Abstract
Small-fiber painful peripheral neuropathy, a complication of chronic ethanol ingestion, is more severe in women. In the present study, we have replicated this clinical finding in the rat and evaluated for a role of estrogen and second messenger signaling pathways. The alcohol diet (6.5% ethanol v:v in Lieber-DeCarli formula) induced hyperalgesia with more rapid onset and severity in females. Following ovariectomy, alcohol failed to induce hyperalgesia in female rats, well past its time to onset in gonad intact males and females. Estrogen replacement reinstated alcohol neuropathy in the female rat. The protein kinase A (PKA) inhibitor (WIPTIDE) only attenuated alcohol-induced hyperalgesia in female rats. Inhibitors of protein kinase Cε (PKCε-I) and ERK1/2 (PD98059 and U0126) attenuated hyperalgesia in males and females, however the degree of attenuation produced by PKCε-I was much greater in females. In conclusion, estrogen plays an important role in the expression of pain associated with alcohol neuropathy in the female rat. In contrast to inflammatory hyperalgesia, in which only the contribution of PKCε signaling is sexually dimorphic, in alcohol neuropathy PKA as well as PKCε signaling is highly sexually dimorphic.
Keywords: Ethanol, Hyperalgesia, Estrogen, Second Messenger Signaling
Introduction
The neurological effects of ethanol consumption are complex, encompassing both the central (Pentney and Quackenbush, 1990, Zou et al., 1993) and peripheral (Victor, 1975) nervous system. Although centrally alcohol acts as an analgesic (James et al., 1978, Woodrow and Eltherington, 1988), in the periphery it produces a small-fiber dying back painful neuropathy (Bosch et al., 1979, Dina et al., 2000). Over time, pain far outweighs analgesia, producing a neuropathic pain syndrome with symptoms that have been described as “like tearing flesh off the bones” (Brain and Walton, 1969).
In recent years, ethanol consumption in Western industrialized countries has increased among women with an associated increase in the rates of alcohol-related health problems (Fillmore, 1987, Gomberg, 1993). Thus, understanding the basis of the more severe clinical expression of ethanol-induced neuropathy in females (Ammendola et al., 2000) is an issue of growing importance and, adequate treatment of this symptom may require therapeutic strategies tailored to gender-specific targets. We have evaluated, in a rat model, mechanisms that may contribute to the increased severity of symptoms in females with alcohol neuropathy.
Experimental Procedures
Animals
Experiments were performed on male and female Sprague-Dawley rats (Charles River, Hollister, CA, USA). Three-week (21-day) old female rats were ovariectomized and used in experiments 4 weeks later, when they were adults. In all other cases, same aged adult male and female (250–400g) gonad-intact rats were used. At the commencement of ethanol diet, male and female animals were weight-matched. They were housed individually in a controlled environment in the Animal Care Facility of the University of California, San Francisco, under a 12 h light/dark cycle. Care, health, general condition and use of experimental animals conformed to NIH guidelines. Experimental protocols were approved by the UCSF Committee on Animal Research. All efforts were made to minimize both animal suffering and number used.
Chronic alcohol consumption
The protocol for feeding the alcohol-containing diet to rats has been previously described (Lieber and DeCarli, 1982, Lieber and DeCarli, 1989, Lieber et al., 1989, Dina et al., 2000). Briefly, all experimental rats were fed an ethanol-containing Lieber-DeCarli liquid diet (ED, 6.5% ethanol; Dyets Inc., Bethlehem, PA, USA) daily over a period of 12 weeks. The control (CD) rats were pair-fed (i.e. calorically-matched to the ethanol-fed rats) by giving them a diet in which equal calories of maltose-dextrin was consumed in place of ethanol (Lieber, 1995). The ethanol used in this study (190-proof ethyl alcohol) was obtained from Gold Shield Distributors, Hayward, CA, USA. Alcohol-containing diet or corresponding control diet was fed only to adult rats. Three-week old ovariectomized (Ovx) or estrogen-implanted ovariectomized (OvxE) female rats were fed standard laboratory chow until they were adults (i.e., 4 weeks after Ovx or OvxE). Animals in all the experimental groups were healthy, and displayed normal reflexes and alertness. Ethanol diet-fed rats showed signs of normal grooming behavior, albeit, were more alert than naïve or control diet fed rats. Rats on alcohol diet were hyperactive - a response which was enhanced by sound stimuli - without apparent sexual dimorphism. No similar findings of hyperactivity were observed in control diet fed animals of either sex. Because the rats were pair-fed, rats fed Lieber-DeCarli liquid diet with ethanol (6.5%, v:v ethanol; ED) did not gain weight faster than those consuming control Lieber-DeCarli diet (CD) (data not shown, (Dina et al., 2000)).
Ovariectomy
Ovariectomy (Ovx) was performed on 21-day old rats (i.e., before sexual maturity) and the animals used for experiments 4 weeks later (i.e., as adults). The ovariectomy procedure was performed using bilateral, cutaneous, upper flank incisions to access the abdominal cavity (Wayneforth and Flecknell, 1992, Green et al., 1999). The ovaries were located, their vascular bundles tied off with 4-0 silk suture, and they were excised. The fascia was closed with 5-0 chromic gut and the cutaneous incisions closed with 7.5 mm metal wound clips. These procedures were carried out under inhalational anesthesia ((2.5% isoflurane (Matrix, Orchard Park, NY, USA)) in 97.5% O2.
Administration of estrogen
Chronic administration of estrogen by subcutaneous implants, to a subset of Ovx female rats (OvxE) was performed as described previously (Smith et al., 1977, Green et al., 1999, Dina et al., 2001). Briefly, 10-mm-long segments of silastic® tubing (1.67 mm inner diameter x 3.18 mm outer diameter; Fisher Scientific, Santa Clara, CA, USA) were filled with 17β-estradiol (Sigma, St. Louis, MO, USA) and the ends of the implants capped with silastic plugs (Goodfellow, Berwyn, PA, USA). Implants were washed in absolute ethanol and equilibrated in four changes of warm phosphate-buffered saline over a 24-h period before placement in the rat. Implants were placed subcutaneously on the back at the time of ovariectomy to produce sustained levels of estrogen over an extended period of time (Smith et al., 1977). Implants were replaced 6 weeks after the first implants were inserted and remained in place through the remainder of the experiment.
Mechanical nociceptive threshold
The mechanical nociceptive flexion reflex was quantified using the Randall–Selitto paw pressure test (Randall and Selitto, 1957), which produces a force that increases linearly over time (Analgesymeter®, Stoelting, Chicago, IL), applied to the dorsum of the rat’s hind paw, a protocol that has been used in alcohol-naive (Taiwo et al., 1989, Dina et al., 2001, Dina et al., 2004, Hucho et al., 2006), alcohol-fed (Dina et al., 2000) and alcohol-withdrawing (Dina et al., 2006) rats. Animals were lightly restrained using cylindrical Perspex® restrainers designed to provide adequate comfort and ventilation, minimize restraint stress and accommodate size differences between individual rats. All experimental animals used in this study were acclimated to the testing procedure such that restraint and test techniques were parallel across groups. Briefly, animals were placed individually in the restrainers for 1 hour prior to the commencement of each study and for 30 minutes prior to testing on each test day (Dina et al., 2006). The nociceptive threshold was defined as the force in grams at which the rat withdrew its paw. The baseline paw-withdrawal threshold was defined as the mean of three readings before alcohol-containing (or control) liquid diet was administered. Each paw was treated as an independent measure and each experiment was performed on a separate group of rats. Rats in each experimental group were tested weekly in order to determine the change in nociceptive threshold in response to ethanol (ED) or control (CD) diet. All behavioral testing was done between 10 am and 4 pm.
Pharmacological interventions
Since sex-specific differences in second messenger signaling have been described in inflammatory hyperalgesia (Dina et al., 2001, Khasar et al., 2005, Hucho et al., 2006) and protein kinase C epsilon (PKCε), protein kinase A (PKA) and extracellular-signal related kinase (ERK) signaling pathways are known to mediate mechanical hyperalgesia (Dina et al., 2001), we determined the sexual dimorphism for the effects of a selective PKCε inhibitor peptide (PKCε-I; EMD Biosciences, La Jolla, CA, USA; (Johnson et al., 1996)), the PKA inhibitor Walsh inhibitor peptide (WIPTIDE; Bachem Biosciences Inc., San Carlos, CA, USA; (Dragland et al., 1985, Glass et al., 1989)) and ERK pathway inhibitors, PD98059 (2′-amino-3′-methoxyflavone; a selective inhibitor of mitogen and ERK kinase (MEK) (Kultz et al., 1998)) and U0126 (1,4-Diamino-2, 3-dicyano-1, 4-bis (2-aminophenylthio) butadiene; a specific inhibitor of ERK 1/2 (DeSilva et al., 1998)) in alcohol-induced hyperalgesia. ERK pathway inhibitors were obtained from EMD Biosciences.
Drug preparation and administration
WIPTIDE and PKCε-I were dissolved in distilled water; PD98059 and U0126 were dissolved in 10% dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and diluted in distilled water before use (final concentration of DMSO <1%). Stock solutions (1 μg/μl) of inhibitors were stored at −20°C. With the exception of estrogen, which was administered by subcutaneous implants, all injections were made by the intradermal route in the hind paw as previously described (Taiwo et al., 1989, Dina et al., 2003). In the subsets of animals used in this study, all inhibitors were injected at a concentration of 1 μg/2.5 μl after alcohol-induced mechanical hyperalgesia had been established. These inhibitors have been shown previously to produce a significant attenuation of mechanical hyperalgesia induced by inflammatory agents in male (Khasar et al., 1999b, Dina et al., 2005) and female (Dina et al., 2001, Khasar et al., 2005) rats. Because they are less membrane permeable, injections of the protein kinase inhibitors were always preceded by administration of 2.5 μl of distilled water in the same syringe to produce hypo-osmotic shock, thereby transiently enhancing cell membrane permeability (Tsapis and Kepes, 1977, Widdicombe et al., 1996). Protein kinase inhibitors were separated from the distilled water by drawing up a small air bubble (<1μl) into the syringe after drawing up the protein kinase inhibitor but before drawing up the distilled water; thus the distilled water was injected first, before the kinase inhibitor.
Statistical analysis
Repeated measures ANOVA with between-subjects factors, as appropriate, was employed to compare the effects of interventions in experimental groups (α=0.05). For each ANOVA the Mauchly criterion was used to determine if the assumption of sphericity for the within-subjects effects was met; if the Mauchly criterion was not satisfied, Greenhouse-Geisser adjusted p values were calculated.
Results
Onset of alcohol-induced hyperalgesia
To induce neuropathy, the Lieber-DeCarli liquid diet with ethanol (ED) or control Lieber-DeCarli diet (CD) was consumed by gonad-intact male and female rats, over a period of 12 weeks, a protocol that has been previously shown to produce mechanical hyperalgesia in male rats (Dina et al., 2000). Before the initiation of ED or CD, the mean baseline paw-withdrawal threshold of male rats (104.9±1.1 g; n=36) was greater than that of females (92.4±0.9 g; n=12) (p < 0.05; Fig. 1a). Consumption of ED resulted in a reduction in mechanical nociceptive thresholds in both male and female rats (both p<0.05, Fig. 1a and 1b). Repeated measures ANOVA with one within-subjects factor (time with 6 levels, including the beginning of the first week) and two between-subjects factors (diet with two levels, ethanol (ED) and control (CD), and sex with two levels, male and female) demonstrated a significant three-way (time × diet × sex) interaction (F(5,400)=2.897, p<0.003). To determine the basis of this significant difference, 2 two-way repeated measure ANOVAs were performed, one for males and females receiving the ethanol diet, the other for males and females receiving the control diet. Rats receiving the ethanol diet demonstrated a significant time × sex interaction (F(5,230)=3.932, P<0.006) as well as a significant main effect of sex (F(1,46)=8.624, p<0.005), indicating that the hyperalgesic effect of the ethanol diet was significantly greater in females. In both sexes, a significant decrease in mechanical nociceptive threshold was produced after 4 weeks on ED and was maximal at 12 weeks, when nociceptive threshold was 84.4±1.1 g (n=36) and 63.9±1.5 g (n=12) in males and females, respectively. However, beginning at 4 weeks, there was a significantly greater decrease in nociceptive threshold in female rats consuming ED, a difference that was sustained until week 12, the final week of the study (Fig. 1a). There was no significant change (p>0.05) from baseline in the paw-withdrawal threshold, in rats of either sex, consuming CD, in the same 12-week study period.
Figure 1. Nociceptive effects of ethanol diet.
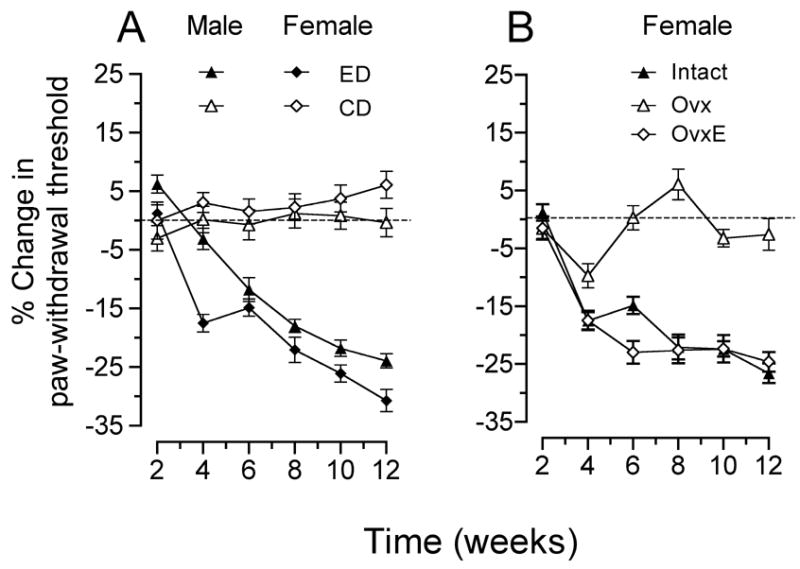
(A) Ethanol diet (ED) reduces the mechanical nociceptive threshold in male and female rats. Males and females receiving the control diet (CD) did not differ significantly. In this and subsequent figures data are plotted as mean ± standard error of the mean (s.e.m.). (B) Ovariectomy (Ovx) prevents ethanol diet-induced hyperalgesia in females. Scheffé post hoc analysis indicated that the ovariectomized group was significantly different from the intact females and the ovariectomized group that received estrogen supplement (OvxE) (p<0.001, in both cases), but the intact group was not significantly different from the OvxE group.
Role of estrogen
Having established a significantly more rapid onset and greater magnitude of hyperalgesia in female rats consuming alcohol, we next examined the role of estrogen in determining the severity of mechanical hyperalgesia in females (Fig. 1b). In separate groups of female rats the effect of ovariectomy and estrogen replacement were studied. Prior to the consumption of ED, the baseline mechanical nociceptive threshold of ovariectomized female rats (114.7±1.8 g, n=12) was significantly higher (p<0.05) than that of gonad-intact (92.4±0.9 g, n=12) or estrogen-replaced ovariectomized (97.9±1.7 g, n=12) rats. Unexpectedly, even after 12 weeks on ED or CD there was no decrease (p>0.05) in the mechanical threshold of ovariectomized female rats (baseline:114.7±1.8 g vs. ED: 111.4±1.8 g, or CD: 112.2±2.2 g, all n=12). When estrogen was administered to ovariectomized female rats, the female phenotype was reconstituted and the paw-withdrawal threshold of the ovariectomized, estrogen-replaced females (97.9±1.7 g, n=12) closely approximated that in gonad-intact females (92.4±0.9 g, n=12). Compared to gonad-intact females, estrogen-replaced ovariectomized rats on ED did not differ significantly with respect to mechanical nociceptive threshold (p>0.05). Two-way repeated measures ANOVA, with one within subjects factor (time with 6 levels) and one between subjects factor (estrogen status with three levels, intact, ovariectomized, and ovariectomized with estrogen supplement) demonstrated a significant time × estrogen status interaction (F(10,165)=20.529, p<0.001) and a significant main effect of estrogen status (F(2,33)=41.520, p<0.001).
Role of PKCε and PKA
In gonad-intact female rats we found that both PKCε–1 μg/2.5 μl) as well as WIPTIDE (1 μg/2.5 μl), injected intradermally at the site of nociceptive testing after establishing alcohol-induced hyperalgesia significantly inhibited alcohol-induced hyperalgesia (p<0.05, Fig. 2a and 2b). The testing was performed by the 9th week of the study, on rats that were hyperalgesic by the 4th week. The somewhat greater effect of inhibitors in estrogen-treated rats, may be due to slightly supraphysiological levels of estrogen. WIPTIDE also attenuated alcohol-induced hyperalgesia in estrogen-replaced female rats (Fig. 2a and 2b). However, as reported previously in the male rat (Dina et al., 2000), the PKCε inhibitor, but not WIPTIDE, attenuated alcohol-induced hyperalgesia (Fig. 2a and 2b, respectively). In addition, the magnitude of analgesia induced by PKCε inhibitor was greater in female rats. In Figure 2a, two-way ANOVA demonstrated a significant two-way interaction (F(2,27)=21.379, p<0.001). Post-hoc one-way repeated measures ANOVAs showed a significant difference between pre- and post-PKCε-inhibitor administration responses for gonad intact females (IF; F(1,11)=193.633, p<0.001; n=12) and ovariectomized estrogen-implanted females (OvxE; F(1,5)=101.104, p<0.001; n=6), and for gonad intact males (IM; F(1,11)= 7.106, p<0.03; n=12). To determine if there was a significant difference in the effect of PKCε inhibitor between intact females and males, post-treatment scores were subtracted from the pre-treatment scores and a one-way between subjects ANOVA (difference with two levels, males and females) was performed. This analysis revealed that the effect of PKCε inhibitor was significantly greater for females than for males (F(1,22) = 22.642; p<0.001). In Figure 2b, two-way ANOVA also demonstrated a significant two-way interaction (F(2,27)=106.237, p<0.001) for the effect of the PKA-inhibitor, WIPTIDE. Post-hoc one-way repeated measures ANOVAs showed significant difference between pre-and post-WIPTIDE administration responses for intact females (F(1,11)=298.820, p<0.001; n=12) and ovariectomized estrogen-implanted females (F(1,5)=161.252, p<0.001; n=6), but not for males (n=12).
Figure 2. Protein kinase inhibitors differentially modulate alcohol-induced hyperalgesia.
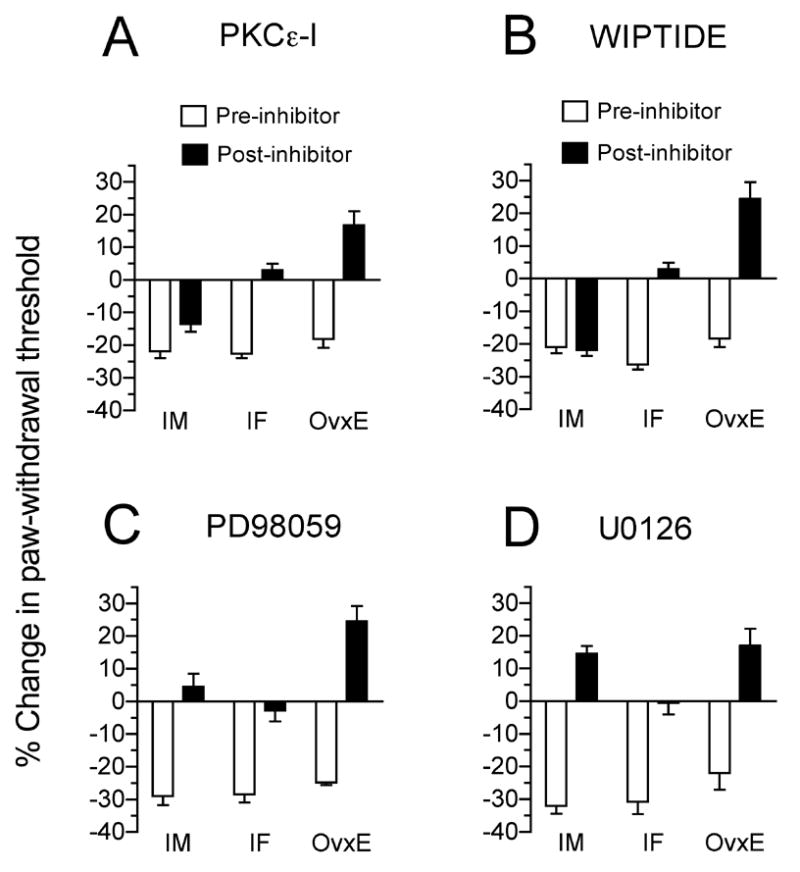
Baseline responses were measured in intact males (IM), intact females (IF) and ovariectomized females with estrogen implants (OvxE) prior to commencement of the ethanol diet; pre-inhibitor and post-inhibitor responses are plotted as percentage change from the baseline scores. Data were analyzed by two-way repeated measures ANOVA with one within-subjects factor (treatment with two levels, pre-inhibitor and post-inhibitor) and sex with three levels (males, females, and ovariectomized females plus estrogen). If there was a significant treatment × sex interaction, separate one-way repeated measures were performed for each sex to determine the basis of the significant differences. Effect of the inhibitors, namely; PKCε, WIPTIDE, PD98059 and U0126 are shown in Figures 2a, 2b, 2c and 2d, respectively. Statistical analysis and data are described in the results, as appropriate.
Role of MEK/ERK
Since previous reports have described a role for MEK/ERK signaling in inflammatory pain in male (Aley et al., 2001, Dina et al., 2003, Dina et al., 2005, Zhuang et al., 2005) and female (Dina et al., 2001) rats, we also evaluated the contribution of MEK/ERK to alcohol-induced peripheral neuropathy. We found that intradermal injection of PD98059 (1 μg/μl) and U0126 (1 μg/μl) after establishing a state of hyperalgesia in alcohol-fed rats of either sex, attenuated ED-induced hyperalgesia similarly in male and female rats (Fig. 2c and 2d), consistent with a comparable role for MEK/ERK signaling in chronic alcohol-induced hyperalgesia in rats of both sexes. In Figure 2c, two-way ANOVA demonstrated a significant two-way interaction (F(2,21)=7.514, p<0.005). Post-hoc one-way repeated measures ANOVAs showed a significant difference between pre-and post-PD98059 administration responses for intact females (F(1,5)= 65.649, p<0.001; n=6) and ovariectomized estrogen-implanted females (F(1,11)= 97.107, p<0.001; n=6), and for males (F(1,5)=110.100, p<0.001; n=12). In Figure 2d, two-way ANOVA also demonstrated a significant two-way interaction for the effect of U0126 (F(2,15)= 4.382, p<0.005) while post-hoc one-way repeated measures ANOVAs showed significant difference between pre- and post-U0126 administration responses for intact females (F(1,5)= 143.146, p<0.001; n=6) and ovariectomized estrogen-implanted females (F(1,5)=55.592, p<0.001; n=6), and for males (F(1,5)= 185.327, p<0.001; n=6).
Discussion
Using a model of painful peripheral neuropathy induced by an ethanol-containing diet (Dina et al., 2000), we have replicated in rats the human clinical finding that females develop a more severe painful alcoholic polyneuropathy than males (Ammendola et al., 2000). The pain phenotype of alcohol neuropathy (Dina et al., 2000) is, at least in part, consequent to alcohol-induced primary afferent hypersensitivity in which there is a lowered mechanical threshold for C-fibers in ethanol diet-fed rats (Dina et al., 2000). Ethanol fed female rats developed mechanical hyperalgesia more rapidly and the mechanical hyperalgesia was more severe. This delayed onset and difference in magnitude of hyperalgesia, albeit statistically significant if not large, was sufficient to inform the need to analyze the intracellular mechanisms underlying hyperalgesia in male and female rats, which are markedly different. To evaluate the role of sex hormones in determining the severity of symptoms in females, we evaluated the effect of ovariectomy with and without estrogen replacement. Quite unexpectedly, we observed a complete loss of the ability of the ethanol diet to induce hyperalgesia even up to a point in time well beyond that required to induce hyperalgesia in gonad-intact and male and female rats (Dina et al., 2000). That this dramatic phenotypic switch was estrogen-dependent was confirmed using estrogen-replaced ovariectomized female rats. Interactions between estrogen and alcohol-induced neurotoxicity have been reported. Thus, while estrogen tends to be neuroprotective in the central nervous system (Jung et al., 2005, Rewal et al., 2005), it can enhance toxic effects of alcohol in peripheral tissues (Bershtein et al., 2002, Enomoto et al., 2004). Although it is conceivable that both estrogen and alcohol are major determining factors for consideration in interpreting the present data, it is known that ethanol exerts a direct neurotoxic action on the peripheral nervous system, resulting in a neuropathy that mostly involves small-diameter fibers (Diamond, 1994, Monforte, 1995, Kielhorn, 1996, Ortiz-Plata et al., 1998, Tredici et al., 1999). While, on the other hand, estrogen receptors have been described on DRG neurons (Sohrabji et al., 1994, Taleghany et al., 1999, Papka and Storey-Workley, 2002, Purves-Tyson and Keast, 2004), whether the effect of this sex hormone is direct or indirect has not been addressed by the present experiments, even with the demonstrated changes in second messenger signaling. While the explanation for the complete loss of ED-induced hyperalgesia will have to await additional experiments, our findings do suggest that the incidence and severity of alcohol-induced painful peripheral neuropathy may be less in post-menopausal women, which could lead to an underestimation of the severity of alcohol neuropathy in younger women. While evaluation of fiber loss is beyond the scope of the present study, it is an interesting point to consider. In fact, pain is frequently an early manifestation of peripheral neuropathy, when anatomical changes are less likely to have occurred. Thus, elucidating the mechanism of the pain associated with peripheral neuropathy may provide insight into the mechanisms that produce the late manifestations such as nerve fiber loss.
To evaluate the contribution of specific signaling pathways to alcohol-induced mechanical hyperalgesia we used selective inhibitors of different protein kinases. We confirmed our previous observation that PKCε mediates alcohol-induced hyperalgesia (Dina et al., 2000) in males. This resembles the important role PKCε also plays in mediating inflammatory hyperalgesia in male rats and mice (Khasar et al., 1999a). However, in female rats the role of PKCε is quite different. Here we observed a major contribution of PKCε to alcohol-induced hyperalgesia in females, whereas previously we found no role for PKCε in inflammatory hyperalgesia in female rats (Dina et al., 2001); only after ovariectomy did we observe an inhibitory effect of PKCε-I which was attenuated by estrogen replacement (Dina et al., 2001). That the contribution of PKCε shown in the present study was significantly greater for females than for males not only contrasts with our findings for inflammatory-mediated hyperalgesia induced by β2-adrenergic receptor agonists (Dina et al., 2001) but also by hyperalgesic priming (Joseph and Levine, 2003b) - a model of chronic inflammatory pain. In a study of diabetic neuropathy, the contribution of PKCε was similar in male and female rats (Joseph and Levine, 2003a). However, in a model of chemotherapy neuropathy, induced by vincristine, PKCε did not contribute to hyperalgesia in female rats (Joseph et al., 2003). Thus, sexual dimorphism in the contribution of PKCε may be dependent on the pain syndrome being studied. Whether estrogen activates PKCε in females or recruits a PKCε-dependent pathway in female nociceptors remains to be determined. However we predict either is likely since estrogen stimulates translocation of PKCε in cultured DRG neurons and produces a PKCε-dependent hyperalgesia in male rats (Hucho et al., 2006).
We also observed a gender-specific effect of PKA on alcohol-induced hyperalgesia. In males the PKA inhibitor had no effect, whereas in intact females and in OvxE females it had a dramatic effect, reversing alcohol-induced in intact females and elevating the mechanical threshold in OvxE females. Of note, estrogen regulates PKA in other types of cells, and PKA in turn mediates several actions of alcohol (Martinez et al., 2003, Belcher et al., 2005, Sedej et al., 2005), including actions in the nervous system (Mize and Alper, 2002, Shingo and Kito, 2002, Shingo and Kito, 2005). The mechanism underlying the sexually dimorphic contribution of PKA and PKCε to pain associated with alcohol-induced neuropathy remains to be determined. While we and others have described a significant role for ERK signaling in rodent models of inflammatory (Aley et al., 2001, Obata and Noguchi, 2004, Dina et al., 2005, Karim et al., 2006, Seino et al., 2006) or neuropathic (Ciruela et al., 2003, Ji, 2004, Ma and Quirion, 2005) pain, our present studies have determined for the first time a major role, albeit not sexually dimorphic, for ERK signaling in alcohol-induced neuropathy, the significance of which will await further investigations.
In conclusion, the present experiments establish a model to study the mechanism underlying the greater severity of alcohol-induced neuropathy in females compared to males. Our results also raise several questions for future research to identify mechanisms that underlie: (1) sexual dimorphism in inflammatory versus alcohol-induced hyperalgesia, (2) the differential contribution of PKA to alcohol-induced neuropathy in males and females and, perhaps of most interest, and (3) the profound resistance of ovariectomized females to alcohol-induced peripheral neuropathy.
Acknowledgments
We thank Mr. Dennis Mendoza for technical assistance with alcohol diet protocols and animal care. This study was supported by NIAAA and ABMRF.
List of abbreviations
- ED
ethanol (6.5 %, v:v) diet
- CD
control diet
- PKA
protein kinase A
- PKCε
protein kinase C (epsilon, ε isoform)
- MEK
mitogen and ERK (extracellular-signal related kinase) kinase
- IM
gonad-intact male
- IF
gonad-intact female
- Ovx
ovariectomized female rat
- OvxE
ovariectomized and estrogen replaced female rat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F. Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol Alcohol. 2000;35:368–371. doi: 10.1093/alcalc/35.4.368. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Bershtein LM, Tsyrlina EV, Poroshina TE, Bychkova NV, Kalinina NM, Gamayunova VB, Kryukova OG, Kovalenko IG, Vasil’ev DA. Induction of the estrogen effect-switching phenomenon by ethanol and its correction. Neurosci Behav Physiol. 2002;32:603–607. doi: 10.1023/a:1020405610682. [DOI] [PubMed] [Google Scholar]
- Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, Munsat TL, Bradley WG. Animal models of alcoholic neuropathy: morphologic, electrophysiologic, and biochemical findings. Muscle Nerve. 1979;2:133–144. doi: 10.1002/mus.880020208. [DOI] [PubMed] [Google Scholar]
- Brain WR, Walton JN. Brain’s Diseases of the Nervous System. Oxford University Press; London, New York: 1969. Disorders of peripheral nerves: Alcoholic Polyneritis. [Google Scholar]
- Ciruela A, Dixon AK, Bramwell S, Gonzalez MI, Pinnock RD, Lee K. Identification of MEK1 as a novel target for the treatment of neuropathic pain. Br J Pharmacol. 2003;138:751–756. doi: 10.1038/sj.bjp.0705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- Diamond I, Messing RO. Alcoholism is the cause of a varirty of neurological disorders. West J Med. 1994;161:279–287. [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Dina OA, McCarter GC, de Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron. 2003;39:613–624. doi: 10.1016/s0896-6273(03)00473-2. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur J Neurosci. 2006 doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Dragland MC, Rothermel JD, Houlihan MJ, Botelho LH. Inhibition of cyclic AMP-dependent protein kinase-induced changes in the kinetic properties of hepatic pyruvate kinase by the specific cyclic AMP antagonist, (Rp)-diastereomer of adenosine cyclic 3′ ,5′-phosphorothioate. J Cyclic Nucleotide Protein Phosphorylation Res. 1985;10:371–382. [PubMed] [Google Scholar]
- Enomoto N, Takei Y, Yamashina S, Ikejima K, Suzuki S, Kitamura T, Sato N. [Gender difference in alcoholic liver injury] Nihon Arukoru Yakubutsu Igakkai Zasshi. 2004;39:163–167. [PubMed] [Google Scholar]
- Fillmore KM. Women’s drinking across the adult life course as compared to men’s. Br J Addict. 1987;82:801–811. doi: 10.1111/j.1360-0443.1987.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Glass DB, Lundquist LJ, Katz BM, Walsh DA. Protein kinase inhibitor-(6-22)-amide peptide analogs with standard and nonstandard amino acid substitutions for phenylalanine 10. Inhibition of cAMP-dependent protein kinase J Biol Chem. 1989;264:14579–14584. [PubMed] [Google Scholar]
- Gomberg ES. Women and alcohol: use and abuse. J Nerv Ment Dis. 1993;181:211–219. doi: 10.1097/00005053-199304000-00001. [DOI] [PubMed] [Google Scholar]
- Green PG, Dahlqvist SR, Isenberg WM, Strausbaugh HJ, Miao FJ, Levine JD. Sex steroid regulation of the inflammatory response: sympathoadrenal dependence in the female rat. J Neurosci. 1999;19:4082–4089. doi: 10.1523/JNEUROSCI.19-10-04082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006 doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- James MF, Duthie AM, Duffy BL, McKeag AM, Rice CP. Analgesic effect of ethyl alcohol. Br J Anaesth. 1978;50:139–141. doi: 10.1093/bja/50.2.139. [DOI] [PubMed] [Google Scholar]
- Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004;5:71–75. [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003a;119:831–838. doi: 10.1016/s0306-4522(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Sexual dimorphism in the contribution of protein kinase C isoforms to nociception in the streptozotocin diabetic rat. Neuroscience. 2003b;120:907–913. doi: 10.1016/s0306-4522(03)00400-7. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Jung ME, Gatch MB, Simpkins JW. Estrogen neuroprotection against the neurotoxic effects of ethanol withdrawal: potential mechanisms. Exp Biol Med (Maywood) 2005;230:8–22. doi: 10.1177/153537020523000102. [DOI] [PubMed] [Google Scholar]
- Karim F, Hu HJ, Adwanikar H, Kaplan DR, Gereau RWt. Impaired Inflammatory Pain and Thermal Hyperalgesia in Mice Expressing Neuron-Specific Dominant Negative Mitogen Activated Protein Kinase Kinase (MEK) Mol Pain. 2006;2:2. doi: 10.1186/1744-8069-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Estrogen regulates adrenal medullary function producing sexual dimorphism in nociceptive threshold and beta-adrenergic receptor-mediated hyperalgesia in the rat. Eur J Neurosci. 2005;21:3379–3386. doi: 10.1111/j.1460-9568.2005.04158.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999a;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999b;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Kielhorn FW. The history of alcoholism: Bruhl-Cramer’s concepts and observations. Addiction. 1996;91:121–128. [PubMed] [Google Scholar]
- Kultz D, Madhany S, Burg MB. Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2 J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- Martinez C, Sanchez M, Hidalgo A, de Boto MJ. Mechanisms of diethylstilbestrol-induced relaxation in rat aorta smooth muscle. Vascul Pharmacol. 2003;40:197–204. doi: 10.1016/j.vph.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17beta-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. 2002;76:339–347. doi: 10.1159/000067583. [DOI] [PubMed] [Google Scholar]
- Monforte RE, Valls-Sole R, Nicolas J, Villata L, Urbana-Marquiz L, A Autonomic and peripheral neuropathies in patients with chronic alcoholism: A dose related toxic effect of alcohol. Arch Neurol. 1995;52:45–51. doi: 10.1001/archneur.1995.00540250049012. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ortiz-Plata A, Palencia G, Garcia E, Perez R, Sotelo J. Ultrastructural changes in limb distal nerves of rats with alcoholism and/or malnutrition before and after dietary correction. J Appl Toxicol. 1998;18:89–92. doi: 10.1002/(sici)1099-1263(199803/04)18:2<89::aid-jat479>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319:71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- Pentney RJ, Quackenbush LJ. Dendritic hypertrophy in Purkinje neurons of old Fischer 344 rats after long-term ethanol treatment. Alcohol Clin Exp Res. 1990;14:878–886. doi: 10.1111/j.1530-0277.1990.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Keast JR. Rapid actions of estradiol on cyclic amp response-element binding protein phosphorylation in dorsal root ganglion neurons. Neuroscience. 2004;129:629–637. doi: 10.1016/j.neuroscience.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflammed tissue. Arch Int Pharmacodyn. 1957;61:409–419. [PubMed] [Google Scholar]
- Rewal M, Wen Y, Wilson A, Simpkins JW, Jung ME. Role of parvalbumin in estrogen protection from ethanol withdrawal syndrome. Alcohol Clin Exp Res. 2005;29:1837–1844. doi: 10.1097/01.alc.0000183013.64829.2e. [DOI] [PubMed] [Google Scholar]
- Sedej S, Rose T, Rupnik M. cAMP increases Ca2+-dependent exocytosis through both PKA and Epac2 in mouse melanotrophs from pituitary tissue slices. J Physiol. 2005;567:799–813. doi: 10.1113/jphysiol.2005.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–203. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estrogen induces elevation of cAMP-dependent protein kinase activity in immortalized hippocampal neurons: imaging in living cells. J Neural Transm. 2002;109:171–174. doi: 10.1007/s007020200012. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. J Neural Transm. 2005;112:1469–1473. doi: 10.1007/s00702-005-0371-8. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JA. Hormone administration: peripheral and intracranial implants. Methods in Psychobiology. 1977:259–279. [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, MAP kinases and neuronal cells: might wine be a neuroprotectant? Drugs Exp Clin Res. 1999;25:99–103. [PubMed] [Google Scholar]
- Tsapis A, Kepes A. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta. 1977;469:1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- Victor M. Polyneuropathy due to nutritional deficiency and alcoholism. Saunders; London: 1975. [Google Scholar]
- Wayneforth HB, Flecknell PA. Experimental and surgical techniques in the rat. Academic Press; London: 1992. [Google Scholar]
- Widdicombe JH, Azizi F, Kang T, Pittet JF. Transient permeabilization of airway epithelium by mucosal water. J Appl Physiol. 1996;81:491–499. doi: 10.1152/jappl.1996.81.1.491. [DOI] [PubMed] [Google Scholar]
- Woodrow KM, Eltherington LG. Feeling no pain: alcohol as an analgesic. Pain. 1988;32:159–163. doi: 10.1016/0304-3959(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zou J, Rabin RA, Pentney RJ. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993;72:75–84. doi: 10.1016/0165-3806(93)90161-3. [DOI] [PubMed] [Google Scholar]


