Abstract
We have recently demonstrated that the severity of sleep-disordered breathing in obstructive sleep apnoea hypopnoea syndrome (OSAHS) can be reduced by lowering the surface tension (γ) of the upper airway lining liquid (UAL). Morning xerostomia (related to oral breathing during sleep) is reported by most OSAHS patients. In the present study we examine relationships between breathing route, oral mucosal ‘wetness’ and the γ of UAL. We studied eight healthy subjects (age, 25 ± 5 years [mean ± s.d.]; body-mass index, 23 ± 2 kg m−2) during a 120 min challenge of both nasal-only breathing (mouth taped) and oral-only breathing (nose clip), each on a separate day (randomized). Both oral mucosal ‘wetness’ (5 s contact gravimetric absorbent paper strip method) and the γ (‘pull-off’ force technique) of 0.2 μl samples of UAL obtained from the posterior pharyngeal wall were measured at 15 min intervals (mouth tape removed and replaced as required). Upper airway mucosal ‘wetness’ increased during 120 min of nasal breathing from 4.0 ± 0.4 (mean ± s.e.m.) to 5.3 ± 0.3 μl (5 s)−1 but decreased from 4.5 ± 0.4 to 0.1 ± 0.2 μl (5 s)−1 with oral breathing (both P < 0.001, repeated-measures ANOVA, Tukey's multiple comparison test, post hoc test). Concurrently, the γ of UAL decreased from 59.3 ± 2.2 to 51.8 ± 0.98 mN m−1 with nasal breathing but increased from 64.4 ± 2.7 to 77.4 ± 1.1 mN m−1 with oral breathing (P < 0.001). For the group and all conditions studied, γ of UAL values strongly correlated with upper airway mucosal ‘wetness’ (correlation coefficient, r2=−0.34, P < 0.001; linear regression). We conclude that oral breathing increases and nasal breathing decreases the γ of UAL in healthy subjects during wakefulness. We speculate that nasal breathing in OSAHS patients during sleep may promote a low γ of UAL that may contribute to reducing the severity of sleep-disordered breathing.
We have previously shown that the surface tension (γ) of upper airway lining liquid (UAL) plays an important role in the control of upper airway patency (Kirkness et al. 2003a,b,c). For example, in both awake (Van der Touw et al. 1997) and anaesthetized humans (Kirkness et al. 2003b) the application of exogenous surfactant decreases the intraluminal pressure required to reopen a closed pharyngeal airway. In anaesthetized rabbits upper airway reopening and closing pressures are both strongly correlated with γ of UAL values (Kirkness et al. 2003a). In addition, a number of studies, including our own, have shown that the instillation of exogenous surfactant into the upper airway of patients with obstructive sleep apnoea hypoponea syndrome (OSAHS) reduces the severity of the associated sleep-disordered breathing (Jokic et al. 1998; Morrell et al. 2002; Kirkness et al. 2003c).
Oral versus nasal breathing has been long recognized as a potential influence on upper airway patency during sleep. Mouth opening occurs during sleep in both healthy subjects and OSAHS patients (Miyamoto et al. 1998, 1999) and may increase UA collapsibility, thereby contributing to the occurrence of sleep-related breathing abnormalities (Meurice et al. 1996). Route dependence of upper airway muscle activity (Basner et al. 1989) and upper airway wall compliance (Meurice et al. 1996) have both been suggested as mechanisms that may increase upper airway collapsibility during mouth breathing. In addition, Olson et al. 1981 (Olson & Strohl, 1988) have pointed out that mouth breathing during sleep has the potential to increase the ‘dryness’ of the upper airway mucosal surface. Subsequent increased wall ‘stickiness’ may then make the upper airway more difficult to reopen after closure.
Xerostomia upon awakening from sleep is reported by ∼74% of OSAHS patients (Kales et al. 1985; American Sleep Disorders Association, 1997), suggesting that upper airway mucosal drying, presumably associated with mouth breathing, may be relatively common in these patients. We have also recently demonstrated that older individuals have increased oronasal breathing during sleep (Madronio et al. 2004) and that, in both healthy subjects and patients with OSAHS, nasal breathing during sleep, as opposed to mouth breathing, is associated with a lower γ of UAL (Kirkness et al. 2005b). When this finding is combined with our previous work describing the effects of adding surfactant to the upper airway, it seems plausible that changes in γ of UAL may be implicated in breathing route influences on sleep-disordered breathing. However, there are no reported studies linking breathing route, upper airway mucosal ‘wetness’ and γ of UAL. These relationships are not understood in healthy human subjects, let alone OSAHS patients. Consequently, the aim of the present study was to define the normal physiological impact of nasal versus oral breathing on both the ‘wetness’ of the pharyngeal wall and γ of UAL values.
Methods
Subjects
We studied eight healthy, awake, volunteer subjects (2 male, 6 female; age, 25.0 ± 5.3 years [mean ± s.d.], body-mass index, 23 ± 2 kg m−2). Subjects were non-smokers, were not taking any medications, and had no history of OSAHS. Written, informed consent was obtained, the protocol was approved by the Western Sydney Area Health Service Human Ethics Committee and the study conformed with the Declaration of Helsinki.
Study design
Subjects performed separate 120 min challenge periods of enforced nasal and enforced oral breathing on two separate days while ventilation, breathing route and swallowing were monitored continuously, and perception of ‘dry mouth’, upper airway mucosal ‘wetness’, and γ of UAL measures were obtained at 15 min intervals. The 120 min study period was chosen in order to have the intervention duration long enough to allow collection of time course data over a period with some relevance to a sleep cycle period and also represented a maximum tolerable mouth breathing period for awake subjects.
Ventilation
Ventilation was monitored using respiratory inductance plethysmography. Inductive bands were fitted around the ribcage and the abdomen, and the system was calibrated using the isovolume method as described by Millman et al. (1986). Data were recorded as the sum of the ribcage and abdomen signals.
Swallowing
Swallows were detected via a recording of the submental electromyogram (EMG). Electrodes were positioned on the skin surface below the chin and connected to an amplifier system (Neotrace NT, 1900, Neomedix Systems, Sydney, NSW, Australia). The EMG signals were filtered (50 Hz to 1 kHz), rectified and integrated with a time constant of 100 ms. Correct placement of the electrodes was established by confirming that a phasic EMG signal occurred in association with a voluntary swallow. The integrated EMG signal was then monitored continuously throughout the protocol. The number of swallows occurring during each challenge period was quantified as the number of phasic submental EMG peaks detected.
Breathing route
Breathing route was monitored using a dual-channel thermocouple device (F-ONT2A, Grass Telefactor, West Warwick, RI, USA) with separate oral and nasal sensors.
Perception of ‘dry mouth’
Two minutes prior to the collection of each upper airway mucosal ‘wetness’ measurement, subjects rated, using a 19 cm visual analog scale (Chandra et al. 1994), their response to the instruction: rate the dryness of your mouth from ‘not dry at all’ to ‘very dry’.
Upper airway mucosal ‘wetness’
Upper airway mucosal ‘wetness’ measurements were made using a timed, gravimetric absorbent paper strip method (Ciantar & Caruana, 1998). A purpose-designed paper strip (SialoPaper Collection Strips, Ora Flow, Inc., Plainview, NY, USA); total surface area, 44.15 mm2; dry weight, 8.5 mg) was placed in contact with the back of the tongue for 5 s. The volume of absorbed fluid (in μl) was obtained by comparing the weight of the strip before and after fluid absorption.
Surface tension measurements
Samples of UAL for the γ measurement were collected from the posterior pharyngeal wall using fine-bore polyethylene tubing (i.d. 0.5 mm; o.d. 0.8 mm) with an attached 1 ml syringe (1 ml, Terumo Medical Corp., Albertion, MD, USA), which was used to draw a small quantity (∼1.0 μl) of the UAL into the tubing (Kirkness et al. 2005a). Samples were then transferred with a 1 μl syringe (7500.5N, Hamilton Company, Reno, NV, USA) to the surface force measurement device.
The γ of UAL was measured via the ‘pull-off’ force technique according to the procedure described by Kirkness et al. (2005a). This approach uses the force required to separate two silica surfaces bridged by a droplet (∼0.2 μl) of the test liquid to estimate the γ of the liquid sample.
Protocol
Subjects were studied on two separate days, randomized to mouth breathing on one day and nasal breathing on the other, and were instructed not to consume any food or drink for 30 min prior to study. Following instrumentation, subjects rated mouth ‘dryness’ and a measurement of upper airway mucosal ‘wetness’ and γ of UAL was obtained. Subjects then completed a 30 min ‘run-in’ period of nasal-only breathing, followed by a 120 min challenge period of either enforced nasal-only (mouth taped) or oral-only breathing (nose clip). During both the run-in and challenge periods, ventilation, submental EMG and thermocouple signals were monitored continuously, while measurements of subject perception of mouth ‘dryness’ (unmarked scale presented on each occasion), upper airway mucosal ‘wetness’ and γ of UAL were made at 15 min intervals. For sampling purposes, mouth tape was removed and replaced as required. Inductive plethysmography, EMG and thermocouple signals were digitized at 1 kHz (PowerLab/8sp, ADInstruments, Sydney, NSW, Australia), and stored on a Macintosh computer for further analysis.
Data analysis
For each 15 min breathing period, the following data were obtained: minute ventilation; swallowing frequency (number of swallows per 15 min); perception of mouth ‘dryness’; upper airway mucosal ‘wetness’; and γ of UAL. Individual subject data were pooled to obtain group mean (± s.e.m.) values.
Repeated-measures ANOVA with Tukey's post hoc tests were used to determine differences between nasal and oral breathing at the same time point and to examine differences between time points for each breathing route. Univariate relationships were explored using unadjusted linear regression. P ≤ 0.05 was considered significant.
Results
Breathing route
In all subjects, for all conditions, exclusive nasal or oral breathing was confirmed via inspection of the nasal and oral thermocouple signals (Fig. 1).
Figure 1. A 1 min raw data period obtained from one subject during both the nasal and oral breathing challenges.
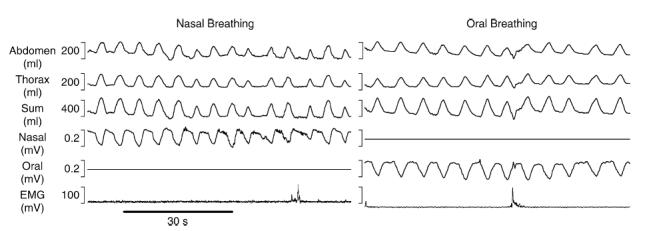
The traces show signals from respiratory inductance plethysmography (thorax, abdomen, thorax + abdomen = sum), breathing route thermocouples (nasal and oral) and submental electromyography (EMG). Note that there is no oral breathing during the nasal breathing challenge and similarly no nasal breathing during the oral breathing challenge. A single swallow is evident on the EMG trace during nasal and oral breathing.
Ventilation
There was no significant change in ventilation from the ‘run-in’ period during either the oral or nasal challenge period (all P > 0.05). Group mean ventilation at all time points (including during the nasal breathing ‘run-in’ period) was ∼10% higher on the oral breathing day (e.g. at 120 min, 4.5 ± 0.2 l min−1) than on the nasal breathing day (e.g. at 120 min, 4.1 ± 0.2 l min−1) but this difference between nasal and oral breathing was not statistically significant (P > 0.05).
Swallowing frequency
Swallowing frequency did not change during either ‘run-in’ period (all P > 0.05) and was not significantly different for the ‘run-in’ periods on the nasal breathing day and the oral breathing day (P > 0.05). However, swallowing frequency progressively increased with oral breathing such that during the oral breathing challenge period, the swallowing frequency significantly increased from 14 ± 1 swallows (15 min)−1 at 0 min to 25 ± 2 swallows (15 min)−1 at 120 min (P < 0.001). For the nasal breathing challenge, there was a decrease in swallowing frequency from 17 ± 1 swallows (15 min)−1 at 0 min to 10 ± 1 swallows (15 min)−1 at 120 min (P < 0.05). A significant difference between the oral and nasal breathing data for swallowing frequency was first achieved at 90 min into the challenge period (P < 0.001).
Upper airway mucosal ‘wetness’
Upper airway mucosal ‘wetness’ values during the ‘run-in’ period progressively increased on both test days (P < 0.001) but were not significantly different between test days (P > 0.08). During the nasal breathing challenge, upper airway mucosal ‘wetness’ increased from 4.0 ± 0.4 μl (5 s)−1 at 0 min to 5.3 ± 0.3 μl (5 s)−1 at 120 min (P < 0.001; Fig. 2). However, during the oral breathing challenge, ‘wetness’ values progressively decreased from 4.5 ± 0.4 μl (5 s)−1 at 0 min to 0.10 ± 0.20 μl (5 s)−1 at 120 min (P < 0.001). A significant difference between the oral and nasal breathing data for upper airway mucosal ‘wetness’ was first achieved at 15 min into the challenge period (P < 0.001; Fig. 2).
Figure 2. Group mean ± s.e.m. upper airway mucosal ‘wetness’ values during nasal and oral breathing challenge periods.
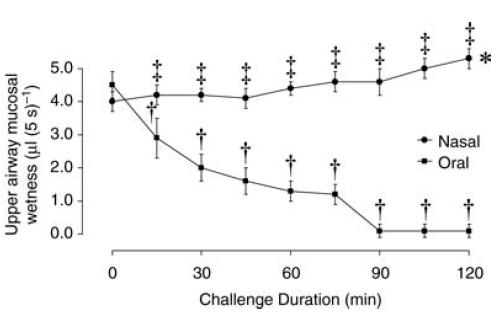
*P < 0.001 compared to time 0 min nasal breathing challenge; †P < 0.001 compared to time 0 min oral breathing challenge; and ‡P < 0.001 nasal compared to oral breathing challenge at respective 15 min intervals.
Perception of ‘dry mouth’
Mouth ‘dryness’ perception values at the commencement of the ‘run-in’ periods were not significantly different between the oral breathing challenge (1.3 ± 0.6 cm) and the nasal breathing challenge (1.5 ± 0.3 cm, P > 0.05) and also did not change during the ‘run-in’ period on either day (P > 0.08). However, perception of mouth ‘dryness’ progressively increased during the oral breathing challenge period, reaching 11.7 ± 1.9 cm at 120 min (P < 0.001 compared with nasal breathing at 120 min), whereas, mouth ‘dryness’ ratings did not change significantly with nasal breathing (P > 0.05). A significant difference between the oral and nasal breathing data for perception of mouth ‘dryness’ was first achieved at 60 min into the challenge period (P < 0.05).
Surface tension of UAL
Values for γ of UAL at the commencement of the ‘run-in’ period were not significantly different between the oral breathing challenge (63.4 mN m−1) and the nasal breathing challenge (62.5 mN m−1; P > 0.08) and did not change during the ‘run-in’ on either day (P > 0.05). However, during the nasal breathing challenge, γ of UAL values progressively decreased from 59.3 ± 2.2 mN m−1 at 0 min to 51.8 ± 0.9 mN m−1 after 120 min (P = 0.05; Fig. 3). By contrast, during the oral breathing challenge the γ of UAL value increased from 64.4 ± 2.7 mN m−1 at 0 min to 77.4 ± 1.1 mN m−1 at 120 min (P < 0.001; Fig. 3). However, a significant difference between the oral and nasal breathing data for γ of UAL was not achieved until 90 min into the challenge period (P < 0.001; Fig. 3). When the data from both challenges were combined, γ of UAL values were found to correlate negatively with upper airway mucosal ‘wetness’ measurements (correlation coefficient, r2=−0.34, P < 0.001; Fig. 4).
Figure 3. Group mean ± s.e.m. γ of UAL values during nasal and oral breathing challenge periods.
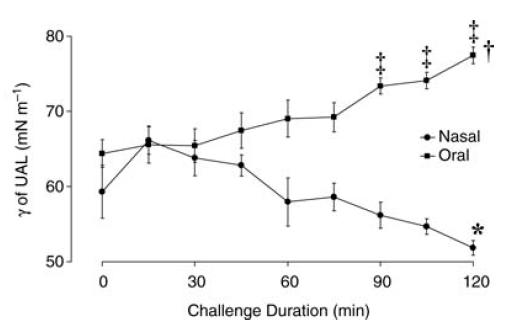
*P < 0.001 compared to time 0 min nasal breathing challenge; †P < 0.001 compared to time 0 min oral breathing challenge; and ‡P < 0.001 nasal compared to oral breathing challenge at respective 15 min intervals.
Figure 4. There was a highly significant, inverse (negative) relationship between UA mucosal ‘wetness’ and γ of UAL.
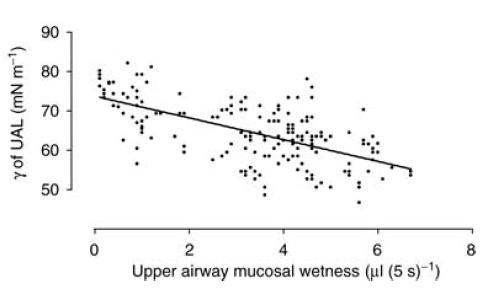
r2 = −0.34, P < 0.001. The line represents the regression line.
Discussion
This study is the first to examine relationships between breathing route and UAL properties. We found that, compared with nasal breathing, oral-route breathing leads to: (1) increased swallowing frequency; (2) increased perception of oral ‘dryness’; (3) decreased upper airway mucosal ‘wetness’; and (4) increased γ of UAL. When combined with our previously published study demonstrating a significant relationship between breathing route during sleep and overnight change in γ of UAL (Kirkness et al. 2005b), the present findings provide support for the hypothesis that breathing route during sleep influences γ of UAL.
Critique of methods
There is no ‘gold-standard’ method for the measurement of posterior pharyngeal wall mucosal ‘wetness’, although methods have been developed to provide semi-quantitative assessments of saliva production from measurements taken in the oral cavity (Wolff & Kleinberg, 1998). Our methodology is a modification of an absorbent paper strip technique previously described for the measurement of oral cavity ‘wetness’ (Ciantar & Caruana, 1998). We used commercially available, purpose-designed, absorbent paper strips and a 5 s contact, gravimetric approach. In preliminary studies, we found that we could detect an absorbed sample volume change of ∼0.1 μl. The technique was reproducible with a coefficient of variation for repeated measures of ∼6.0%.
The UAL samples for the γ measurements were obtained using a small polyethylene catheter positioned against the posterior pharyngeal wall in the same manner as we have previously described (Kirkness et al. 2005a). While the γ values of UAL samples were obtained from the posterior pharyngeal wall, the upper airway mucosal ‘wetness’ measurements were obtained from the very back of the tongue. Thus, although it would appear unlikely, our results may have been influenced by regional differences in mucosal surface behaviour throughout the upper airway. Our methodology for the measurement of γ of UAL has been validated and described in detail previously (Kirkness et al. 2005a).
Route of breathing
Previous studies have shown that most human subjects demonstrate at least some oral breathing during sleep and that this breathing pattern is more prevalent in men than in women (Gleeson et al. 1986). We (Madronio et al. 2004) and others (Gleeson et al. 1986) have also shown that the prevalence of oral-route breathing during sleep increases with age. In their study, Gleeson et al. (1986) concluded that oral breathing during the night may be associated with OSAHS in men and, along with (Nishino & Kochi, 1994), they also concluded that the resistive load imposed by nasal breathing may be an important factor in determining ventilation levels during sleep. The mechanisms linking breathing route and sleep-disordered breathing are not clear, although degree of nasal obstruction (Fitzpatrick et al. 2003; Shikata et al. 2004) breathing route effects on ventilatory drive (Douglas et al. 1982), upper airway muscle recruitment and pharyngeal airway mechanical properties (Basner et al. 1989) have been considered. The present study highlights another potential mechanism, i.e. breathing route effects on γ of UAL. In this context, while oral breathing may contribute to elevated γ of UAL levels, nasal breathing lowers γ of UAL. Therefore, exclusive nasal breathing may be beneficial in maintaining upper airway patency during sleep via, at least in part, preservation of a low γ of UAL.
Ventilation
Quantification of ventilation in this study required respiratory inductance plethysmography, so as to avoid the potential confounding influence of applying a face-mask or mouthpiece. Previous studies have shown that ventilation during oral breathing is higher than with nasal breathing (Gleeson et al. 1986; Schwab et al. 1993). In the present study, the ventilatory levels were slightly, but not significantly, higher (∼10%) during the oral breathing challenge period compared with the nasal breathing challenge period, but this also applied to the nasal breathing run-in period immediately prior to the oral breathing challenge. Nevertheless, differing effects of oral versus nasal breathing on γ of UAL may have been influenced by this slight difference in ventilatory levels.
Swallowing
Accumulation of fluid in the mouth triggers swallowing as part of a normal reflex (Gemba et al. 1996). We monitored swallowing in order to assess the impact of breathing route on a potential delivery mechanism for saliva to coat the posterior pharyngeal wall. During the day, swallowing frequency in normal humans has been reported at a rate of more than 25 swallows h−1 (Lichter & Muir, 1975). However, there have been no previous studies that directly compare swallowing frequency during enforced nasal versus oral breathing. In the present study, oral breathing increased swallowing frequency from 14 to 25 swallows (15 min)−1. However, this increased swallowing rate did not prevent the development of decreased oral mucosal ‘wetness’ or an increase in γ of UAL.
Upper airway mucosal ‘wetness’
In the present study we demonstrated that upper airway mucosal ‘wetness’ increased slightly during 120 min of enforced nasal breathing, but decreased significantly, to barely detectable levels, with a similar period of enforced oral breathing. Since breathing via the mouth bypasses the humidification and warming processes that operate in the nose, this drying of the upper airway mucosa during the oral breathing challenge is probably related to the promotion of evaporative water loss from the mucosal surface.
Nasally inspired air becomes almost saturated with water vapour at body temperature by the time it arrives in the pharynx (Irlbeck et al. 1997). Water added to nasally inspired gas is evaporated from the vast nasal mucosal surface area associated with the complex and highly vascular nasal turbinate structures. However, when low-humidity gas is inspired through the mouth, water is evaporated from the oral mucosa, a process that is usually insufficient to saturate the inhaled air before it reaches the pharynx. As a consequence, water is generally evaporated from the pharyngeal mucosa during mouth breathing, but not during nasal breathing. This leads to pharyngeal upper airway mucosal drying and increased perception of ‘dry mouth’. The extent of these processes will be influenced by, among other things, the temperature and relative humidity of the inspired gas, ventilatory flow rates and, in the case of oronasal breathing, the percentage of inspired gas passing through the mouth versus the nose.
An alternative explanation for decreased upper airway mucosal ‘wetness’ during mouth breathing is a reduction in saliva production itself. Studies have also assessed xerostomia and reported that there was an abnormal dryness of the mouth resulting from decreased secretion of saliva in Sjogrens patients. Saliva production was not measured in the present study, so we are unable to distinguish whether altered saliva production contributed to our findings (Kleinberg et al. 2002; Dawes, 2004).
Perception of ‘dry mouth’
Xerostomia was assessed utilizing a visual analog scale. Our approach in providing an unmarked scale for each assessment allowed the subject to rate their perception without visual feedback from their previous responses. Perception of mouth ‘dryness’ progressively increased during the 120 min oral breathing challenge period (progressive decrease in upper airway mucosal ‘wetness’), whereas there was no change during the nasal breathing challenge period (progressive increase in upper airway mucosal ‘wetness’). This difference in perception response may be related to the inability of subjects to perceive increases in upper airway mucosal ‘wetness’ in the same manner as decreases in ‘wetness’. However, the strength of the stimulus was greater for oral breathing (∼98% decrease in ‘wetness’ over the challenge period) than for nasal breathing (∼32% increase in ‘wetness’), and the perception rating question focused subjects on decreased, rather than increased, mucosal ‘wetness’. These findings demonstrate that, at least for mucosal drying, the graded changes in upper airway mucosal ‘wetness’ obtained in the present study were of sufficient magnitude to induce a graded increase in perception. Thus, our data apply to changes in upper airway mucosal ‘wetness’ that are within perception levels for healthy human subjects.
Suface tension of UAL
There are several methods by which γ can be measured, many of which require at least millilitre volumes of the sample for study. The oropharyngeal mucosa is lined by a relatively thin layer (∼15 μm) of liquid, which makes single-sample large volumes of UAL difficult to obtain (Widdicombe & Widdicombe, 1995). Therefore, in this study we used our previously validated technique for determining the γ of UAL of small-volume liquid samples (∼0.2 μl; Kirkness et al. 2000, 2005a).
Very little is known about the γ of posterior pharyngeal mucosal lining liquid in healthy subjects or OSAHS patients. Recent studies have shown that the γ of the UAL is similar to that of saliva (Kirkness et al. 2000, 2005a). The liquid lining the upper mucosa is derived from a combination of oral and nasopharyngeal secretions. Saliva, secreted from the sublingual and submandibular salivary glands into the oral cavity, is composed of water (> 99%) but contains biologically active components such as mucins, electrolytes, enzymes and phospolipids. Saliva is known to be composed of proteins such as albumin, lactoferrin, salivary peroxidase and myeloperoxidase and electrolytes such as Mg+, Ca+, K+ and Na+. Since saliva is known to contain surface-active phospholipids and to have a relatively low γ (∼57 mN m−1), this its presence in the oral cavity may result in a low γ for the liquid lining the pharyngeal wall. The quantity of saliva produced and the processes that result in the maintenance of a salivary film on the pharyngeal mucosal surfaces may all contribute to the preservation of a low γ of UAL. The main functions of saliva in healthy individuals are to protect the epithelium in the oral cavity and to assist in the predigestion of food (Ciantar & Caruana, 1998). Other functions include water balance, digestion and antimicrobial activity (Mandel, 1987; Lamkin & Oppenheim, 1993). Maintenance of the γ of the UAL may constitute yet another function for the salivary film coating the upper airway mucosal surface.
In the present study, γ of UAL increased by ∼14 mN m−1 during oral breathing but decreased by ∼10 mN m−1 during nasal breathing. These changes are quite large, given that in our previous studies a decrease in γ of UAL of up to 16 mN m−1 was achieved by instillation of exogenous surfactant into the pharynx of anaesthetized humans (Kirkness et al. 2003b). In the study of Kirkness et al. (2003b), a change in γ of UAL of this magnitude was associated with an ∼1 cm H2O decrease in pharyngeal airway opening pressures.
An increase in γ of UAL with oral breathing may result from evaporative water loss changing the surface activity of saliva, while the demonstrated fall in γ of UAL with nasal breathing is likely to be associated with increased retention of phospholipid-containing saliva on the pharyngeal wall. These findings are similar to those in our previous study, where a preponderance of nasal breathing during sleep was associated with an overnight fall in γ of UAL (Kirkness et al. 2005b). Baseline values for γ of UAL measured in the present study are similar to values we (Kirkness et al. 2005b) and others (Glantz, 1970) have previously reported for saliva. It would seem plausible that the γ of UAL is maintained by transfer of saliva to the posterior pharyngeal wall via the swallowing mechanism. Both the occurrence of swallows and the production of saliva continue to occur during sleep, albeit at a somewhat decreased rate (Thie et al. 2002). However, in the present study, an increase in the occurrence of swallows during mouth breathing (probably in response to decreases in ‘wetness’) was unable to maintain mucosal ‘wetness’ levels and, concurrently, γ of UAL increased substantially. Thus, breathing route appears to exert a major impact on maintenance of a low-surface tension liquid lining layer covering the pharyngeal mucosa. While the most likely cause is the evaporative influence of cold dry air, inspired via the oral route, other possibilities, including changes in saliva production rates and composition, require further investigation.
This study is the first to examine the relationship between breathing route, upper airway mucosal ‘wetness’ and the γ of UAL. We found that as upper airway mucosal ‘wetness’ decreased with the duration of exclusive oral breathing, the γ of UAL increased. The opposite was true during nasal breathing, where upper airway mucosal ‘wetness’ increased and γ of UAL decreased. Thus, we conclude that breathing route influences γ of UAL and speculate that nasal breathing during sleep may contribute to reduction of the severity of sleep-disordered breathing via promotion of a low γ of UAL.
Acknowledgments
This study was supported by the Garnett Passe and Rodney Williams Memorial Foundation, The Westmead Millennium Foundation and National Health and Medical Research Council of Australia.
References
- American Sleep Disorders Association. The International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Rochester, MN, USA: American Sleep Disorders Association; 1997. pp. 52–58. [Google Scholar]
- Basner RC, Simon PM, Schwartzstein RM, Weinberger SE, Weiss JW. Breathing route influences upper airway muscle-activity in awake normal adults. J Appl Physiol. 1989;66:1766–1771. doi: 10.1152/jappl.1989.66.4.1766. [DOI] [PubMed] [Google Scholar]
- Chandra A, Coggeshall JW, Ravenscraft SA, Marini JJ. Hyperpnea limits the volume recruited by positive end-expiratory pressure. Am J Respir Crit Care Med. 1994;150:911–917. doi: 10.1164/ajrccm.150.4.7921462. [DOI] [PubMed] [Google Scholar]
- Ciantar M, Caruana DJ. Periotron 8000: calibration characteristics and reliability. J Periodontal Res. 1998;33:259–264. doi: 10.1111/j.1600-0765.1998.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Dawes C. How much saliva is enough for avoidance of xerostomia? Caries Res. 2004;38:236–240. doi: 10.1159/000077760. [DOI] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Zwillich CW. Effects of breathing route on ventilation and ventilatory drive. Respir Physiol. 1982;51:209–218. doi: 10.1016/0034-5687(83)90041-5. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick MF, McLean H, Urton AM, Tan A, O'Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003;22:827–832. doi: 10.1183/09031936.03.00047903. [DOI] [PubMed] [Google Scholar]
- Gemba H, Teranaka A, Takemura K. Influences of emotion upon parotid secretion in human. Neurosci Lett. 1996;211:159–162. doi: 10.1016/0304-3940(96)12741-5. [DOI] [PubMed] [Google Scholar]
- Glantz PO. The surface tension of saliva. Odontol Revy. 1970;21:119–127. [PubMed] [Google Scholar]
- Gleeson K, Zwillich CW, Bendrick TW, White DP. Effect of inspiratory nasal loading on pharyngeal resistance. J Appl Physiol. 1986;60:1882–1886. doi: 10.1152/jappl.1986.60.6.1882. [DOI] [PubMed] [Google Scholar]
- Irlbeck M, Iwai T, Lerner T, Zimmer HG. Effects of angiotensin II receptor blockade on hypoxia-induced right ventricular hypertrophy in rats. J Mol Cell Cardiol. 1997;29:2931–2939. doi: 10.1006/jmcc.1997.0528. [DOI] [PubMed] [Google Scholar]
- Jokic R, Klimaszewski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant: a randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 1998;157:1522–1525. doi: 10.1164/ajrccm.157.5.9708070. [DOI] [PubMed] [Google Scholar]
- Kales A, Cadieux RJ, Bixler EO, Soldatos CR, Velabueno A, Misoul CA, Locke TW. Severe obstructive sleep-apnea – I: Onset, clinical course, and characteristics. J Chronic Dis. 1985;38:419–425. doi: 10.1016/0021-9681(85)90137-7. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Amis TC, Wheatley JR, Christenson HK. Determining the surface tension of microliter amounts of liquid. J Colloid Interface Sci. 2000;232:408–409. doi: 10.1006/jcis.2000.7146. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Christenson HK, Garlick SR, Parikh R, Kairaitis K, Wheatley JR, Amis TC. Decreased surface tension of upper airway mucosal lining liquid increases upper airway patency in anaesthetised rabbits. J Physiol. 2003a;547:603–611. doi: 10.1113/jphysiol.2002.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness JP, Christenson HK, Wheatley JR, Amis TC. Application of the ‘pull-off’ force method for measurement of surface tension of upper airway mucosal lining liquid. Physiol Meas. 2005a;26:677–687. doi: 10.1088/0967-3334/26/5/009. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Eastwood PR, Szollosi I, Platt PR, Wheatley JR, Amis TC, Hillman DR. Effect of surface tension of mucosal lining liquid on upper airway mechanics in anesthetized humans. J Appl Physiol. 2003b;95:357–363. doi: 10.1152/japplphysiol.01198.2002. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol. 2003c;95:1761–1766. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/hypopnea syndrome. Sleep. 2005b;28:457–463. doi: 10.1093/sleep/28.4.457. [DOI] [PubMed] [Google Scholar]
- Kleinberg I, Wolff MS, Codipilly DM. Role of saliva in oral dryness, oral feel and oral malodour. Int Dent J. 2002;52(Suppl. 3):236–240. doi: 10.1002/j.1875-595x.2002.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Lamkin MS, Oppenheim FG. Structural features of salivary function. Crit Rev Oral Biol Med. 1993;4:251–259. doi: 10.1177/10454411930040030101. [DOI] [PubMed] [Google Scholar]
- Lichter I, Muir RC. Pattern of swallowing during sleep. Electroenceph Clin Neurophysiol. 1975;38:427–432. doi: 10.1016/0013-4694(75)90267-9. [DOI] [PubMed] [Google Scholar]
- Madronio MR, Di Somma E, Stavrinou R, Kirkness JP, Goldfinch E, Wheatley JR, Amis TC. Older individuals have increased oro-nasal breathing during sleep. Eur Resp J. 2004;24:71–77. doi: 10.1183/09031936.04.00004303. [DOI] [PubMed] [Google Scholar]
- Mandel ID. The functions of saliva. J Dent Res. 1987;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–259. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- Millman RP, Chung DCC, Shore ET. Importance of breath size in calibrating the respiratory inductive plethysmograph. Chest. 1986;89:840–845. doi: 10.1378/chest.89.6.840. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ozbek MM, Lowe AA, Sjoholm TT, Love LL, Fleetham JA, Ryan CF. Mandibular posture during sleep in healthy adults. Arch Oral Biol. 1998;43:269–275. doi: 10.1016/s0003-9969(97)00122-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ozbek MM, Lowe AA, Sjoholm TT, Love LL, Fleetham JA, Ryan CF. Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch Oral Biol. 1999;44:657–664. doi: 10.1016/s0003-9969(99)00057-6. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Arabi Y, Zahn BR, Meyer KC, Skatrud JB, Badr MS. Effect of surfactant on pharyngeal mechanics in sleeping humans: implications for sleep apnoea. Eur Resp J. 2002;20:451–457. doi: 10.1183/09031936.02.00273702. [DOI] [PubMed] [Google Scholar]
- Nishino T, Kochi T. Breathing route and ventilatory responses to inspiratory resistive loading in humans. Am J Resp Crit Care Med. 1994;150:742–746. doi: 10.1164/ajrccm.150.3.8087346. [DOI] [PubMed] [Google Scholar]
- Olson LG, Strohl KP. Airway secretions influence upper airway patency in the rabbit. Am Rev Respir Dis. 1988;137:1379–1381. doi: 10.1164/ajrccm/137.6.1379. [DOI] [PubMed] [Google Scholar]
- Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–1400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- Shikata N, Ueda HM, Kato M, Tabe H, Nagaoka K, Nakashima Y, Matsumoto E, Tanne K. Association between nasal respiratory obstruction and vertical mandibular position. J Oral Rehabil. 2004;31:957–962. doi: 10.1111/j.1365-2842.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- Thie NM, Kato T, Bader G, Montplaisir JY, Lavigne GJ. The significance of saliva during sleep and the relevance of oromotor movements. Sleep Med Rev. 2002;6:213–227. doi: 10.1053/smrv.2001.0183. [DOI] [PubMed] [Google Scholar]
- Van der Touw T, Crawford AB, Wheatley JR. Effects of a synthetic lung surfactant on pharyngeal patency in awake human subjects. J Appl Physiol. 1997;82:78–85. doi: 10.1152/jappl.1997.82.1.78. [DOI] [PubMed] [Google Scholar]
- Widdicombe JH, Widdicombe JG. Regulation of human airway surface liquid. Respir Physiol. 1995;99:3–12. doi: 10.1016/0034-5687(94)00095-h. [DOI] [PubMed] [Google Scholar]
- Wolff M, Kleinberg I. Oral mucosal wetness in hypo- and normosalivators. Arch Oral Biol. 1998;43:455–462. doi: 10.1016/s0003-9969(98)00022-3. [DOI] [PubMed] [Google Scholar]


