54 species × distance analyses as a function of the similarity scores of adjoining plots. There is a strongly significant tendency for plots most dissimilar from their neighbors to show the strongest (that is, most negative) regression results (F ratio = 8.37, P = 0.006, R2 = 0.122). A very similar pattern appears if exponential regression coefficients are used (F ratio = 8.03, P = 0.007, R2 = 0.117). Including the absolute difference in species richness between adjoining plots improves the linear model somewhat (overall: F ratio = 7.048, P = 0.002, R2 = 0.186; similarity effect: P = 0.004; difference in species number effect: P = 0.029) by accounting for the differences in potential for mass effect on the two sides of a given boundary (percent similarity scores are symmetrical). This effect is not found, however, in a stepwise analysis of exponential regression scores.
Figure 3.
Regression coefficients from analysis of species richness × distance from plot boundaries at the Rothamsted Park Grass experiments, plotted as a function of the botanical similarity of the adjoining plots. Plot similarity is expressed as percent similarity (Renkonen’s index) between pooled samples of all quadrats >2.5 m from each side of the boundary. Shaded symbols indicate transects with individually significant diversity × distance trends when no correction is made for multiple comparisons (light shading, P < 0.05; dark shading, P < 0.01). Note that, overall, regressions are much more often negative than positive, and that the most dissimilar plots tend to show the strongest (that is, most negative) effects.
Species accumulation curves provide a very similar picture to that displayed by the quadrat scale data. Significantly more species were generally sampled in the outer part of each transect (that is, the section nearest to plot boundaries) than in the corresponding inner segment (paired t = 2.294, df = 53, P = 0.0258). Similar patterns emerge from analyses of jackknifed species richness estimates; values tended to be highest in the outer portion of transects (paired t = 2.351, df = 53, P = 0.0225).
Species Abundance Patterns.
The 7 transects exhibiting significant negative regressions of species diversity as a function of distance from plot boundaries contained between 2 and 22 species, for a total of 88 species transects. Of these, only 11 exhibited even marginally significant (P < 0.1) patterns of population decline with distance (Table 1). Many of the other species contributed toward the community patterns of diversity decline with distance but were too rare to provide statistically significant abundance declines when considered alone. Such species may or may not be exhibiting mass effects, but they are not amenable to statistical analysis if they are; however, the 11 species with measurable declines are clear candidates for the source–sink population dynamics that drive mass effects. However, for mass effects to be responsible for the observed population trends, the adjoining subplots must have populations of the species concerned to serve as sources of propagules. This further restricts the set of possible examples; in four cases, the focal species was entirely absent from the putative source plot. In another three cases, the species population was not appreciably commoner in the source than in the assumed sink population, a pattern inconsistent with the propagule density hypothesis suggested in the Introduction. Indeed, whereas it is possible for sink populations to exist at higher densities than the source populations that support them under special circumstances (e.g., where habitats differ greatly in competitive pressures; refs. 9 and 10), such conditions certainly do not suggest mass effects as the most likely mechanism for observed population patterns. This leaves only four cases of significant species patterns clearly consonant with predictions; significant declines in abundance with distance from a neighboring plot where the species concerned is reasonably common.
Table 1.
Patterns of population decline in 11 species transects
| Plot (neighbor) | Species | F | P value | Status in putative source | Mass effect? |
|---|---|---|---|---|---|
| 1d (2) | Luzula campestris | 5.8 | 0.030 | Rare (similar to sink) | Unlikely |
| 4/1a (4/2) | Briza media | 13.7 | 0.002 | Absent | No |
| L. campestris | 3.9 | (0.068) | Absent | No | |
| 11/1a (10) | Anthoxanthum oderatum | 9.9 | 0.007 | Common | Yes |
| Poa pratensis | 7.1 | 0.018 | Rare (similar to sink) | Unlikely | |
| 11/1d (10) | No significant species regressions | ||||
| 11/2a (12) | P. pratensis | 5.9 | 0.028 | Rare (rarer than sink) | No |
| Rumex acetosa | 4.7 | 0.047 | Fairly common | Yes | |
| Taraxacum officinale | 9.2 | 0.008 | Common | Yes | |
| 12d (11/2) | Alopecurus pratensis | 6.0 | 0.027 | Absent | No |
| 17d (16) | Conopodium majus | 4.8 | 0.044 | Fairly common | Yes |
| R. acetosa | 7.9 | 0.013 | Rare (similar to sink) | Unlikely | |
| Total: Yes 4 | |||||
| Unlikely 3 | |||||
| No 4 |
DISCUSSION
My results serve both to support the existence of mass effects and to question their importance in maintaining local species diversity.
This paper constitutes the first clear demonstration of the pattern of increased species diversity near experimentally imposed habitat boundaries—the most important prediction of mass effect models. The only previously published tests of the phenomenon are seriously flawed. Shmida and Wilson (3) showed that a valley transect placed near the bordering hillsides contained more species, and specifically more species typical of hillsides, than an otherwise similar valley area distant from the surrounding slopes. The pattern is certainly illustrative and may, indeed, be caused by mass effects, but the case is weakened by lack of replication and experimental control. It seems likely, for instance, that valley soil near hill slopes was shallower, rockier, or otherwise different than soil in the central regions of the valley. Such differences could have made the area more hospitable to hillside species without invoking mass effects as a mechanism. Hatton and Carpenter (21) examined native species invading a recently reclaimed coal mine, which had been reseeded with a mixture of pasture species not native to the site. They found a gradual decrease in diversity as they moved farther into the reclaimed area and away from the native sagebrush steppe vegetation. Their study, however, is not strictly relevant to testing the efficacy of mass effects. By their account, it appears that the reclaimed area was gradually being colonized by native species as succession proceeded, and thus was not serving as a sink population for them as required by mass effect models. Mass effects are defined as “the flow of individuals from areas of high success … to unfavorable areas” (3). Yet there is no reason to conclude that the reclaimed area in Hatton and Carpenter’s study (21) was in any way unfavorable; it was simply unoccupied by native species because of its recent history of disturbance.
The most effective way to test the power of mass effects to enhance local plant diversity is to examine an experimentally controlled habitat boundary at equilibrium. The treatment boundaries at the Park Grass plots at Rothamsted are probably the best such test cases available. The predicted diversity patterns do seem to occur there. Among the transects examined here, negative trends in species richness with increasing distance from interplot boundaries occurred much more often than would be expected by chance alone. With all but the most similar of neighbors, there seems to be a weak but pervasive trend toward the increase in species diversity near habitat boundaries predicted by mass effect models.
Before accepting this evidence as unambiguous support for the existence of mass effects, however, an important caveat must be considered. This study, in common with those cited above (3, 21), looks only for a pattern predicted by the mass effect model (increased diversity at an environmental boundary) and does not document the process (or processes) responsible for creating that pattern. I have no direct evidence that the increases in species diversity observed near treatment boundaries were because of the sorts of source-sink dynamics envisioned by theorists. Indeed, there are at least two other processes that might explain patterns of this sort; internal heterogeneity and intermediate conditions. I will present each briefly, and explain why I expect it not to be important in this study.
Internal heterogeneity. No management regime, however precisely applied, could produce truly discrete habitat treatments. The borders between Rothamsted’s subplots exhibit environmental gradients, albeit very steep ones. Consequently, quadrats sampled near the boundary between two plots contain a wider range of environmental conditions than do samples from plot centers, because of the changes in environmental conditions that occur across them. Thus, such marginal quadrats might be expected to support a wider array of species because of their greater internal habitat heterogeneity.
Intermediate conditions. Very extreme environmental conditions tend to result in depauperate plant communities. If two neighboring treatments are extreme in different ways, the border between them might be more hospitable than either area in its pure form (as the edge of a desert stream could prove intermediate in hydrology between the saturated conditions in the stream itself and the arid conditions in the surrounding terrain), and so may support a more diverse community. Some of the treatments at Rothamsted have produced very extreme (e.g., highly acidified) environments, with consequently depauperate plant communities. With any blurring of treatment boundaries, the edges of such treatments might be less extreme, and might therefore be capable of supporting a greater diversity of plant species.
Both of these difficulties apply only in areas where treatment conditions are in transition, which in the case of the Park Grass plots seems to be restricted to approximately 0.5 m on each side of interplot boundaries (Fig. 2). Consequently, by eliminating the first quadrat of each transect from consideration, I should have almost completely removed both effects. Examining those excluded quadrats, however, does not significantly change the regression results reported above. Thus, it appears that neither internal environmental heterogeneity nor intermediate condition effects are likely to be responsible for the increases in species richness found near plot boundaries at Rothamsted. Unless another competing explanation can be found, the data seem to strongly indicate that mass effects are responsible for the observed trends in species richness. Follow-up studies are under way to document the mechanistic basis of the observed pattern on one of the studied boundaries.
Although the data outlined above reveal patterns consistent with mass effects, the strength of the apparent effects detected was less than overwhelming. Where the patterns were strongest (in plots with completely nonoverlapping plant communities) the mean strength of mass effects according to the linear regression resulted in a drop of only about 0.3 species per meter, a drop of approximately 1.5 species over the 5-m survey distance. The exponential regression analysis suggests that these most dissimilar neighbors averaged a 20.1% reduction in species over the transect length (although a few plots lost 50% or more of their species over this distance). Most boundaries were considerably less extreme than these, and showed correspondingly weaker effects. Averaged over all the plots, the mean linear effect was only 0.1 species per meter, an average loss of only 0.5 species per quadrat sample (agreeing nicely with the exponential estimate of a 6.9% loss) over the 5-m length of the transects. Such contributions are not insubstantial, but they do not contribute much to the overall explication of species coexistence at the site.
It remains possible that stronger mass effects might have been detected by using other survey techniques. As mentioned above, my decision to count only reproductive plants makes my analysis conservative, ignoring species that may have invaded across boundaries without surviving to reproduce. This, however, seems unlikely to explain the model’s poor showing. In the second survey season (1994), additional records were kept of species found without flowers or fruits, but this component of species richness did not decrease significantly with distance from plot boundaries. Another potential difficulty with my techniques is the scale chosen for analysis. Mass effects are phenomena of intermediate spatial scales (3); perhaps the 5-m transects used here were too small to pick them up. The relevant scale, however, should be set by the dispersal distance of the plants involved. With the exception of a few wind dispersed species (most notably T. officinale and Tragopogon pratensis), most of the species in this study lacked any obvious mechanism for long distance seed dispersal. Seed movements of more than a few meters in such species would be surprising. Even among wind-dispersed species, most seeds seem to be deposited relatively close to the maternal plant (18), suggesting that a 5-m transect should be ample for sampling dispersal-dependent population effects even for these most mobile members of my species sample. The clear implication is that mass effects at Rothamsted, where they occur, are surprisingly weak.
This weakness makes it more difficult to test the factors modulating mass effects. In the Introduction, two types of possible explanations for the relative strength of mass effects were considered; the propagule density hypothesis and the intermediate difference hypothesis. The first of these concerned species properties likely to affect the number of propagules moving across habitat boundaries; abundance in source populations, seed number, and dispersal ability. To evaluate the hypothesis would require a comparison of species successfully invading across subplot boundaries with those that did not. Unfortunately, the species abundance analyses reported above provide us with very few individual species cases for consideration. Of the seven cases listed in Table 1 for which source-sink dynamics are plausible, three come from transects adjoining plots in which the species involved is relatively rare; observations clearly inconsistent with the predictions of the propagule density hypothesis. The remaining four cases are too few to allow for any meaningful interspecific patterns of mass effect strength to be tested. It is nonetheless suggestive that one of these four involves the wind-dispersed species T. officinale, one of the best dispersers among Rothamsted’s plants.
We can, however, test the proposed effect of boundary type on mass effect strength. The intermediate difference hypothesis predicts that mass effects should be strongest where interplot differences are moderate. I had suggested that, if neighboring plots were too similar, there should be few species available nest door to colonize a given plot that are not already living there; whereas, if plots are too dissimilar, propagules from across plot boundaries should have little or no chance of establishment. I found evidence of the first, but not the second, of these phenomena. The clearest evidence of mass effects came from the subplot boundaries where species compositions were most dissimilar. Of course, even the most dissimilar pairs studied here were structurally quite similar; all plots were grass-dominated pastures on structurally similar soils. It may be that more extreme environmental boundaries (e.g., between peat bogs and limestone cliffs, as suggested above) would show the predicted downturn in mass effect strength.
If the treatment boundaries examined were never as dissimilar as the most disparate natural neighbors, some were nonetheless quite pronounced. The most striking contrasts (where evidence of mass effects was most often noted) involved treatments where hundreds of kilograms of nitrogen, potassium, or sodium silicate and tens of kilograms of potassium, sodium, and magnesium are applied per hectare annually. These treatment differences dwarf the range of microhabitat diversity within any one field. This fact, combined with the observed trends in mass effect strength, casts serious doubt on the applicability of mass effects models at a microhabitat scale. Source-sink dynamics across abrupt habitat boundaries (such as those studied here) may produce a few curiously misplaced plants, but for such processes to play an important role in promoting α-diversity in nature, they must also occur across the subtler microhabitat boundaries that characterize even apparently “uniform” habitats in a nonuniform world. To extrapolate from experimentally imposed ecotones (which are amenable to study) to the smaller and less abrupt transitions typical of natural microsites, one needs to know not only how strong spillover effects are in general, but also how they respond to the severity of an environmental boundary.
The results of this study suggest that such microsite mass effects may be of little consequence at Rothamsted. Not only are the effects found between plots weak and hazy overall, but they get weaker and hazier where interplot differences in species composition (and soil chemistry) are small. That being the case, it is difficult to imagine that the subtle variation typically found between microsites will produce any stronger effects than those produced at relatively subtle plot boundaries—which is to say, hardly any. If mass effects between Rothamsted plots are fairly weak in all but the severest of boundaries, mass effects between microhabitats within each plot cannot reasonably be taken to explain the coexistence of diverse plant communities there. Other mechanisms for coexistence must be sought (22–24).
This study provides both encouragement and discouragement for those proposing mass effects as a mechanism maintaining local plant species diversity. On one hand, my results are the first clear evidence of the pattern of augmented diversity at environmental boundaries, the principle prediction of mass effect models. Nonetheless, my results suggest that such mechanisms make only a minor contribution to the maintenance of diverse plant communities, at least at Rothamsted. There is a temptation in science to portray one’s findings in polarized terms, stressing either how they strongly support a theory or how they debunk it. My findings in this case, however, do both, leaving me in the uncomfortable position of burying Ceaser and praising him at the same time. Plant species diversity is augmented at environmental boundaries, suggesting that mass effects do exist. But if the Rothamsted Park Grass plots are at all indicative of the situation elsewhere, they are not likely to be very important in maintaining diversity within the subtle habitat mosaics that typify the natural world.
Acknowledgments
Gebreselasie Asefa provided field assistance, along with Quentin Paynter, Catherine Long, and Gillian Sinclair. Soil pH samples were taken and analyzed by Dr. Paul Poulton. Edward Bacon provided assistance in gaining approval for this project, Jacqueline Potts helped clean up the data set, and George Cussans served as my official collaborator. Mick Crawley provided encouragement and assisted in identifying difficult taxa. Deborah Goldberg, Gordon Orians, and seven anonymous reviewers provided useful advice toward improving earlier drafts of this paper.
References
- 1.Grubb P J. Biol Rev. 1977;52:107–145. [Google Scholar]
- 2.Shmida A, Ellner S. Vegetatio. 1984;58:29–55. [Google Scholar]
- 3.Shmida A, Wilson M. J Biogeogr. 1985;12:1–20. [Google Scholar]
- 4.Pulliam H R. Am Nat. 1988;132:652–661. [Google Scholar]
- 5.Pulliam H R, Danielson B J. Am Nat. 1991;137:S50–S56. [Google Scholar]
- 6.Holt R D. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 77–88. [Google Scholar]
- 7.Cody M L. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 147–158. [Google Scholar]
- 8.Levin S A. Ecology. 1992;73:1943–1967. [Google Scholar]
- 9.Keddy P A. J Ecol. 1981;69:615–630. [Google Scholar]
- 10.Keddy P A. Oecologia (Berlin) 1982;52:348–355. doi: 10.1007/BF00367958. [DOI] [PubMed] [Google Scholar]
- 11.Kadmon R, Shmida A. Am Nat. 1990;135:382–397. [Google Scholar]
- 12.Dias P C. Trends Ecol Evol. 1996;11:326–330. doi: 10.1016/0169-5347(96)10037-9. [DOI] [PubMed] [Google Scholar]
- 13.Pulliam H R. In: Population Dynamics in Ecolological Space and Time. Rhodes O E Jr, Chesser R K, Smith M H, editors. Chicago: Univ. of Chicago Press; 1996. pp. 45–69. [Google Scholar]
- 14.Lawes J B, Gilbert J H. Philos Trans R Soc London A and B. 1880;171:289–415. [Google Scholar]
- 15.Tilman D. Resource Competition and Community Structure. Princeton, NJ: Princeton Univ. Press; 1982. [PubMed] [Google Scholar]
- 16.Dodd M, Silvertown J, McConway K, Potts J, Crawley M. J Ecol. 1995;83:277–285. doi: 10.1016/0169-5347(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 17.Thurston J M, Williams E D, Johnston A E. Ann Agron. 1976;27:1043–1082. [Google Scholar]
- 18.Willson M F. Vegetatio. 1993;108:261–280. [Google Scholar]
- 19.Krebs C J. Ecological Methodology. New York: Harper & Row; 1989. [Google Scholar]
- 20.Wilkinson L. SYSTAT: The System for Statistics. 2nd Ed. Evanston, IL: SYSTAT; 1989. [Google Scholar]
- 21.Hatton T J, Carpenter A T. Vegetatio. 1986;68:33–36. [Google Scholar]
- 22.Tilman D. Ecology. 1994;75:2–16. [Google Scholar]
- 23.Tilman D, Pacala S. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 13–25. [Google Scholar]
- 24.Chesson P L. In: Community Ecology. Diamond J, Case T, editors. New York: Harper & Row; 1986. pp. 240–256. [Google Scholar]