Abstract
Cartilage ultrastructure is based on collagen fibrils tied together by proteoglycans (PGs). Interfibrillar orthogonal PG bridges (‘shape modules’) were located by electron histochemistry using Cupromeronic blue methodology. Their frequency and size, similar to those in tendon, cornea, etc., were compatible with biochemical estimates of tissue decoran (formerly decorin), the PG component of shape module bridges. Digestion by hyaluronanase and chondroitinase AC helped to identify aggrecan and decoran and exemplified the destruction of shape modular organization by glycan-splitting agents. The anionic glycosaminoglycan (AGAG) of decoran, dermochondan sulphate (DS, formerly dermatan sulphate), contains l-iduronate, an elastic sugar unit. Chondroitan, keratan (present in aggrecan) and hyaluronan are not similarly elastic but can participate in sliding-filament reversible deformability. Mechanical properties predicted for the interfibrillar bridges accord with anisotropic stress/strain responses of articular cartilage to compressive or tensile stresses. We propose that fluid from pericellular aggrecan-rich domains moves under pressure into the interterritorial fibrillar arrays against the elastic resistance of the shape modules, which return the fluid, post-compression, to its original position. Cartilage is tendon-like, with the addition of expansile aggrecan-rich reservoirs of aqueous shock absorber fluid. Rupture or loss of interfibrillar ties would allow expansile PG to force the collagenous matrix apart, imbibing water, increasing swelling and fissuring – characteristic manifestations of osteoarthrosis (OA), a joint disease of major economic importance. Decoran may be a primary target of the OA disease process.
Cartilages transmits stresses elastically, while maintaining shape in the long term. Collagen fibrils therein resist and transmit tensile forces. Compression is opposed by carbohydrate-rich polyanionic PGs that expand in solution (a) because of the mutual repulsion of their many negative charges and (b) to increase their solution entropy, like all polymers in good solvents. Donnan osmotic pressure of PG-associated cations adds to the turgor. Space-filling PGs lose water from their domains under pressure, regaining it after loads are removed (Stockwell & Scott, 1965; Scott, 1975). Turgor or elasticity are lost if PGs are precipitated (i.e. rendered insoluble) in the tissue (Hedbys, 1961; Sokoloff, 1963). Interfibrillar links must prevent fibrils from dissipating under PG swelling forces, and stable relationships between PGs and fibrils must prevent PGs moving like beans in a beanbag (Scott, 1975).
PGs themselves provide the links, organizing collagen matrices and thereby fulfilling a vital morphological role. Electron histochemistry using Cupromeronic blue (Scott, 1985) showed bridges between collagen fibrils in extracellular matrices (ECMs) (Scott, 1988), e.g. tendon, consisting of AGAG attached to the PG protein (decoron), which in turn associates with the fibril (Fig. 1). These, the only specific interfibrillar connections repeated periodically along the collagen fibrils, were called shape modules (Scott & Thomlinson, 1998) because of their tissue-organizing function. Human cells unable to express the decoron gene did not produce an organized ECM matrix (Scott et al. 1998).
Figure 1. Scheme showing elastic shape modules at rest (left) and under tensile stress (right), not to scale.
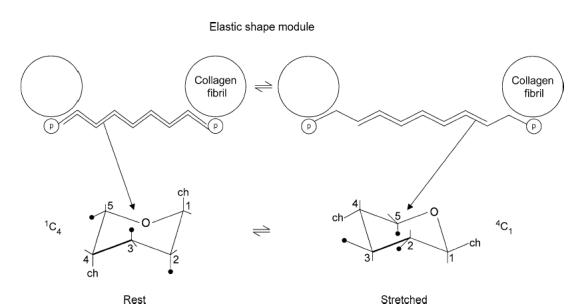
PG proteins (p) are attached non-covalently to specific binding sites on collagen fibrils, seen in cross section and linked anti-parallel via AGAG chains. ch, AGAG chain. The AGAG aggregates are stabilized by H-bonds and hydrophobic bonds. l-iduronate in the unstretched DS AGAG is in the 1C chair form (left), elongating under tension to the longer C1 chair form in the stretched shape module (right). This occurs at relatively low tension (200 pN, Haverkamp et al. 2005). Tensioned AGAG chains slip with rupture of H- and hydrophobic bonds (right), slipping back to the unstretched form, post deformation, in which these bonds have been re-formed (Fischer et al. 2002).
Four PG binding sites are present on collagen fibril surfaces in every D period, in the a, c, d and e bands. Decorans in tendons, skin, cornea, etc. associate at each d or e band. In corneal stroma a and c bands are also occupied, by keratan sulphate (KS)-conjugated PGs (Scott, 1988). Thus, more polyanion is attached to collagen fibrils in cornea than in tendon, giving more turgor and hence a stiffer structure. Cartilage is richer in PG than cornea, resulting in yet greater turgor.
AGAG interfibrillar bridges of shape modules must be reversibly deformable (Scott, 2003) for the ECM to be elastic. Two mechanisms were suggested: (1) reversible slippage between anti-parallel AGAG chains in the bridges (Fig. 1) and (2) concertina-like expansion and contraction of l-iduronate units in DS (Fig. 1). Mechanism (2) was observed directly by stretching single AGAG molecules using atomic force spectroscopy (Haverkamp et al. 2005). l-iduronate is present in varying amounts in DS from different tissues, increasing with elasticity of the tissue (Scott, 2003).
Figure 2. Electron micrographs of rabbit femoral condyle articular cartilage middle zone (a–c) rat nasal septal cartilage (d), stained with Cupromeronic blue.
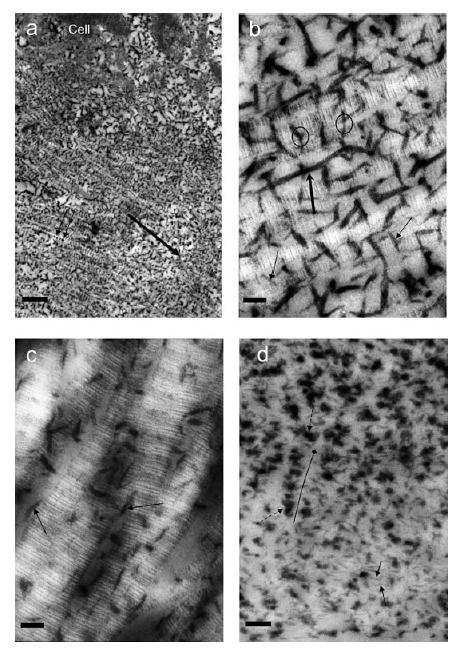
a–c, in 0.3 m MgCl2; d, in 0.1 m MgCl2). a–c were counterstained with uranyl acetate. Scale bars: in a, 450 nm; in b and c, 50 nm; in d, 150 nm. b is the control for hyaluronanase digestion (c), immersed in buffer plus proteolytic inhibitors for the same time as c. a, parallel collagen fibrils orientated left to right (large arrow) carrying regularly spaced (50–60 nm, D periodicity) orthogonally arrayed Cupromeronic blue-stained PGs (small arrows). b, parallel collagen fibrils showing a–e banding. Orthogonal PG AGAGs (thin arrows) associated mainly at the d and e bands bridge between fibrils (ringed). Thick AGAGs stretching ≥ 4-D periods along the fibrillar surfaces (thick arrow) appear to fuse with transfibrillar AGAGs. c, after hyaluronanase digestion the ordered orthogonal arrays seen in b are mainly absent although AGAGs are seen parallel to the fibrils (arrowed). Chondroitinase AC digestion removes almost all AGAG (data not shown). d, regularly spaced (D periodic) PG AGAGs (small arrows, possibly decoran), similar to those in a and b, are thin and orthogonal to collagen fibrils (light areas), contrasting with densely stained clumps (dashed arrows) of AGAGs (probably aggrecan, see text). These clumps are frequently in linear groups, probably following collagen fibrils (long diamond). Aggrecan-rich regions were clearly delimited from the zone in which decoran-like PGs are seen. Such intensely polyanionic regions were uncommon in rabbit articular cartilage.
The distribution of AGAGs in cartilages is more complex than in tendon, and collagen fibril arrangements are more varied. Although the fibrillar collagen matrix in cartilage sometimes appears disordered, there are many stretches of parallel fibrils (Chen & Broom, 1998), particularly in the interterritories (see Results and Discussion, below). The territories show honeycomb fibrillar meshworks. Chondroitan sulphate (CS) and KS distribute differentially. The pericellular territories are rich in CS, staining characteristically in the Alcian blue–critical electrolyte concentration (CEC) method (Stockwell & Scott, 1965). Aggrecan is probably the major PG in the territories, since these contain much CS and aggrecan carries the greater part of the tissue CS. The interterritories contain lower concentrations of AGAGs, including KS in mature human articular and horse nasal septal cartilages, identified by its resistance to hyaluronanase and high CECs. Whereas CS is present in cartilages of all ages and species, KS is not found in small animals (mouse, rat; Venn & Mason, 1985) and levels increase greatly with age in human cartilage (Kaplan & Meyer, 1959). A third AGAG, DS, is present as part of decoran in smaller quantities but similar molar concentrations to that of aggrecan (Roughley & White, 1989; Stanescu, 1990). Nothing was known about its ultrastructural location partly because it is quantitatively overshadowed by CS and KS, which hinder penetration by immunochemical stains into the tissue.
Limited data suggested that cartilage, too, might be based on shape modules. Cupromeronic blue–CEC methodology showed unidentified polyanion at d bands of thick collagen fibrils in deep zones of dog femoral condylar cartilage (Orford & Gardner, 1984). Decoran was isolated from cartilages (Rosenberg et al. 1981; Stanescu, 1990). Our results shows that cartilage consists of AGAG-bridged shape modules as do all non-mineralized ECMs.
Our finding widens discussion of cartilage elasticity. We suggest that shape module elasticity returns cartilage to the original morphology, post-stress, in collaboration with the aggrecan-based inflated-matrix mechanism. New insights into the aetiology of OA, the most common crippling joint disease, are thereby obtained (see Discussion). Biomechanical data on cartilages provide good bases on which to test predictions of mechanical properties of shape modules.
Methods
Cartilaginous tissues were taken from mature rabbits and rats post mortem after they had been used for raising antibodies (rabbits) or as ex-breeders (rats) by other groups in the Medical School. The animals were killed with sodium pentobarbitone (3 ml of 200 mg ml−1i.v.) and were dissected within an hour of death. Cartilages dissected from rabbit and rat femoral condyles or nasal septa were immersed in isopentane at −183°C. Frozen sections 10 μm thick were cut and stained in 0.05% w/v Alcian blue or Cupromeronic blue in 0.05 m sodium acetate buffer pH 6.5 containing 0.1 m or 0.3 m MgCl2 and 2.5% glutaraldehyde. Sections were examined by light microscopy and trimmed to leave selected areas, which were embedded in Taab resin (Haigh & Scott, 1986). Ultrathin sections were then examined by electron microscopy. Costal cartilage taken post mortem from a 70-year-old human male (prior to 1990) was similarly treated. Frozen sections 10 μm thick of articular cartilage were fixed with 2.5% buffered aqueous formaldehyde (30 min), washed briefly in 0.05 m sodium acetate and digested at 37°C for 4 h with sheep testicular hyaluronanase (EC3.2.135; Sigma, type V) in 0.05 m sodium acetate buffer pH 6.5 containing 0.5 m NaCl, 0.5 mg ml−1 bovine serum albumin and the protease inhibitors disodium EDTA (1.6 mm), benzamidine (0.8 mm) and soyabean trypsin inhibitor (0.4 mg ml−1); then stained as above. Digestion with chondroitinase AC (Seikagaku, chondroitin AC lyase, EC4.2.2.5; 2.5 units ml−1,) was carried out at pH 8.0 (0.3 m ammonia–ammonium acetate buffer) with similar concentrations of protease inhibitors, NaCl and albumin. Some ultrathin sections were counterstained with UO22+.
Results
Our methodology enables investigations to be performed on selected and precisely defined areas of tissue. Large numbers of parallel collagen fibrils of similar thicknesses (∼50 nm) orientated towards the surface were observed in the interterritories of rabbit femoral condylar articular cartilage middle zones. Similar swathes of parallel fibrils were seen in the interterritories of rat nasal septa and aged human costal cartilage.
These fibrils carried filaments densely stained with Cupromeronic blue, orthogonal to the fibrils at regular intervals of ≤ D periodicity (Fig. 2a). The filaments were sulphated polyanions, since they stained at 0.3 m MgCl2 (Scott, 1985). Many, located at d bands (Fig. 2b), were mostly removed by hyaluronanase (Fig. 2c) or more completely by chondroitinase AC under these conditions, proving that they contained CS/DS. Thus, CS/DS associated at the d bands is most probably part of decoran, by analogy with similarly placed AGAGs in tendon, cornea, sclera, etc.
Counts of the number of orthogonal AGAGs per unit length of fibril, performed at low magnification to increase numbers, suggested that the average interorthogonal AGAG separation was ≥ 50 nm, implying that about one orthogonal AGAG was present per D period (∼60 nm). Long (> 150 nm) thick (∼10 nm) sulphated polyanions were frequently associated with fibrils, parallel to their axes. After hyaluronanase digestion many, but not all, were lost (Fig. 2c). Chondroitinase AC digestion was more complete under the conditions, removing almost all stainable AGAG in rabbit cartilage.
The orthogonal interfibrillar AGAGs were relatively thin (∼5 nm), whereas perifibrillar clumps or globules occurring in contiguous groups were much thicker (∼30 nm). These were very probably aggrecan because (a) they stained at 0.3 m MgCl2), (b) were largely digested by hyaluronanase and chondroitinase AC, and (c) were much bigger than the orthogonal decoran AGAGs and closely packed near to chondrocytes (Fig. 2d). The densely stained ‘aggrecan’ globules were often distributed periodically vis-a-vis the fibrils, sometimes approximating D periodicity.
The different AGAG morphologies were all associated with collagen fibrils. The collagen fibril–PG supra-molecular matrix is based on PGs bound to the fibrils which in turn are orientated by the PGs – in contrast to fibrils being merely embedded in an AGAG gel.
Discussion
Collagen fibrils in cartilages often occur in extensive parallel arrays (Chen & Broom, 1998), which were suggested to be maintained by interfibrillar interactions of unknown nature (see their Fig. 10). PG bridges provide these connections (Results). Our demonstration of extensive regions of parallel collagen fibrils D-periodically linked by orthogonal PG bridges establishes the fundamental unity of cartilages with all ECMs, as shape module-based structures typical of tissues such as tendon, skin, sclera and cornea (Scott, 1988).
Important similarities between shape modules in cartilage and, e.g. tendon, appear at several levels. Eleven-aminoacid sequences of putative decoran-binding sites on type I collagen fibrils were identified in the d and e bands. Analogous sequences are present in cartilage type II collagen, identically placed in the primary structure (Scott & Glanville, 1993). Therefore, cartilage decoran, which resembles skin and tendon decorans in primary structure (Roughley & White, 1989), probably combines non-covalently with type II collagen fibrils by the same mechanism as other decorans do to type I fibrils (Scott, 1988). Although decoran constitutes a small amount by weight of cartilage compared with the much larger molecule aggrecan, the molar amounts of the two PGs are similar (Roughley & White, 1989; Stanescu, 1990). Electron micrographs (Fig. 2a) show interfibrillar bridges that are as abundant in cartilages as in tendons. Therefore the ratio of decoran to collagen fibrillar surface area should be similar in cartilage and in tendon, given that decoran is regularly attached at the fibril surface. Thus, 1 g cartilage with fibrils of ∼50 μm diameter contains 2–4 mg decoran (g wet wt)−1 (Roughley & White, 1989). Assuming ∼20% of cartilage wet weight is collagen and ∼40% of decoran is DS, this is equivalent to 4–8 mg DS (g dry cartilage collagen)−1. One gram of collagen in the form of 50 μm fibrils in dry tendon is associated with 4–5 mg DS (Scott, 1984). Hence, this calculation supports the view that cartilage is a shape-module-based structure similar to tendon. It is relevant that tendon produces aggrecan and becomes cartilage-like when it is relocated to a pressure-bearing position under the rabbit heel (Flint et al. 1980).
The proposed structure suggests that decorans orientate fibrils, helping to maintain cartilage shape. As yet, there is no evidence that aggrecan acts similarly. Figure 2d shows a regular perifibrillar distribution of a large PG, presumably aggrecan because of its size and high tissue concentration. Heavily stained globular polyanionic material often touches neighbouring globules. Their size suggests several (> 10) AGAG chains were in each globule. No banding pattern was identifiable because ‘aggrecan’ blots out fibril details, so it was not possible to assign binding sites.
KS PG (fibromodulan) similar to that (lumican) in cornea is present in cartilage (Svensson et al. 2000) in larger animals and by analogy it might associate at the a or c bands, in addition to decoran at the d band. However, KS is almost absent from rabbit (see Results) and small mammal cartilages and this parallels the situation in their corneas (Scott & Haigh, 1988). Our results therefore do not contribute to this discussion. In the human, KS levels increase with increasing thickness of cartilage during maturation, particularly in the interterritory (Stockwell & Scott, 1965), where fibromodulan may have a bridging role as do KS PGs in corneas (Scott & Haigh, 1988).
Shape modular and articular cartilage mechanics compared
The proposed structure (Fig. 3) implies that cohesion against pulling forces orthogonal to the collagen fibrils depends on the AGAG bridges (Figs 1 and 3) which are elastic, whereas pulling forces parallel to the fibrils are taken by the fibrils which are practically inextensible (Fig. 3A), i.e. the response of cartilage to stress is anisotropic. Force–extension plots (Kempson, 1975) support this prediction. Moreover, the force needed to pull the tissue apart orthogonal to the fibrils is decreased by prior digestion of the tissue by cathepsin D (Kempson, 1975), which attacks decoron (J. D. Sandy, personal communication), whereas cathepsin D is without effect on force–extension properties parallel to the collagen fibrils (Kempson, 1975). Articular cartilage was less resistant to initial low stress than to higher stress, compatible with the presence of low tension–low resistance elements (l-iduronate) in this model (Fig. 1). Under compression, cartilage deforms reversibly to a greater extent orthogonally to the fibre axis than along it (Maroudas et al. 1985), as expected from our proposed structure (Fig. 3D) and from the mechanism of elastic elongation of the AGAG bridges (see below, and Fig. 1; Scott, 2003). This is crucial, since the cartilage role is to cope primarily with compression. Biomechanical data therefore provide direct support for the physiological function of shape modules.
Figure 3. Proposed schemes of shape module-based stresses, elasticity and damage.
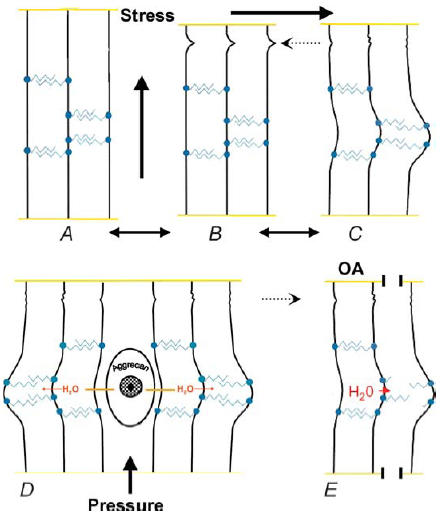
A–C, schemes showing predicted responses of shape module-based tissue to tensile mechanical stresses. Collagen fibrils (vertical black lines) are linked together by anti-parallel AGAG bridges (blue zigzag lines) attached non-covalently via protein cores (filled blue circles) to the fibrils (see also Fig. 1). Crimps in the fibrils indicated by dotted dashed arrows. B is the resting state. A, stress applied parallel to the fibrils straightens the crimps at low stress, allowing small extension (∼10%) of the tissue but then resistance from the almost inextensible fibrils stiffens. C, stress applied orthogonally to the fibrils stretches the tissue against low and slowly increasing resistance from the elastic AGAG bridges to a larger extent than similar stress applied as in A. The deformation is reversible, i.e. elastic. These patterns are seen on cartilage under tensile stress (Kempson, 1975; see text). The mechanisms are anisotropic, implying that elastic deformation orthogonal to the fibrils should be greater than along the fibril direction (see text). D, proposed scheme of cartilage elasticity under compression. Under pressure, the aggrecan-rich reservoir (the territory) of aqueous fluid forces water into the shape modules of the interterritory against elastic resistance (see C above). The compressed aggrecan domains exert swelling pressure, sucking back the water from the shape modules, helped by the AGAG bridge elasticity, which ensures that the tissue organization stays intact (see text). E, proposed scheme of damage to osteoarthrotic cartilage (OA). The AGAG bridges between the fibrils on the right are disrupted or lost, depriving the tissue of elastic deformability, permitting swelling, increased water content, softening and fissuring to take place (see text).
Cartilage elasticity
Pericellular territories are rich in AGAG, as shown by their uptake of cationic dyes which is much greater than that of interterritories. The great part of the territorial AGAG is sulphated and hyaluronanase digestible, hence largely CS. It was suggested that the territory was one compartment of a shock absorber, into which the aqueous shock absorber fluid was sucked by the strong swelling pressure of the AGAG (Stockwell & Scott, 1965; Scott, 2003). Under pressure the fluid was driven out of the AGAG-rich domain into other parts of the tissue – the second compartment of the shock absorber, to be sucked back on cessation of the stress (Scott, 1975). It is crucial that the AGAG chains are free to expand and this is deducable from the 13C NMR spectra of CS in cartilage, which were sharp (Brewer & Keiser, 1975), resembling CS in free solution, proving that many CS chains were freely mobile and potentially expansile. In particular, the acetamido carbonyl resonances were sharp, showing that they were not involved in rigid intermolecular H-bonds (Scott & Heatley, 1999).
The second shock absorber compartment must be completely, rapidly and reversibly deformable; able to revert quickly to the original shape after release of compression. We suggest that the intrinsic elasticity of the PG bridges provides that property. Shape module bridges are seen as anti-parallel aggregates of AGAG chains (Fig. 1), stabilized by hydrophobic and hydrogen bonding (Scott & Thomlinson, 1998). rheoNMR showed that analogous HA tertiary structures were disrupted by shear stress and reformed spontaneously at rest (Fischer et al. 2002). CS and KS of low sulphation can aggregate similarly (Scott et al. 1992; Scott, 2003). Thus the low energy structure, with an optimum number of interchain bonds, could slip ratchet-wise, reforming the interchain bonds on removal of the stress (Figs 1 and 3). In addition to this intermolecular mechanism DS chains are intramolecularly elastic (Scott, 2003; Haverkamp et al. 2005), since l-iduronate residues therein can switch between equi-energetic conformations (1C, C1 and 2S0; Figures illustrated in Scott 2003) of different lengths in the polymer chain (Casu et al. 1988). Elongation and retraction are reversible (Scott, 2003) at low tensile stress (200 pN), as shown by atomic force spectroscopy on stretched DS single molecules (Haverkamp et al. 2005). CS does not have this property. The two mechanisms could work together.
In this context compressive forces are converted into tensile stresses (Fig. 3). Fluid forced from one space stretches shape module bridges elsewhere in the tissue, until compression ceases and they return to their original length, giving up the stored energy (Fig. 3D) (Scott, 2003).
Proposed OA aetiology and the role of decoran
The shape-modular concept suggests new ways in which stress and pathology could affect function and durability, inviting re-examination of investigations in which aggrecan was the only PG thought to be involved. Damage to decoran may be primary in the breakdown of the matrix, e.g. in OA. A few glycanase or free radical-mediated cleavages in AGAG bridges could loosen the fibrillar matrix (see Figs 2c and 3E), diminishing load-bearing capacity and facilitating development of clefts parallel to the fibril axes (Fig. 3E). Such clefts (or fibrillations) are characteristic of osteoarthrosic degeneration in deeper zones of cartilage. ‘Fibrillar parting’ was observed in osteoarthrosic surface zones, where the fibrils run parallel with the cartilage surface (Stockwell et al. 1983). By contrast, orthogonal clefts, implying cleavage of collagen fibrils, are less common, although sometimes found at the tidemark. Glycan-splitting agents attacking bridge DSs would also probably degrade aggrecan CS chains because of their closely similar chemistry.
Rupture of PG bridges would allow fibrils to drift apart under PG expansile pressure, with concomitant swelling and increase in tissue water (Fig. 3E) – general findings in OA, both in animal models (McDevitt & Muir, 1977) and in human disease (Mankin, 1975; Maroudas & Venn, 1977). Osteoarthrosic cartilage swelled much more than normal cartilage on immersion in physiological saline solution (Maroudas & Venn, 1977), as expected if AGAG bridges malfunctioned. A PG with the properties of decoran, present in normal cartilage, was absent from osteoarthrosic and old cartilage (Stanescu, 1990), although not from osteophytes of the same joints. Aggrecan turnover is increased in osteoarthrosic cartilage (Carney et al. 1984), possibly stimulated by the increased available space created by breakage of restraining AGAG bridges and leakage of aggrecan from the loosened fibrillar matrix.
More subtly, loosening of the fibrillar meshwork in the superficial zone is implied in normal reversible short-term swelling after moderate exercise of the joint (Stockwell, 1991), involving uptake of water. The PG bridges can either cycle elastically in response to physiological stress or, where there are unremitting or excessive mechanical loads, develop permanent damage.
The hypothesis of a damaged shape module provides a new aetiology of OA, posing the question, what is the disrupting mechanism? OA may affect decoran function, secondary to removal or damage. Human cells unable to produce decoran were not only unable to make organized shape-modular ECMs, proving its essential role at a tissue level, but also to form collagen fibrils of normal morphology (Scott et al. 1998). If fibromodulan serves the same interfibrillar bridging role in mature human cartilage as the very similar KS PG lumican does in cornea, damage to KS bridges in the human may be as important as that to DS bridges. Fibromodulan and lumican bind to the same collagen sites in vitro (Svensson et al. 2000). Nothing is known about the ultrastructural role of KS PGs in cartilage, but it is relevant that a double knockout of fibromodulin and biglycan proceeded to osteoarthrosis during development of the affected mouse (Ameye & Young, 2002).
The ubiquity of shape modules in connective tissues of all animals so far examined suggests that other pathologies involving damage to the PG bridges will be found, emerging later in life.
Acknowledgments
Thanks to Marian Haigh and Barry Oswald for electron microscopy. We are grateful for Grant No. S0634 from the Arthritis Research Campaign.
References
- Ameye L, Young MF. The biglycan/fibromodulin double-deficient mouse: Characterization of a new animal model of osteoarthritis. Eur Cells Mater. 2002;4(Suppl. 1):8. [Google Scholar]
- Brewer CF, Keiser H. Carbon-13 nuclear magnetic resonance study of chondroitin 4-sulfate in the proteoglycan of bovine nasal cartilage. Proc Nat Acad Sci U S A. 1975;72:3421–3423. doi: 10.1073/pnas.72.9.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney SL, Billingham MEH, Muir H, Sandy JD. Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Ortho Res. 1984;2:201–206. doi: 10.1002/jor.1100020301. [DOI] [PubMed] [Google Scholar]
- Casu B, Petitou M, Provasoli M, Sinay P. Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acidcontaining glycosaminoglycans. Trends Biochem Sci. 1988;13:221–225. doi: 10.1016/0968-0004(88)90088-6. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Broom N. On the ultrastructure of softened cartilage: a possible model for structural transformation. J Anat. 1998;192:329–341. doi: 10.1046/j.1469-7580.1998.19230329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E, Callaghan P, Heatley F, Scott JE. Shear flow affects secondary and tertiary structures in hyaluronan solution as shown by rheo-NMR. J Mol Struct. 2002;602, 603:303–311. [Google Scholar]
- Flint MH, Gillard GC, Merrilies MJ. The effects of local physical environmental factors on connective tissue organisation and glycosaminoglycan synthesis. In: Parry D, Creamer LK, editors. Fibrous Proteins: Scientific, Industrial and Medical Aspects. vol. 2. London: Academic Press; 1980. pp. 107–119. [Google Scholar]
- Haigh M, Scott JE. A method for processing tissue sections for staining with Cupromeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Bas Appl Histochem. 1986;30:479–485. [PubMed] [Google Scholar]
- Haverkamp RG, Williams MAK, Scott JE. Stretching individual molecules of connective tissue glycans to characterise their shape-maintaining elasticity. Biomacromol. 2005;6:1816–1818. doi: 10.1021/bm0500392. [DOI] [PubMed] [Google Scholar]
- Hedbys BO. The role of polysaccharides in corneal swelling. Exp Eye Res. 1961;1:81–91. doi: 10.1016/s0014-4835(61)80012-2. [DOI] [PubMed] [Google Scholar]
- Kaplan D, Meyer K. Ageing of human cartilage. Nature. 1959;182:1267–1268. doi: 10.1038/1831267a0. [DOI] [PubMed] [Google Scholar]
- Kempson GE. The effects of proteoglycan and collagen degradation on the mechanical properties of adult human articular cartilage. In: Burleigh PMC, Poole AR, editors. Dynamics of Connective Tissue Macromolecules. New York: Elsevier; 1975. pp. 277–305. [Google Scholar]
- McDevitt CA, Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Jt Surg. 1977;59B:24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Mankin HJ. The metabolism of articular cartilage in health and disease. In: Burleigh PMC, Poole AR, editors. Dynamics of Connective Tissue Macromolecules. New York: Elsevier; 1975. pp. 327–353. [Google Scholar]
- Maroudas A, Mizrahi J, Weisman N, Ziv I. Some physico-chemical and biomechanical characteristics of normal and osteoarthritic cartilage. In: Peyron JG, editor. Osteoarthritis Current Clinical and Fundamental Problems. Rueil-Malmaison, France: Ciba-Geigy; 1985. pp. 157–164. 92506, ISBN 2-902711-17-4. [Google Scholar]
- Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. Ann Rheum Dis. 1977;36:399–406. 327–353. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford CR, Gardner DL. Proteoglycan association with collagen d band in hyaline articular cartilage. Conn Tiss Res. 1984;12:345–348. doi: 10.3109/03008208409013696. [DOI] [PubMed] [Google Scholar]
- Rosenberg LC, Choi HU, Tang L-H, Johnson TL, Pal S, Webber C, Reiner A, Poole AR. Isolation of dermatan sulphate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1981;260:6304–6313. [PubMed] [Google Scholar]
- Roughley PJ, White RJ. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 1989;262:823–827. doi: 10.1042/bj2620823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Composition and structure of the pericellular environment: physiological function and chemical composition of pericellular proteoglycan (an evolutionary view) Phil Trans Roy Soc Lond B. 1975;271:235–242. doi: 10.1098/rstb.1975.0047. [DOI] [PubMed] [Google Scholar]
- Scott JE. The periphery of the developing collagen fibril. Quantitative relationships with dermatan sulphate and other surface-associated species. Biochem J. 1984;218:229–233. doi: 10.1042/bj2180229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Proteoglycan histochemistry – a valuable tool for connective tissue biochemists. Coll Rel Res. 1985;5:541–598. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- Scott JE. Proteoglycan–fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Chen Y, Brass A. Secondary and tertiary structures involving chondroitin and chondroitin sulphates in solution, investigated by rotary shadowing-electron microscopy and computer simulation. Eur J Biochem. 1992;209:675–680. doi: 10.1111/j.1432-1033.1992.tb17335.x. [DOI] [PubMed] [Google Scholar]
- Scott JE, Dyne KM, Thomlinson AM, Ritchie M, Bateman J, Cetta G, Valli M. Human cells unable to express decoron produced disorganized extracellular matrix lacking ‘shape modules’ (interfibrillar proteoglycan bridges) Exp Cell Res. 1998;243:59–66. doi: 10.1006/excr.1998.4089. [DOI] [PubMed] [Google Scholar]
- Scott JE, Glanville RW. Homologous sequences in fibrillar collagens may be proteoglycan binding sites. Biochem Soc Transactions. 1993;21:123S. doi: 10.1042/bst021123s. [DOI] [PubMed] [Google Scholar]
- Scott JE, Haigh M. Identification of specific binding sites of keratan sulphate and chondroitin/dermatan sulphate proteoglycans on collagen fibrils in cornea, using Cupromeronic blue in critical electrolyte concentration techniques. Biochem J. 1988;253:607–610. doi: 10.1042/bj2530607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Heatley F. Hyaluronan forms specific stable cooperative tertiary structures in solution. A 13C NMR study. Proc Nat Acad Sci U S A. 1999;96:4850–4855. doi: 10.1073/pnas.96.9.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (‘shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Elasticity of articular cartilage, effect of ions and viscous solutions. Science. 1963;141:1055–1057. doi: 10.1126/science.141.3585.1055. [DOI] [PubMed] [Google Scholar]
- Stanescu V. The small proteoglycans of cartilage matrix. Seminars Arthritis Rheumatism. 1990;20:51–64. doi: 10.1016/0049-0172(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Stockwell RA. Cartilage failure in osteoarthritis: Relevance of normal structure and function. A Rev Clin Anat. 1991;4:161–191. [Google Scholar]
- Stockwell RA, Billingham MEH, Muir H. Ultrastuctural changes in articular cartilage after experimental section of the anterior cruciate ligament of the dog knee. J Anat. 1983;136:425–439. [PMC free article] [PubMed] [Google Scholar]
- Stockwell RA, Scott JE. Observations on the acid glycosaminoglycan (mucopoly: saccharide) content of the matrix of ageing cartilage. Ann Rheum Dis. 1965;24:341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Narlid I, Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- Venn G, Mason RM. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 1985;228:443–450. doi: 10.1042/bj2280443. [DOI] [PMC free article] [PubMed] [Google Scholar]


