Abstract
Carboplatin produces progressive damage to auditory nerve fibers, spiral ganglion neurons (SGNs) and inner hair cells (IHC) in the chinchilla cochlea but leaves outer hair cells intact. Within 1 h after injection, many afferent terminals beneath IHCs and myelin lamellae surrounding SGN processes are vacuolated. One day after injection, approximately half of the nerve fibers are missing. IHCs are intact at 2 d, but 20–30% are missing at 3 d. We studied the electrophysiological correlates of this progressive morphological damage by recording cochlear microphonics (CM), distortion product otoacoustic emissions (DPOAE), summating potentials (SP), compound action potentials (CAP), and midbrain evoked potentials (IC-EVP) before and 1 h, 12 h, 1 d, 3 d, 5 d, 7 d and 14 d after carboplatin injection (75 mg/kg IP) in four chinchillas. CM and DPOAEs tended to be unchanged or enhanced. CAP and SP showed little change until Day 3, when amplitudes were reduced in all animals and CAP thresholds were elevated by 9 dB; amplitudes declined further between Days 3 and 5 but not thereafter. IC-EVP amplitudes decreased on Days 3 or 5 but thresholds were relatively unchanged. All animals showed some recovery of IC-EVP between Days 7 and 14, including one with 70% enhancement on Day 14. The results indicate that threshold and amplitude measures fail to detect peripheral pathology until some relatively high threshold level of damage has been exceeded. This has important implications for monitoring peripheral damage and interpreting electrophysiological test results in animals and humans.
Keywords: Chinchilla, electrophysiology, cochlea, myelin, ganglion neurons, plasticity
1. Introduction
Carboplatin, a widely used platinum-based chemotherapy agent, produces an unusual and well documented pattern of damage in the peripheral auditory system of the chinchilla that is unlike the damage produced in other species, including rats, guinea pigs and humans. When administered at relatively low doses, carboplatin destroys sensory inner hair cells (IHCs) and spiral ganglion neurons (SGNs) while leaving the outer hair cells (OHCs) intact and functioning (Ding et al., 1999, 2001; Hofstetter et al., 1997a,b; Trautwein et al., 1996; Takeno et al., 1994; Wake et al., 1993, 1996; Wang et al., 1997, 2003). Damage to the chinchilla cochlea and auditory nerve is rapid and progressive, with vacuolization of nerve terminals beneath IHCs and separation of myelin lamellae surrounding nerve fibers being the earliest observable effects (Ding et al., 1999, 2001; Wang et al., 2003). Wang et al. (2003) observed many small vacuoles in afferent terminals beneath IHCs and separation of myelin layers surrounding nerve fibers in the internal auditory meatus at 1 h after low-dose carboplatin injection (50 mg/kg IP). At 6 h post injection, IHCs still appeared normal while afferent terminals were frequently swollen and myelin was more severely and extensively disrupted. Counts of nerve fibers in the habenula perforata showed losses of approximately 45% at 24 h, 55% at 48 h, and 75% at 72 h post injection, consistent with losses of approximately 50%, 60% and 80% at 24 h, 48 h, and 72 h, respectively, following injection of a moderate dose (100 mg/kg) in a previous study (Ding et al., 2001). At 72 h, nearly all afferent terminals were severely swollen, ruptured or undergoing autolysis. In contrast to the rapid loss of nerve fibers (approximately half within 1 d following injection), IHC counts remained normal until 3 d post-injection, when approximately 20–30% of IHCs were missing.
In most previous studies of carboplatin-treated chinchillas, electrophysiological measurements were made weeks or months after injection, after SGN and IHC loss had stabilized. The aim of the present study was to investigate the effects of early morphological damage in the chinchilla cochlea and auditory nerve on peripheral and central auditory system functioning. Cochlear microphonics (CM) and distortion product otoacoustic emissions (DPOAE) were used to assess OHC function; summating potentials (SP) were used to monitor IHC function; compound action potentials (CAP) were measured to assess the functional integrity of the IHC/afferent fiber synapses and auditory nerve; and evoked potentials measured from the inferior colliculus (IC-EVP) were used to assess central auditory system functioning. By measuring changes in auditory physiology at various levels of the auditory system over time in carboplatin-treated chinchillas, we hoped to identify functional consequences attributable to myelin damage and nerve fiber loss (i.e., the early effects of carboplatin) and compare them to changes following SGN and IHC loss in the same animals.
2. Results
Hair cell loss 14 d after carboplatin injection is shown in Figure 1. The pattern of damage was similar across the four animals, with IHC loss generally increasing from the apical end of the basilar membrane to the basal region. Losses peaked at 77–96% in the region of the basilar membrane representing frequencies between approximately 3 kHz to 9 kHz. OHC loss was negligible (< 2% across the cochlea) in all animals. Averaged across the four animals, IHC loss was 20.5% ± 6.0% in the apical half of the cochlea and 50.7% ± 13.8% in the basal half of the cochlea; mean OHC loss was 1.1% ±1.0% across the entire cochlea.
Figure 1.
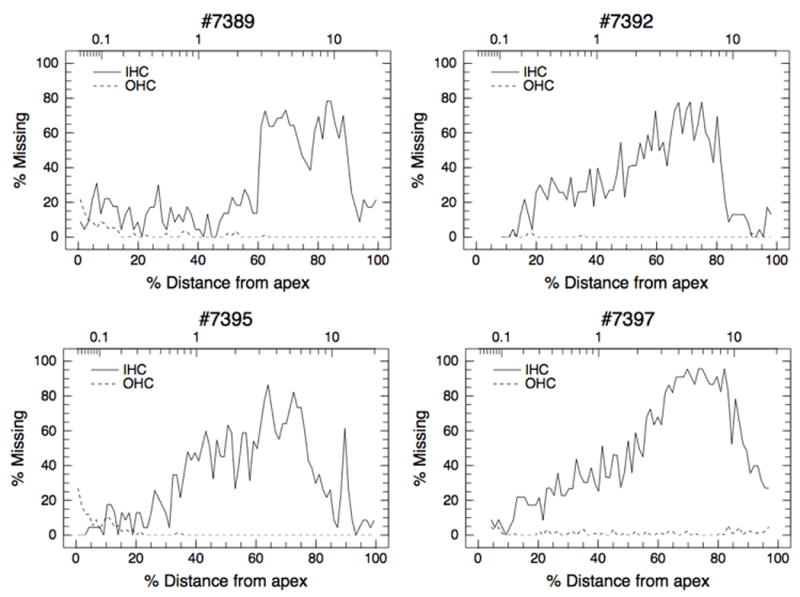
Cochleograms of the individual animals showing hair cell loss two weeks after carboplatin injection. OHC counts were averaged across the three rows.
Figure 2 shows the input/output (I/O) functions for the CM, DPOAE, SP, CAP, and the IC-EVP measured from each animal before carboplatin injection. Figure 3 shows amplitudes (averaged across 70, 75, and 80 dB SPL stimuli and shown as percent of pre-injection values) as a function of carboplatin injection time. Because the results of electrophysiological testing were similar across the three frequencies used in this study (1, 4 and 8 kHz), only the results for 8 kHz stimuli are shown. Post-injection values within 10% (CM, SP, CAP, IC-EVP) or 1 dB SPL (DPOAE) of the pre-injection values are considered to be within the normal range. Note that IHC loss in the 8 kHz region, located approximately 80% distance from the apex along the basilar membrane, ranged from approximately 36% for Chinchilla #7395 to 88% for Chinchilla #7397 (Fig. 1); mean IHC loss in the 8 kHz region was 58.4% ± 21.7%.
Figure 2.
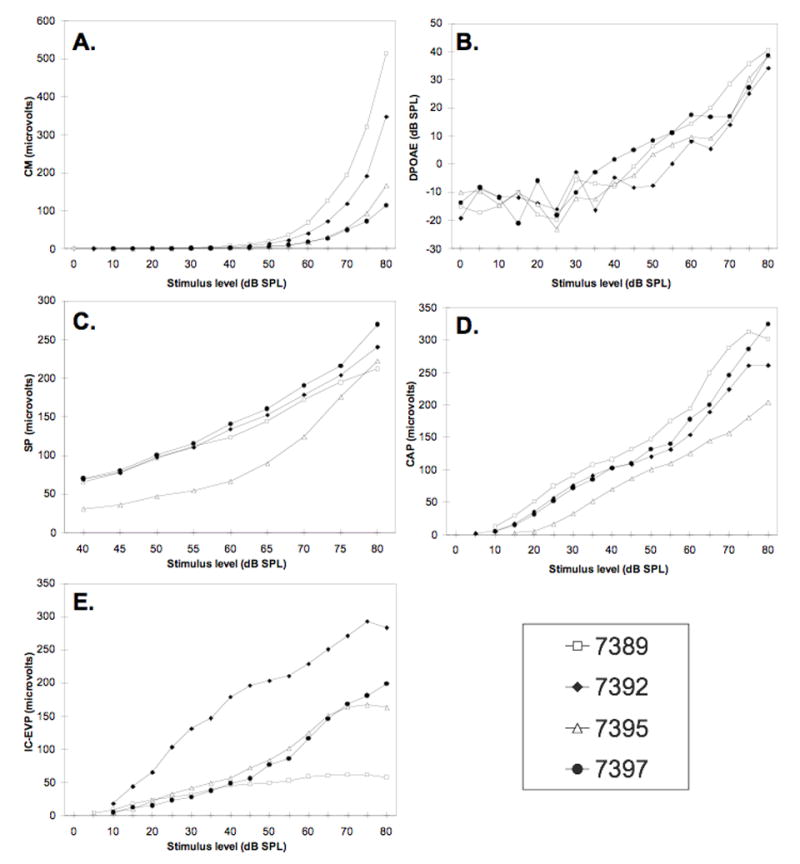
Input/output functions for 8 kHz stimuli measured before carboplatin injection. A. CM; B. DPOAEs; C. SP; D. CAP; E. IC-EVP.
Figure 3.
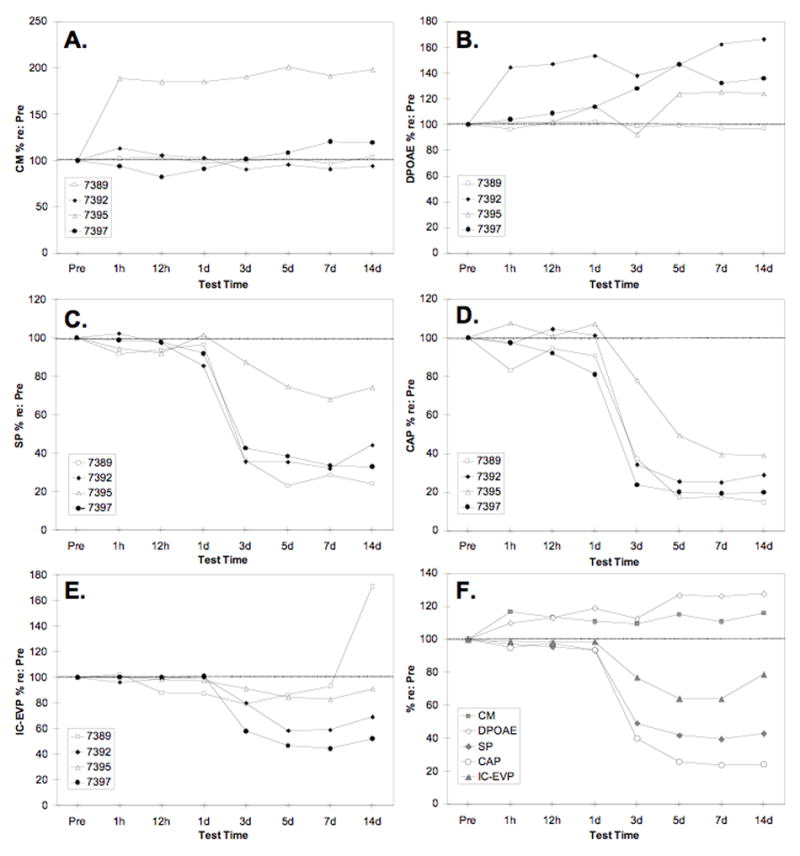
Response amplitudes for 8 kHz stimuli as a function of time relative to carboplatin injection. Amplitudes are expressed as a percentage of pre-injection values (averaged for 70, 75 and 80 dB SPL stimuli; see Fig. 1). Panels A–E show amplitudes of individual animals; panel F shows amplitudes averaged across the four animals. A. CM; B. DPOAEs; C. SP; D. CAP; E. IC-EVP; F. Average CM, DPOAE, SP, CAP and IC-EVP compared.
2.1. The CM and DPOAE
Changes in the CM and DPOAE varied across animals following carboplatin injection (Fig. 3A). Except for a decrease in CM amplitude at 12 h for Chinchilla #7397, however, all post-injection values were either within the normal range or enhanced. Two animals (#7389 and #7392) had CM values within 10% of pre-injection values at all post-injection times; a third animal (#7397) showed an 18% decrease at 12 h, followed by an increase of 20% above the pre-injection value at 7 d; and #7395, the animal with the least amount of IHC loss in the 8 kHz region (Fig. 1C), showed increases of 85–101%, starting 1 h after injection and persisting over the 14 d test period. Three of the four animals showed enhanced DPOAE after carboplatin (Fig. 3B). Whereas #7389 showed little change at any time post-injection, the other three animals demonstrated increases exceeding 10% beginning as early as 1 h (44% for #7397) or 1 d after injection (14% for #7392 and #7395) and generally persisting over the 14 d test period.
Averaged across animals, CM and DPOAE were enhanced at 1 h post-injection and thereafter (Fig. 3F). One-way repeated measures ANOVA (using absolute values of amplitude across 8 measurement times; Greenhouse-Geisser correction used to adjust df for all tests) showed a significant enhancement of the CM, F(1.58, 17.41) = 4.276, p =.038, and the DPOAE, F(2.53, 27.87) = 7.58, p = 0.001, with effect sizes (partial eta2) of .28 and .41, respectively.
2.2. The SP and CAP
Changes in the SP after carboplatin injection are shown in Figure 3C. Three animals showed a relatively steep drop in SP amplitude between Days 1 and 3, with a slower decline over the next 2–4 days. The decline in SP was more gradual for the fourth animal, with an initial decline of 12% on Day 3, 25% on Day 5, and 32% on Day 7; this was also the animal with the least IHC loss in the 8 kHz region of the cochlea (see Fig. 1C). Averaged across animals (Fig. 3F), SP amplitude was reduced by 7% or less until 3 d after the injection, when a reduction of 51% occurred. A further reduction of 7% occurred between Days 3 and 5. Two weeks after injection, SP amplitude was 43% of the pre-injection value. One-way ANOVA confirmed that SP was significantly reduced after carboplatin, F(1.52, 16.76) = 58.23, p < 0.001, eta2 = 0.84. Tukey pairwise comparisons (p < 0.05) showed that the SP declined significantly on Day 3 and between Days 3 and 5, with no further significant reduction thereafter.
The CAP demonstrated the same pattern of changes as the SP, but the magnitude of change was greater (Fig. 3F). As with the SP, the CAP declined precipitously between Days 1 and 3 for chinchillas #7389, 7392 and 7397, and more gradually for #7395 (Fig. 3D). Averaged across animals, CAP amplitude was reduced by 7% or less until Day 3, when a 60% reduction occurred. CAP amplitude declined 24% more between Days 3 and 5 with no further change thereafter. Two weeks after carboplatin injection, CAP amplitude was 24% of the pre-injection value. One-way repeated measures ANOVA, F(1.36, 14.97) = 113.38, p < 0.001, eta2 = 0.98, and Tukey multiple comparison tests (p < 0.05) indicated that the changes described above were statistically significant.
CAP threshold (Fig. 4) was essentially unchanged (± 1 dB SPL) until Day 3, when an elevation of 9 dB SPL occurred. CAP threshold remained relatively stable between Days 3 and 14, with threshold shifts ranging from 9.5 dB on Day 5 to 7.5 dB on Day 14. Repeated measures ANOVA did not detect significant differences in threshold as a function of test time; however, the small sample size limits the power of our statistical test to detect a significant effect.
Figure 4.

Thresholds for 8 kHz stimuli as a function of time. A. CAP thresholds; B. IC-EVP thresholds. Bars show SD.
2.3. The IC-EVP
Changes in the amplitude of the IC-EVP are shown in Figure 3E. For #7389, IC-EVP was normal at 1 h but had decreased by 12% when measured 12 h after injection. The IC-EVP remained depressed on Days 1 and 3 then returned to the normal range on Days 5 and 7. On the final day of measurement, the IC-EVP of #7389 was enhanced 71% above the pre-injection value. In contrast to #7389, chinchillas #7392 and #7397 showed no change until Day 3, when amplitudes declined by 20% and 42%, respectively. Chinchilla #7395, with the least IHC loss, showed the smallest change over the 14 day period; amplitudes of the IC-EVP fell below the normal range on Days 5 and 7 only (reductions of 16% and 17%, respectively). All four animals showed a substantial recovery of response amplitude between 7 and 14 days after injection.
Averaged across animals, the IC-EVP remained normal until 3 d after injection, when a reduction of 23% occurred (Fig. 3F). IC-EVP amplitude declined 13% further between Days 3 and 5, then recovered by 15% between Days 7 and 14. At 14 d post-injection, the amplitude of the IC-EVP was 79% of the pre-injection value. One-way repeated measures ANOVA, F(1.88, 20.69) = 8.58, p = 0.002, eta2 = .44, and Tukey tests (p < 0.05) showed that the declines from Day 1 to 3 and Day 3 to 5 and the recovery between Days 7 and 14 were statistically significant.
Threshold of the IC-EVP changed by less than ±5 dB SPL after injection (Fig. 4). However, consistent with the CAP thresholds, the initial threshold shifts at 1 h and 12 h were in the negative direction (improved thresholds) whereas shifts on Days 3 and thereafter were in the positive direction (elevated thresholds).
3. Discussion
We investigated the peripheral and central auditory effects of progressive damage to myelin, nerve fibers and IHCs in the chinchilla cochlea by recording CM, DPOAE, SP, CAP and IC-EVP at multiple times after carboplatin injection. In addition to showing the early physiological effects of carboplatin in this animal model, the results provide a novel perspective on the sensitivity and specificity of the various physiological measures themselves.
3.1. CAP can be normal despite significant nerve fiber loss and myelin disruption
Previous studies (Ding et al., 1999, 2001; Wang et al., 2003) have shown that carboplatin produces rapid and severe morphological damage to myelin and nerve fibers in the cochlea. Within 1 h after injection, the layers of myelin surrounding nerve fibers are separated and many afferent nerve terminals beneath IHCs are severely swollen. Afferent nerve fibers deteriorate rapidly, with approximately 45–50% of distal fibers missing at 1 d and 55–60% missing at 2 d after injection. Despite the widespread early damage to myelin and loss of approximately 50% of the afferent nerve fibers, there were no significant changes at 1 h, 12 h or 1 d in the threshold or amplitude of the CAP, a measure commonly assumed to be sensitive to neural dysfunction and demyelination. The mean amplitude of the CAP, as well as the SP and IC-EVP, changed by less than 7% until Day 3 and therefore did not exceed changes expected from normal test-retest variability. This result shows that in ears with intact OHCs and IHCs, CAP is surprisingly insensitive to relatively high levels of nerve fiber loss and myelin abnormality.
The most likely explanation for the insensitivity of the CAP to neural damage is that relatively few nerve fibers are required to sustain ostensibly normal function under simple listening conditions. The high degree of divergence from each IHC to multiple afferent nerve fibers (Spoendlin, 1967) may ensure that basic functionality remains despite a 50% or greater reduction of neural channels. An additional factor may be hyperactivity of remaining afferent fibers. Wang et al. (2003) found that surviving auditory nerve fibers have significantly higher mean driven discharge rates than normal at 1, 3, 4 and 5 h after injection, followed by a return to normal levels at 8 h. After cochlear damage has increased and stabilized, surviving auditory nerve fibers have significantly lower mean spontaneous and driven discharge rates than normal (Wang et al., 1997). Thus, it is possible that temporary hyperactivity in surviving afferent nerve fibers contributed to an ostensibly normal CAP in the face of massive nerve fiber damage. Regardless of the underlying reason, the results have important implications for using CAP as the sole indicator of auditory nerve fiber integrity or for monitoring the onset and progression of neural damage when IHCs and OHCs are intact. By the time electrophysiological changes are observed, cochlear damage may be more advanced than previously recognized.
3.2. Late cochlear damage is associated with reliable depression of CAP and SP
The first significant decline of CAP and SP amplitudes occurred between Day 1 (when approximately 50% of afferent nerve fibers were missing) and Day 3 (when approximately 75–80% of afferent fibers and 20–30% of IHCs were missing and nearly all afferent terminals were morphologically abnormal). It appears that the additional loss of afferent nerve fibers and the loss of IHCs between Days 1 and 3 exceeded a critical level of damage for producing measurable changes in electrophysiological potentials. After the critical damage level had been exceeded, changes in SP and CAP were consistent across animals. Changes in CAP were large and reliable, resulting in an effect size of .98, followed by an effect size of .84 for the SP.
Previous studies have suggested that CAP amplitude recorded from the RW is a sensitive index of the magnitude of IHC loss (Wang et al., 1997; Qui et al., 2000). Consistent with this, the animal with the smallest decrease in CAP amplitude (#7395; Fig. 3D) also had the least IHC loss in the 8 kHz region (Fig. 1). However, it is important to emphasize that the correlation between CAP amplitude and IHC loss is indirect, mediated by the loss of nerve fibers or disruption of IHC/afferent fiber synapses rather than a loss of IHCs per se. In chinchillas treated with kainic acid (Zheng et al., 1997, 1999), CAP was abolished even though IHCs were morphologically normal and SP recorded from the round window was within the normal range. Thus, CAP can be affected independently of IHC loss by disrupting the synapse between the IHC and the afferent fibers innervating them or by damaging afferent nerve fibers, and CAP depression should not be interpreted as a sign of IHC loss.
Bearing in mind that our results are based on a small sample of four animals, the SP appears to be an equally sensitive, and perhaps more specific, index of IHC loss when OHCs are intact. Overall, a 58% loss of IHCs in the 8 kHz region of the cochlea resulted in a 57% reduction in the amplitude of the SP. These results are consistent with evidence from other studies suggesting that IHCs are the major source of the SP recorded from the round window (Durrant et al., 1998; Zheng et al., 1997). Zheng et al. (1997) recorded SP, DPOAE, CM and CAP from chinchillas that underwent surgery for application of kainic acid to the round window membrane or transection of the auditory nerve. Kainic acid produced selective excitotoxic damage to most if not all IHC/afferent fiber synapses but spared IHCs, whereas surgical deafferentation caused secondary loss of IHCs, primarily in the basal half of the cochlea. CM and DPOAE were relatively unchanged in both groups; SP was somewhat enhanced in the kainic acid group with morphologically normal IHCs but significantly decreased in the group with IHC loss. Results such as these suggest that the decline of SP may serve as a specific marker of IHC damage in ears with intact OHCs.
3.3. CM and DPOAE may be enhanced after nerve fiber loss
As in previous studies (e.g., Jock et al., 1996; Trautwein et al., 1996; Takeno et al., 1994), there was no significant depression of CM or DPOAE at any time following carboplatin injection. The results provide further evidence that OHCs are the major source for the CM and DPOAE with little or no contribution from the IHCs or the auditory nerve afferents (Dallos et al., 1972; Martin et al., 1987; Schrott et al., 1991). When substantial changes in CM or DPOAE occurred, they were in the direction of enhancement. However, the effects of cochlear damage on CM and DPOAE were variable across animals and time. Two animals showed substantial enhancement of the CM, but with different time courses and magnitudes (Fig. 3A). Three animals showed clearly enhanced DPOAE, again with different time courses and magnitudes (Fig. 3B). For both CM and DPOAE, enhancement could be seen as early as 1 h after carboplatin injection. Averaged across animals, both the CM and DPOAE enhancements were significant at 1 h post-injection, although the effect size was larger for the DPOAE (eta2 = .41) than the CM (eta2 = .28).
Enhancement of the OAE after carboplatin injection was reported by Takeno et al. (1994) for the click evoked otoacoustic emission (CEOAE) and by Jock et al. (1996) for the DPOAE. These investigators speculated that enhancement was due to the loss of tonic medial olivocochlear (MOC) efferent feedback to the OHCs as a result of IHC loss and reduced input from the afferent arm of the feedback loop. The MOC feedback circuit involves sound-driven neurons in the medial superior olivary region that synapse with OHCs in the cochlea and decrease cochlear output by modulating basilar membrane movement (McFadden, 2007). Reduced input to these brainstem neurons as a result of peripheral damage might be expected to decrease or eliminate the inhibitory effects of MOC efferents on OHC activity, resulting in enhanced OAEs and CM. Our results are not inconsistent with this interpretation, and they show that IHC loss is not a necessary condition to trigger changes consistent with enhanced cochlear sensitivity. However, this explanation involving reduced MOC hyperpolarization of the OHCs may not be sufficient to account for the OAE enhancement observed in the current study. We have shown previously that chronic cochlear deefferentation enhances DPOAE amplitudes at low frequencies (1 and 2 kHz) but not at higher frequencies (4 and 8 kHz) in the chinchilla (Zheng et al., 2000). Thus, the mechanism underlying OAE enhancement remains to be elucidated. It is interesting to note that enhanced sensitivity was also evident in slightly lower thresholds of both the CAP and the IC-EVP at 1 h and 12 h post-injection (Fig. 4).
Although both the CM and DPOAE showed enhancement after carboplatin injection in the current study, the pattern of enhancement was different for each function. This finding suggests that although both functions are linked to the OHC and could involve the MOC efferent system, the process that governs the amplification of each function after peripheral loss is different. The one animal with substantial CM enhancement at 1 h (#7395) did not show reliable DPOAE enhancement until Day 5, and the animal with the earliest (1 h) and largest (50–70%) enhancement of DPOAE (#7392) had normal CM at all times after injection. Dissociation between CM and DPOAE effects following cochlear damage is a well known phenomenon (e.g., Zheng et al., 2000); however, DPOAE is typically normal or enhanced while CM is typically normal or depressed rather than enhanced as in the current study. The most obvious reasons for this discrepancy are sample size and the number of animals showing a particular effect. In the current study, the CM enhancement effect was primarily due to one animal that showed substantial enhancement at all post-injection times. In a large sample, data from this single animal would be lost in the mean. Here, however, we can note that CM enhancement, although apparently rare, is one possible outcome of carboplatin-induced cochlear damage. The factors governing the variety of changes and time courses seen in CM and DPOAE remain to be determined.
3.4. The IC-EVP showed significant recovery after one week
Like the SP and CAP, the first significant decline of IC-EVP amplitude occurred 3 d after injection. However, the IC-EVP was less sensitive to cochlear damage than the CAP or SP, as evidenced by a much smaller effect size (eta2 = .44). As shown in Figure 3, changes in the IC-EVP were less pronounced and more variable among animals than were changes in the CAP or SP. Moreover, there was significant recovery of IC-EVP amplitude between 7 and 14 d. Three animals showed partial recovery of IC-EVP amplitude, and the fourth showed not only recovery but response enhancement at 14 d. It is interesting that the recovery and enhancement occurred after SP and CAP had stabilized (Fig. 3D). The results are consistent with previous data showing faster recovery of IC-EVP amplitudes than CAP amplitudes following temporary deafferentation by kainic acid (Zheng et al., 2000). The results are also consistent with previous studies showing that evoked response amplitudes can decrease, increase or remain unchanged after carboplatin, depending on the recording site. The amplitude of the CAP recorded from the round window decreases in proportion to the magnitude of IHC loss, whereas evoked potentials recorded from the IC and the auditory cortex can decrease, increase or remain unchanged in the same animals (Burkard et al., 1997; McFadden et al., 1998; Qui et al., 2000). It is not clear what governs the recovery or the enhancement of the IC-EVP amplitude after the initial reduction, but we have previously speculated that enhancement may result from a reduction of tonic inhibitory input to IC neurons, most likely via a circuit originating in the cochlear nucleus (McFadden et al. 1998). Why one animal showed an enhancement of the IC-EVP amplitude while others did not is not clear. However, it is interesting to note that the one animal showing IC-EVP enhancement (#7389) also showed the largest reduction of CAP and SP but not the most IHC loss.
3.5. CAP and IC-EVP thresholds
CAP and IC-EVP thresholds were remarkably insensitive to both massive neural damage and IHC loss in animals with intact OHCs. IC-EVP threshold showed a 4 dB improvement at 12 h, and was ±2 dB of the pre-injection threshold at all other times. The maximum CAP threshold elevation was 9.5 dB at 5 d. Even when more than 75% of nerve fibers and 50% of IHCs (Fig. 1) were missing at 14 d, CAP threshold was elevated by less than 8 dB. Similar results were reported by others (e.g., McFadden et al., 1998; Salvi et al. 2000) and can be explained by findings of Wang et al. (1997) and Wake et al. (1996) that auditory nerve fibers and IC neurons have normal thresholds, tuning, and characteristic frequencies despite massive IHC loss. It is clear that the CAP and IC-EVP amplitudes are more sensitive to the effect of IHC and auditory nerve fiber loss than thresholds and that relatively few IHCs and auditory nerve fibers are required to maintain normal threshold of the CAP and IC-EVP (Qiu et al., 2000; Salvi et al., 2000; Wang et al., 1997). The fact that both peripheral (CAP) and central (IC-EVP) threshold measures were relatively unaffected by early morphological damage cautions against invoking “central plasticity” as an explanatory mechanism for preserved central auditory system thresholds in these animals.
3.6. Summary and conclusions
Early morphological damage to auditory nerve fibers and myelin were not associated with reliable changes in the electrophysiological measures used in the current study, even though previous studies indicate that the nerve fiber population was reduced by approximately 50%. As nerve fiber damage progressed and IHC loss occurred, CAP, SP and IC-EVP amplitudes significantly declined. CAP and SP remained depressed, while IC-EVP amplitudes recovered significantly over time, suggesting central auditory system plasticity in the face of peripheral damage. In the same animals, CM and DPOAEs could be normal or enhanced. This collection of results points to a relative insensitivity of the CAP and other measures to cochlear damage when OHCs are intact. The critical level of neural damage for triggering measurable changes in CAP is higher than previously recognized. Thus, results of electrophysiological testing should be interpreted cautiously and in the context of other results when using them to infer or monitor cochlear damage. Furthermore, both peripheral and central measures should be available when interpreting the effects of peripheral damage on the central auditory system, as it cannot be assumed that peripheral damage will result in measurable changes in peripheral auditory function. More sensitive and specific measures of cochlear damage are clearly needed for experimental and clinical use.
4. Experimental Procedure
4.1. Subjects and electrode implantation surgery
Four adult chinchillas (Chinchilla laniger) obtained from a vendor licensed by the U.S. Department of Agriculture (Jarr Chinchilla Inc., Hubbard, OH) underwent surgery for implantation of three recording electrodes. Animals were anesthetized with an intramuscular injection of ketamine (50 mg/kg) and acepromazine (0.3 mg/kg), and placed on a Deltaphase® Isothermal Pad (Braintree Scientific, Inc., Braintree, MA) in a stereotaxic apparatus. A postauricular incision was made to expose the cochlear part of the right bulla and a small hole was made in the dorsal bulla. A silver-ball electrode was placed against the round window (RW) niche and fixed to the bulla with dental cement. The dorsal cranium was then exposed and small holes were drilled in the rostral cranium and in the region above the left inferior colliculus (IC) for implantation of ground and midbrain recording electrodes, respectively (McFadden et al., 1997a). Electrodes for IC recordings were Teflon-coated tungsten wire with a bared tip at one end and a gold-plated male connector pin soldered to the other end. As described elsewhere (McFadden et al., 1998), the low-impedance (typically 20–50 k) electrodes pick up electrical activity from a very wide region of the auditory midbrain and thresholds are independent of electrode placement and impedance. The electrode was inserted into the midbrain to a depth that evoked a clear two-phase waveform in response to clicks delivered to the left ear. Electrodes were fixed to the skull using dental cement. Buprenorphine (0.05 mg/kg) was administered subcutaneously for 3 days after surgery, and animals were allowed to recover for at least 14 days before electrophysiological testing was initiated.
4.2. Electrophysiology and carboplatin injection
Physiological recording was conducted in a single-walled sound booth, with the awake animal placed in a custom-designed animal restraint (Snyder et al., 1994). Electrical activity was recorded in response to stimuli presented to the right ears of the animals through an insert earphone (Etymotic ER-2). The CM, SP and CAP were recorded from the RW electrode, and the IC-EVP was recorded from the IC electrode. DPOAEs generated in response to stimuli presented to the right ears through insert earphones (Etymotic ER-2) were recorded using an Etymotic 10B microphone. After baseline physiological measures were obtained, each animal received a single injection of carboplatin (75 mg/kg IP; LKT Laboratories, Inc.). Electrophysiological measures were then obtained 1 h, 12 h, 1 d, 3 d, 5 d, 7 d, and 14 d after injection.
Stimuli for the CM, CAP, SP and IC-EVP were tone bursts at 1, 4, and 8 kHz, generated digitally using an array processor (TDT AP2) and a 16-bit D/A converter (TDT DA 3–4). The signals were attenuated with a programmable attenuator (TDT PA5), then routed through a headphone buffer (TDT HB7) to the insert earphone. Repetition rates were 10.1/s for the CAP and IC-EVP, 21/s for the SP, and 6.1/s for the CM. Stimulus duration was 5 ms for the CAP and IC-EVP, 15 ms for the SP, and 50 ms for the CM. Rise/fall time was shaped with a cos2 window of 2 cycles of the stimulus frequency. Stimulus level was decremented in 5 dB steps from 80 dB SPL to 0 dB SPL (CM, CAP and IC-EVP) or 40 dB SPL (SP).
Evoked responses were band-pass filtered (100–3000 Hz for the CAP and IC-EVP; 5–300 Hz for the SP; 100 Hz-15 kHz for the CM), amplified 10,000 x using a bioamplifier (TDT DB4), and digitized using an A/D converter (TDT AD1). One hundred sweeps were averaged for each stimulus level.
Input-output functions for the DPOAE were obtained using two primary tones, f1 and f2, where f2 / f1 = 1.2 and f1 frequency ranged from 1 kHz to 8 kHz in octave steps. Stimuli were digitally generated and attenuated (McFadden et al., 1997b). Stimulus level was incremented in 5 dB steps from 0 to 80 dB SPL; L1-L2 = 10 dB SPL.
4.3. Cochleograms
Chinchillas were deeply anesthetized and perfused intracardially with phosphate buffered saline (PBS, phosphate buffer pH 7.4 at 37°C) followed by 2.5% glutaraldehyde in PBS. The animals were then decapitated and the right bulla was quickly removed and dissected. The cochlea was immersed in fixative for 24 h, then dissected to obtain a surface preparation of the basilar membrane. Specimens were stained with hematoxylin and hair cell loss was assessed by microscopic inspection. A cochleogram showing the percent of IHC and OHC loss as a function of percent distance from the apex was constructed for each ear (Ding et al., 2001). Position in the cochlea was translated to frequency using a generalized cochlear frequency-place map (Greenwood, 1990).
All procedures regarding the use and care of animals in this study were reviewed and approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
Acknowledgments
This work was supported by NIDCD grant P01 DC03600 (SLM). The authors gratefully acknowledge Dalian Ding, Center for Hearing and Deafness, University at Buffalo, for preparing the cochleograms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burkard R, Trautwein P, Salvi R. The effects of click level, click rate, and level of background masking noise on the inferior colliculus potential (ICP) in the normal and carboplatin-treated chinchilla. J Acoust Soc Am. 1997;102:3620–7. doi: 10.1121/1.420149. [DOI] [PubMed] [Google Scholar]
- 2.Dallos P, Billone MC, Durrant JD, Wang C, Raynor S. Cochlear inner and outer hair cells: functional differences. Science. 1972;177:356–8. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- 3.Ding D, McFadden SL, Salvi R. Calpain immunoreactivity and morphological damage in chinchilla inner ears after carboplatin. JARO. 2001;3:68–79. doi: 10.1007/s101620020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding D, Wang R, Salvi R, Henderson D, Hu BH, McFadden SL, Mueller M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999;884:152–70. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 5.Durrant JD, Wang J, Ding DL, Salvi RJ. Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am. 1998;104:370–7. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood DD. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 7.Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 8.Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36:301–11. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- 9.Jock BM, Hamernik RP, Aldrich LG, Ahroon WA, Petriello KL, Johnson AR. Evoked-potential thresholds and cubic distortion product otoacoustic emissions in the chinchilla following carboplatin treatment and noise exposure. Hear Res. 1996;96:179–90. doi: 10.1016/0378-5955(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 10.Martin GK, Lonsbury-Martin BL, Probst R, Scheinin SA, Coats AC. Acoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure-tone exposures. Hear Res. 1987;28:191–208. doi: 10.1016/0378-5955(87)90049-9. [DOI] [PubMed] [Google Scholar]
- 11.McFadden SL, Campo P, Quaranta N, Henderson D. Age-related decline of auditory function in the chinchilla (Chinchilla laniger) Hear Res. 1997a;111:114–26. doi: 10.1016/s0378-5955(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 12.McFadden SL, Kasper C, Ostrowski J, Ding D, Salvi RJ. Effects of inner hair cell loss on inferior colliculus evoked potential thresholds, amplitudes and forward masking functions in chinchillas. Hear Res. 1998;120:121–32. doi: 10.1016/s0378-5955(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 13.McFadden SL, Quaranta N, Henderson D. Suprathreshold measures of auditory function in the aging chinchilla. Hear Res. 1997b;111:127–35. doi: 10.1016/s0378-5955(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 14.McFadden SL, editor. Thomson Delmar Learning. 2007. Biochemical Bases of Hearing. K.C.M. Campbell, Pharmacology and Ototoxicity for Audiologists; pp. 86–123. [Google Scholar]
- 15.Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139:153–71. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 16.Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Health. 2000;2:9–26. [PubMed] [Google Scholar]
- 17.Schrott A, Puel JL, Rebillard G. Cochlear origin of 2f1-f2 distortion products assessed by using 2 types of mutant mice. Hear Res. 1991;52:245–53. doi: 10.1016/0378-5955(91)90204-m. [DOI] [PubMed] [Google Scholar]
- 18.Snyder D, Salvi R. A novel chinchilla restraint device. Lab Anim. 1994;23:42–44. [Google Scholar]
- 19.Spoendlin H. The innervation of the organ of Corti. J Laryngol Otol. 1967;81:717–38. doi: 10.1017/s0022215100067669. [DOI] [PubMed] [Google Scholar]
- 20.Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 21.Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 22.Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatoin ototoxicity: an animal model. The Journal of Laryngology and Otology. 1993;107:585–589. doi: 10.1017/s0022215100123771. [DOI] [PubMed] [Google Scholar]
- 23.Wake M, Takeno S, Mount RJ, Harrison RV. Recording from the inferior colliculus following cochlear inner hair cell damage. Acta Otolaryngol. 1996;116:714–20. doi: 10.3109/00016489609137912. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107:67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Xi-Y, Ding D-L, McFadden SL, Henderson D. Evidence that inner hair cells are the major source of cochlear summating potential. Hear Res. 1997:76–88. doi: 10.1016/s0378-5955(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 27.Zheng XY, McFadden SL, Henderson D, Ding DL, Burkard R. Cochlear microphonics and otoacoustic emissions in chronically de-efferented chinchilla. Hear Res. 2000;143:14–22. doi: 10.1016/s0378-5955(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 28.Zheng XY, Salvi RJ, McFadden SL, Ding DL, Henderson D. Recovery of kainic acid excitotoxicity in chinchilla cochlea. Ann N Y Acad Sci. 1999;884:255–69. doi: 10.1111/j.1749-6632.1999.tb08647.x. [DOI] [PubMed] [Google Scholar]


