Abstract
The aim of the present study was to examine the regulation of exercise intensity in hot environments when exercise is performed at a predetermined, fixed subjective rating of perceived exertion (RPE). Eight cyclists performed cycling trials at 15°C (COOL), 25°C (NORM) and 35°C (HOT) (65% humidity throughout), during which they were instructed to cycle at a Borg rating of perceived exertion (RPE) of 16, increasing or decreasing their power output in order to maintain this RPE. Power output declined linearly in all three trials and the rate of decline was significantly higher in HOT than in NORM and COOL (2.35 ± 0.73 W min−1, 1.63 ± 0.70 and 1.61 ± 0.80 W min−1, respectively, P < 0.05). The rate of heat storage was significantly higher in HOT for the first 4 min of the trials only, as a result of increasing skin temperatures. Thereafter, no differences in heat storage were found between conditions. We conclude that the regulation of exercise intensity is controlled by an initial afferent feedback regarding the rate of heat storage, which is used to regulate exercise intensity and hence the rate of heat storage for the remainder of the anticipated exercise bout. This regulation maintains thermal homeostasis by reducing the exercise work rate and utilizing the subjective RPE specifically to ensure that excessive heat accumulation does not occur and cellular catastrophe is avoided.
Exercise performance is impaired in hot compared to cool conditions (Nielsen et al. 1990; Tatterson et al. 2000; Marino et al. 2000, 2004), and this has been attributed to ‘central fatigue’ (Nybo & Nielsen, 2001a) in which there is a failure of motor-unit recruitment after body temperature reaches a critical limiting value of approximately 40°C (Nielsen et al. 1990; Nybo & Nielsen, 2001a). In support of this, if exercise is performed at a constant work rate until volitional fatigue, skeletal muscle motor-unit recruitment, measured indirectly using surface electromyography (EMG) during sustained isometric contractions, is lower in hyperthermic (40°C) than in normothermic (38°C) subjects (Nybo & Nielsen, 2001a). This suggests that the ‘hot brain’ is unable to recruit motor units to allow exercise to continue at the required work rate.
Recently, it has been found that power output and integrated EMG(iEMG) activity during self-paced cycling exercise in the heat decrease well before the core temperature reaches 40°C (Marino et al. 2000; Tatterson et al. 2000; Tucker et al. 2004). Further, Tucker et al. (2004) found that power output and iEMG amplitude were greatest in the final kilometre of a 20-km cycling time-trial, when the rectal temperatures were also the highest. In another study, isometric force production and voluntary activation percentage have been found to decrease progressively during a passive heating protocol (Morrison et al. 2004), even at body temperatures below 39°C. Todd et al. (2005) found that reduced force output occurred as a result of a failure of voluntary drive during passive heating despite the availability of additional motor cortical output, which would, in theory, allow increased force output. Collectively, these studies suggest that when force output or exercise work rate are self-selected, rather than being fixed, an anticipatory mechanism (Tatterson et al. 2000; Tucker et al. 2004; Marino, 2004; Morrison et al. 2004; Cheung & Sleivert, 2004) adjusts the work rate by regulating the degree of motor-unit recruitment to prevent body temperature from rising to levels which may cause harm or premature fatigue (Marino et al. 2004). The brain thus acts pre-emptively to ensure that a catastrophic failure of thermoregulation does not occur.
Such regulation represents homeostatic control, the goal of which would be to prevent an abnormal rise in body temperature by regulating the rate of heat production (Marino et al. 2004; Tucker et al. 2004). It has also been found that the conscious sensation of fatigue, measured as the rating of perceived exertion (RPE), is correlated with changes in brain electrical rhythms (Nybo & Nielsen, 2001b) and increases in body temperature during both dynamic exercise (Galloway & Maughan, 1997; Nybo & Nielsen, 2001a) and passive heating (Gonzalez-Alonso et al. 1999; Armada-da-Silva et al. 2004). During self-paced exercise, the RPE has been shown to increase similarly in hot and cool conditions, even though power output was lower in the hot condition (Tucker et al. 2004). We proposed that, rather than acting solely as a measure of exercise intensity, the conscious perception of effort may play a regulatory function to ensure that the work rate remains at an intensity that can be safely sustained for the expected duration of the exercise (Tucker et al. 2004). If this is correct, then this regulatory control should be evident during exercise at a constant RPE. We have termed this the RPE clamp protocol.
Accordingly, we aimed to examine the regulation of exercise performance in hot (35°C) compared to normal (25°C) and cool (15°C) conditions during cycling at a predetermined, fixed rating of perceived exertion (RPE). In this protocol, the subject is free to vary the work rate, but only to ensure that the RPE remains constant throughout exercise. We have previously shown that the RPE clamp produces repeatable results with respect to trial duration, rate of power output decline, average power output, heart rate, oxygen consumption and skeletal muscle recruitment (authors' unpublished observations).
We hypothesized that during exercise at a fixed RPE, self-selected power output would decrease most rapidly in the hot condition, which would ensure that the rate of heat storage would be similar in all environmental conditions. The more-rapid decrease in power output in the heat would occur soon after the onset of exercise, and even when the rectal temperature was the same as values measured during exercise in the cooler conditions.
Methods
Subjects
Eight well-trained male cyclists were recruited from local cycling clubs and training facilities and from participation in previous studies. The subjects mean age, height, body mass and peak power output were 23.4 ± 4.2 years, 177.1 ± 7.1 cm, 70.57 ± 7.14 kg and 369.1 ± 32.8 W, respectively. Prior to participation in the study, the subjects were informed of the risks associated with the study, and informed consent was obtained in writing prior to the initiation of the study. Subjects were also required to refrain from any strenuous exercise on the day prior to or on the day of a trial. All procedures conformed with the declaration of Helsinki. The Research and Ethics Committee of the Faculty of Health Sciences of the University of Cape Town Medical School approved the study.
Testing procedure
Subjects were required to report to the laboratory on five separate occasions. During the first two occasions, subjects underwent preliminary testing consisting of tests of peak power output, familiarization trials and anthropometric measurements. The subjects' height (cm) and body mass (kg) were measured using a precision stadiometer and balance (Model 770, Seca, Bonn, Germany; accurate to 10 g). The third, fourth and fifth trials were experimental trials, during which subjects performed three cycling trials in random order, in an environmental chamber at ambient temperatures of 15°C (COOL), 25°C (NORM) and 35°C (HOT). All cycling trials were performed on a Kingcycle ergometer system, which allows subjects to cycle their own bicycles in the laboratory.
Preliminary testing
Each subject's peak power output (PPO) was determined using a modified protocol as described by Hawley & Noakes (1992). Subjects performed a self-paced warm-up for 10 min prior to beginning the test at a starting power output of 2.5 W (kg body weight)−1. The work load was increased by 20 W min−1 until exhaustion. The test was terminated when the subject was unable to match the required power output. PPO was recorded as the highest mean power output achieved over a 1-min period. The subjects were also requested to refrain from standing while cycling throughout the test.
Within 1 week of performing the PPO trial, subjects reported to the laboratory for a familiarization session, during which they underwent procedures identical to the experimental trials. Subjects completed a cycling trial at a fixed RPE in ambient temperatures of 24°C, relative humidity of 60% and a wind velocity of 10 km h−1 under the same conditions and procedures as the experimental trials (described below). During both the PPO and the familiarization trials, the subjects were familiarized with both the Borg 6–20 RPE scale (Borg, 1982). scale and instructions for the subsequent trials. A standard set of instructions was given to subjects during these two trials.
Experimental protocol
Environmental conditions
Subjects performed three randomized experimental trials, separated by 3–7 days, in an environmental chamber (Scientific Technology Corporation, Cape Town, South Africa), at ambient temperatures of 15°C, 25°C and 35°C and relative humidity and wind speed of 60% and 10 km h−1, respectively. Temperature, humidity and wind velocity were measured every minute. The wind velocity was measured at the level of the subject's head while in their riding position. We have previously shown that this wind speed is suboptimal and causes increased heat storage during exercise in the heat (Saunders et al. 2004). It was assumed that subjects were not heat acclimatized as the testing was conducted during the winter months between April and September, when the average maximum air temperature ranges from 16 to 19°C. Subjects were asked to refrain from strenuous physical exercise, caffeine and alcohol on the day of and the day prior to the trials, and from all products containing ephedrine for the duration of their involvement in the trials. Euhydration was confirmed by a body weight within 200 g of the value in the preceding trials, a resting rectal temperature of within 0.2°C of the value in the preceding trials, and a resting heart rate within six beats of the value in the previous trials (Montain & Coyle, 1992). During the trials, subjects were allowed to ingest water ad libitum.
Cycle trials at a fixed RPE
During experimental trials, subjects cycled on the Kingcycle ergometer at a fixed RPE. Subjects were instructed to cycle from the outset at a power output which was perceived by them to represent an RPE of 16 on the Borg scale. This rating corresponded to the verbal cue of between ‘hard’ and ‘very hard’ on the Borg scale. The power output measured during the first 3 min of the trial was averaged to calculate an initial value. Subjects continued cycling until their power output declined to a value corresponding to 70% of the initial value. The trial was terminated when the power output, measured every minute during the trial, fell below 70% of the initial value for three consecutive minutes. Immediately after the trial was terminated, subjects were requested to perform a maximal sprint, lasting 30 s, during which time they were verbally encouraged to produce the highest power output possible. No feedback in terms of distance covered, time elapsed or power outputs and heart rates was provided to the subject at any time during any trial.
EMG testing
Prior to each experimental session, subjects performed two maximal voluntary contractions (MVC) for normalization of the EMG signal obtained during the subsequent cycling bouts. We have previously used this method for normalization of the EMG signal obtained during maximal (Hunter et al. 2002; Hunter et al. 2003) and submaximal (St Clair Gibson et al. 2001; Tucker et al. 2004) cycling exercise. Briefly, the EMG activity of the vastus lateralis muscle was recorded during the two 5-s maximal isometric contractions using an isokinetic dynamometer (Bio-Dex dynamometer, Bio-Dex, UK). Subjects were firmly strapped into the dynamometer and the right leg attached to the arm of the dynamometer at a level slightly above the lateral malleolus. The arm was set so that the knee was at a 60 deg angle from full leg extension (0 deg). The EMG activity recorded from the contraction producing the highest force was used for normalization of the iEMG signal obtained during the subsequent cycling trials.
During each MVC and the subsequent cycling trials, the EMG activity of the vastus lateralis muscle was recorded. Before placement of the electrodes, the skin was shaved and cleaned with 95% ethanol, according to methods previously described (Kay et al. 2001; St Clair Gibson et al. 2001). A triode electrode (Thought Technology, West Chazy, NY, USA) was placed over the muscle belly of the vastus lateralis and connected to a preamplifier. Outputs from the preamplifier were relayed to a Flexcomp/DSP EMG apparatus (Thought Technology) via a fibre optic cable and stored on computer. EMG signals were captured at 1984 Hz and analysed for 5-s periods during the MVC and for 5-s periods at each measurement period during the trials. For analysis, the raw EMG signals were filtered, using a second-order, 15-Hz Butterworth high-pass filter to remove movement artefact, rectified and then smoothed with a low-pass, second-order Butterworth filter with a cut-off frequency of 5 Hz. This was performed using MATLAB software. All processed iEMG data were normalized by dividing the iEMG obtained at each work load during the trials by the iEMG obtained during the MVC performed before the start of the trial. Therefore, iEMG data are expressed as a percentage of the iEMG measured during the MVC. We have previously shown that this method of EMG normalization is reliable and valid for use in cycling trials (Hunter et al. 2002), and that the neuromuscular responses during self-paced cycling in the heat are reproducible between trials using this methodology (Kay et al. 2001).
Temperature measurements
Following calibration of the Kingcycle ergometer system and the measurement of body mass, the subjects inserted a rectal thermometer (YSI 409AC, Yellow Springs, OH, USA) 10 cm beyond their anal sphincter. Four surface thermocouples (YSI 427, Yellow Springs, OH, USA) were taped to the skin (medial calf, anterior mid-thigh, anterior mid-bicep and on the chest at a point midway between the acromium process and the nipple). The subject was then instructed to perform a self-paced 10-min warm-up on the bicycle outside the chamber at room temperature of 20°C. The duration was standardized to ensure that initial core and skin temperature values taken prior to entering the chamber were not different between the HOT, NORM and COOL conditions. At the completion of the warm-up, initial heart rate and temperature readings were obtained. Skin and rectal temperatures were recorded at 1-min intervals throughout the trial using a telethermometer (YSI 400 series, Yellow Springs, OH, USA accurate to 0.1°C).
The weighted skin temperature from four sites was calculated using the following equation (Mitchell & Wyndham, 1969):
Where Tsk, Tchest, Tarm, Tthigh and Tleg are the temperatures recorded from the skin, chest, arm, thigh and leg, respectively. The total body temperature (Tbody) used in the study was calculated from skin and core temperatures (Trec) using the following equation (Colin et al. 1971):
Heat content was calculated every minute during exercise using the following equation:
where Qc was the heat content, Tbody was the body temperature in °C, m was body mass in kg, and 3.47 was a constant measured in kJ °C−1 kg−1.
Heat storage was calculated with the following equation:
where Qs is heat storage in kJ, Qc,T1 is heat content at time 1 and Qc,T2 is the heat content at time 2.
Heart rates were measured at the start of the trial and every minute throughout the duration of the trial, using a Polar S720i heart rate monitor (Polar Electro OY, Kempele, Finland). Total exercise time was also recorded from the heart-rate monitor.
During the RPE clamp part of each trial, power output was recorded every minute by the Kingcycle ergometer. During the 30-s maximal sprint bout, power output was measured every 5 s and then averaged for the 30-s sprint.
RPE was recorded every 2 min. For the RPE clamp, subjects were required to ride constantly at an RPE of 16 using the Borg 6–20 scale. In addition, a rating of thermal comfort was also recorded at 2-min intervals, using a modified Borg category scale. This scale ranged from values of 1, which corresponded to ‘much too cool’, to 7, which corresponded to ‘much too hot’.
Statistical analysis
All statistical analyses were performed using Statistica 6.0 (Statsoft Inc. 1284–2001). Data are presented as means ± s.d. Power output, skin and body temperatures, heat storage, heart rates and iEMG were analysed using repeated measures ANOVA (trial × time). Where significant interaction effects were found, post hoc analysis was performed using a Tukey's HSD test for pairwise comparisons. Because trials varied in duration, trials were normalized with respect to time by expressing the time of each measurement as a percentage of the total trial duration. Significance was accepted at P < 0.05.
Results
The temperatures for COOL, NORM and HOT were 15.1 ± 0.3, 24.9 ± 0.4 and 35.2 ± 0.6°C, respectively. The average humidity was 68 ± 4%, 66 ± 4% and 65 ± 3% for COOL, NORM and HOT, respectively.
Exercise performance
Total trial duration was significantly shorter in the HOT compared to the NORM and COOL conditions (Fig. 1A) (34.0 ± 10.4, 48.6 ± 14.1 and 50.2 ± 16.3 min, respectively, P < 0.001). Power output decreased at a significantly higher rate in HOT compared to the the COOL and NORM conditions; 2.35 ± 0.73 W min−1 compared to 1.63 ± 0.70 and 1.61 ± 0.80 W min−1, respectively (P < 0.05).
Figure 1. Total duration during trials in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
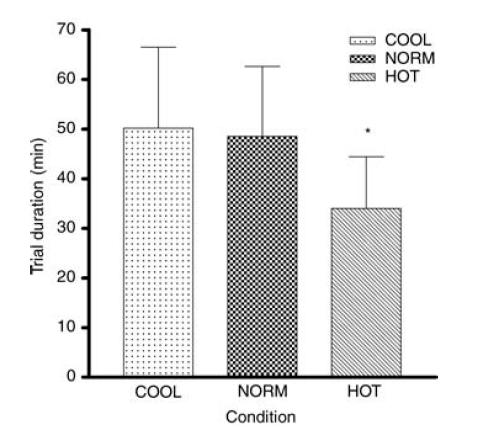
*Significantly different from NORM and COOL (P < 0.001).
Figure 2 shows the decrease in power output expressed as a percentage of total trial duration. The starting power outputs were not different between trials (COOL, 245 ± 35 W; NORM, 250 ± 43 W; HOT, 261 ± 33 W). Power output decreased linearly in all trials, as seen by correlation coefficients of 0.99, 0.97 and 0.95 for the HOT, NORM and COOL trials, respectively. The rate of decrease in power output tended to be highest in HOT, though this was not significantly different (Fig. 2).
Figure 2. Mean power output expressed relative to total trial duration in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
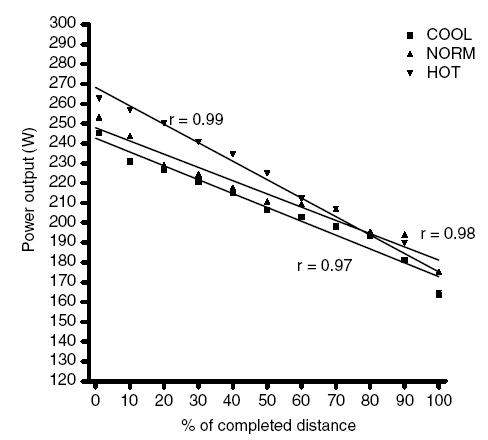
Values are means for 10 subjects, calculated at intervals of 10% of the completed distance.
During the 30-s sprint at the end of the trial, peak power outputs of 437 ± 61 W, 492 ± 78 W and 454 ± 88 W were achieved in COOL, NORM and HOT, respectively. The power outputs achieved during these 30-s sprints were not significantly different between conditions.
Heat storage
During the first 4 min of the trials, the rate of heat storage was significantly greater in the HOT condition than in NORM and COOL (Fig. 3A; P < 0.05). From 5 min until trial completion, there were no differences in rates of heat storage between conditions. When expressed relative to total trial duration, heat storage was significantly higher in HOT at 1% and again at 90% and 100% of the trial duration (Fig. 3B; P < 0.05). No significant differences were found between conditions between 1% and 90%.
Figure 3. Heat storage (kJ min−1) during the first 22 min of the trials (A) and expressed relative to total trial duration (B) for trials in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
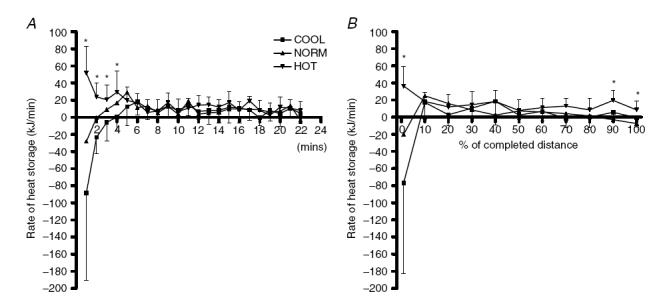
*Significantly different from NORM and COOL (P < 0.05).
The rate of heat storage was significantly higher during the first 10 min in the HOT compared to the NORM and COOL conditions (Fig. 4A; P < 0.05). From 10 to 20 min, the rate of heat storage decreased significantly in the HOT condition and increased significantly in the COOL, resulting in a similar rate of heat storage between conditions during this time period. There were no differences in heat storage between conditions from 20 to 30 min (Fig. 4A).
Figure 4. Heat storage (A) and power output expressed over 10-min intervals (B).
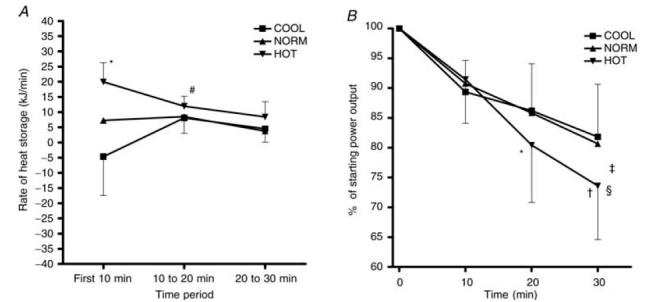
*Significantly different from COOL and NORM (P < 0.0005); #significantly different from first 10 min in HOT and COOL (P < 0.05); †significantly different from 20 min in HOT (P < 0.05); §significantly lower than in COOL at 30 min; ‡time main effect, decrease over time in all conditions (P < 0.005).
Figure 4B depicts power output, expressed as a percentage of initial power output, during 10-min intervals in the trials. Overall, there was a significant decrease in power output in all conditions, with the greatest reduction occurring in the HOT condition (P < 0.01). Over the first 10 min, the decrease in power output from starting values was not different between conditions. From 10 to 20 min, power output decreased significantly in the HOT condition but not in the NORM or COOL conditions. From 20 to 30 min power output continued to decrease at a significantly faster rate in the HOT condition than in the NORM and COOL conditions, so that the decrease from initial power output was significantly greater in HOT compared to NORM and COOL (P < 0.01).
Thermoregulatory variables
Starting rectal temperatures were 37.5 ± 0.2°C, 37.5 ± 0.2°C and 37.6 ± 0.3°C for COOL, NORM and HOT, respectively. Rectal temperatures increased significantly during the first 22 min of the trial, but there was no difference between HOT, COOL and NORM conditions (Fig. 5). When rectal temperature is expressed as a percentage of total trial duration, there was no difference between HOT, NORM and COOL during the first 80% of the duration of the trials. At 90% and 100%, the rectal temperatures were significantly higher in the HOT condition (P < 0.05). The highest rectal temperature recorded in HOT was 39.1 ± 0.6°C. Mean skin temperatures increased upon exposure in the HOT condition and decreased in COOL, resulting in significantly higher skin temperatures in the HOT compared to the NORM and COOL conditions (P < 0.05; Fig. 6). Initial heat content was not different between conditions, but as a result of the significantly higher skin temperatures from the first minute onwards in the HOT condition, the heat content was significantly greater in the HOT condition throughout the trial (P < 0.01, trial effect).
Figure 5. Rectal temperature during the first 22 min (A) and relative to total trial duration (B) in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
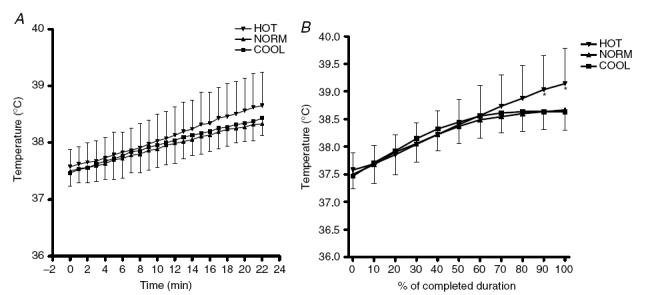
*Significantly different from NORM and COOL (P < 0.05).
Figure 6. Skin temperatures during the first 22 min during trials in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
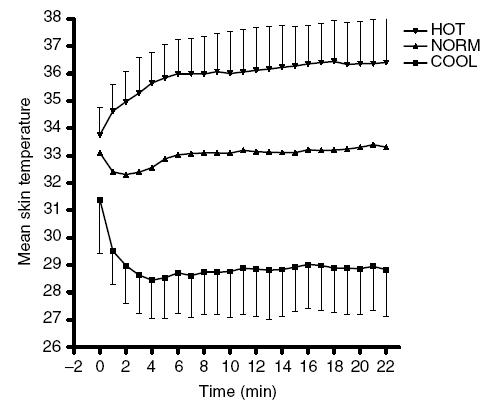
Values are means ± s.d. for 10 subjects.
iEMG activity
iEMG activity was not different at the start of the trials, and no significant differences were found during the first 22 min of trials between conditions (Fig. 7A). Expressed relative to total trial duration, iEMG activity decreased significantly in HOT, but not in NORM and COOL, and the iEMG activity was lower in HOT than in COOL at 60%, 90% and 100% of total trial duration (P < 0.05; Fig. 7B). During the final 30-s sprint, iEMG activity increased significantly in all conditions (P < 0.0001). There were no differences between conditions during the 30-s sprint.
Figure 7. iEMG activity during the first 22 min (A) and relative to total trial duration (B) in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
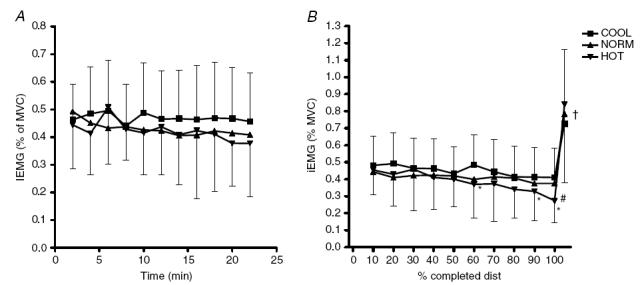
*Significantly different from COOL (P < 0.05); #significant decrease over time in HOT (P < 0.05); †significant increase in all conditions (P < 0.0001).
Heart rates and thermal comfort
Figure 8A shows heart rate during the three trials. Mean heart rates was significantly higher in the HOT compared to NORM and COOL (P < 0.05).
Figure 8. Heart rates (A) and thermal comfort scores (B) during trials in HOT (35°C), NORM (25°C) and COOL (15°C) conditions.
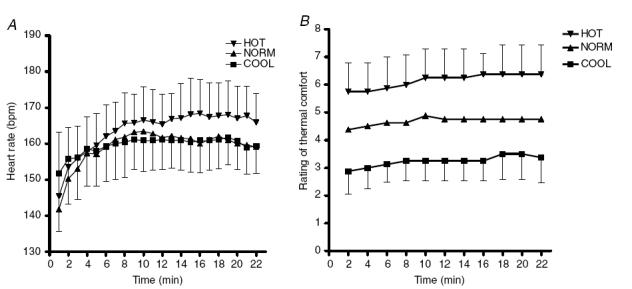
Values are means ± s.d. for 10 subjects.
Figure 8B shows that thermal comfort scores were significantly higher in the HOT compared to the COOL and NORM conditions (P < 0.01). Similarly, thermal comfort scores were significantly lower in the COOL compared to the NORM conditions (P < 0.01). Mean thermal comfort scores were 3.26 ± 0.85, 4.76 ± 0.49 and 5.95 ± 1.05 for COOL, NORM and HOT, respectively.
Discussion
Currently, two different exercise models are used to evaluate the impairment of exercise performance in the heat. In the first model, the exercise work rate is fixed, and fatigue occurs when a critically high core temperature is reached, resulting in a failure to maintain the required level of skeletal-muscle recruitment necessary to maintain the work rate (Bruck & Olschewski, 1987; Nybo & Nielsen, 2001a,b). Fatigue is thus the consequence of excessive heat accumulation, which ultimately forces a centrally mediated reduction in exercise intensity (Nielsen, 1996; Nybo & Nielsen, 2001a). In the second model, exercise is self-paced, and exercise work rate (Tatterson et al. 2000; Marino et al. 2004; Tucker et al. 2004) or force output (Morrison et al. 2004) is reduced in advance of excessive heat accumulation to ensure that a catastrophic rise in body temperature does not occur. In this model, skeletal muscle power output does not decline as a direct result of high body temperatures, but is instead regulated specifically to prevent excessive heat storage. In the present study, we used a third exercise model to evaluate the regulation of exercise performance in the heat. We allowed subjects to adjust their work rate only to maintain their subjective perception of effort (RPE) at a fixed, predetermined level, in order to evaluate the interdependence of power output, the rate of heat storage and the development of fatigue.
The first important finding of this study was that during exercise at a fixed RPE, the work rate decreased as a linear function of exercise duration (Fig. 2) and the absolute rate of decline was fastest in the HOT condition, resulting in a significantly shorter exercise time in the heat, which is a common finding (Nielsen et al. 1984). This suggests that the RPE is not simply a marker of exercise intensity, because power output was falling at different rates in the different environmental conditions even though the RPE was fixed and identical. This linear decrease in power output in the present study began shortly after the onset of exercise (Fig. 2), suggesting that exercise performance is altered well in advance of a critical increase in body temperature (Fig. 5). Therefore, the present findings support the existence of anticipatory regulation of exercise intensity, which occurs before the body temperature is abnormally elevated (Tatterson et al. 2000; Marino et al. 2000, 2004; Tucker et al. 2004; Marino, 2004), rather than reductions in performance only after body temperature has risen to critical levels as a result of excessive heat storage (Nielsen, 1996; Nybo & Nielsen, 2001a).
The second important finding was that the rate of heat storage was different only during the first 4 min of exercise in the different environmental conditions, after which time the rate of heat storage was similar despite a difference in ambient temperature of 20°C between the HOT and COOL conditions (Fig. 3A). Equal rates of heat storage during exercise in very different environmental conditions are achieved by matching rates of heat production to the different rates of heat loss achievable in the different environmental conditions, and we suggest that this is the result of alterations in exercise intensity in the present study.
The rectal temperature in the present study reached a plateau value of 38.5°C in COOL conditions after approximately 60% of the exercise duration (Fig. 5B). However, in the HOT condition, the rectal temperature continued to rise throughout exercise, reaching a value of 39.1°C (Fig. 5B) when the trial was terminated. In order for the rectal temperature to continue rising so that it eventually reached the heatstroke level of 41°C, exercise would have had to continue at this same rate of heat storage for a total of 76 min (extrapolated from the linear increase in rectal temperature in the HOT condition in Fig. 5A), which is more than twice the actual exercise duration. Assuming that the power output decreased at the same rate during this time, the power output after 76 min would be only 70 W (or 27% of starting power output). It seems more probable that prior to reaching that temperature, the power output and hence rate of heat storage, would have declined sufficiently so that the rectal temperature would have stabilized.
However, an essential requirement in order for rates of heat storage and body temperatures to be regulated in anticipation of a limiting hyperthermia is that the precise duration of exercise should be known. In the present study, this was not the case, and we therefore speculate that the exercise intensity was adjusted to ensure that the rate of heat storage was close to zero in all environmental conditions (Fig. 4A). Thus, the high rate of heat storage during the first few minutes of exposure during the HOT trial led to an anticipatory reduction in power output which reduced the rate of heat storage to a level that would not produce a limiting hyperthermia. The greater reduction in exercise intensity in the hot condition may therefore result from calculations based on the rate of heat storage, as part of feedback–feedforward control.
The final important finding was the iEMG activity decreased during exercise in all conditions (Fig. 7), although the fall was significant only in the HOT condition. The reduction in iEMG activity in the HOT condition before the core temperature reached the critical value (Nielsen et al. 2001; Nybo & Nielsen, 2001a; Drust et al. 2005) is similar to previous findings (Tucker et al. 2004). It suggests that impaired performance in the heat is, at least in part, the result of reduced activation of skeletal muscle based on sensory feedback to the central controller. That iEMG activity began to fall early in the exercise bout in the HOT condition whereas the rectal temperatures were the same in all conditions (Fig. 5A), indicates that this was not due to a direct effect of temperature on the brain, but occurred in anticipation of a critical rise in temperature. In COOL and NORM, the decrease in iEMG activity was not significant, though this may be the result of peripheral factors such as changes in metabolite levels. Such changes can result in ‘myographical signs of muscle fatigue’ (Kayser et al. 1994; Kayser, 2003), where power output can decline without equivalent changes in iEMG activity. We do not dispute that such changes are responsible, at least in part, for the observed reduction in power output. However, the important point is that during a 30-s sprint at the end of the trial, both iEMG activity (Fig. 7B) and power output increase significantly in all three conditions. Indeed, power output increased three-fold, whereas the iEMG activity doubled compared to values measured at trial completion. Thus, the power output decreased during the RPE clamp protocol in all conditions, despite the presence of a sizeable reserve capacity for force output and recruitment of skeletal muscle. This suggests that the reductions in power output and in iEMG activity were not the direct result of any failure of either muscle function or motor-unit recruitment but must instead be part of a regulatory process which maintains a reserve of motor units and is sensitive to hot ambient conditions or high rates of heat storage shortly after the initiation of exercise.
The present results support the hypothesis that the rate of heat storage, determined by skin and rectal temperatures, is the sensed variable that regulates the exercise response in all environmental conditions when the endpoint is unknown before the exercise bout begins. This is most apparent when exercise in HOT and COOL conditions is compared. Thus, upon first exposure to the hot environment, the significant increase in skin temperature produces a significantly higher rate of heat storage in the first few minutes in the HOT condition. Power output did not, however, change differently between conditions, until 10 min onwards, when the power output decreased more rapidly in the HOT trial, leading to an overall impairment in performance (Fig. 1A). The decrease in power output during HOT was thus preceded by an elevated rate of heat storage in that condition and then resulted in a reduction in heat storage which ensured that from 10 minutes onwards, the rate of heat storage was similar between the conditions. This suggests that the continual regulation of power output is based on feedback that measures the rate of heat storage.
As the work rate in this study was selected on the basis of the subject's perception of effort, the finding that the initial power output and the early decline in power output were not different between conditions indicates that the regulation of the work output is initially via a feedforward mechanism and is determined by factors other than heat-related variables. However, the work rate was then altered during exercise, on the basis of continuous afferent feedback from temperature sensors and the rate of heat storage. The novel aspect of the present study was that any changes in work rate were achieved specifically to maintain the RPE at the predetermined level, suggesting that afferent feedback, RPE and exercise work rate are interdependent.
We therefore suggest that during exercise in the heat, afferent feedback in the form of increasing skin temperatures and the significantly higher rate of heat storage in HOT result in a relative ‘up-regulation’ of the RPE. However, because the RPE must be kept constant in this RPE clamp protocol, the power output declined more rapidly in HOT than in NORM and COOL. As a result, the rate of heat storage was reduced and from 5 min onwards, there were no differences in the rates of heat storage, and hence in body temperature changes, until completion of the trials. This was demonstrated by a similar increase in the rectal temperature in all trials (Fig. 5). Thus, the regulation of exercise intensity is the result of both afferent feedback signalling and a feedforward anticipatory strategy which serves to defend thermal homeostasis while using the RPE as the mediator of this response.
Previous research supports the concept that the RPE is sensitive to changes in core and skin temperature, and hence to changes in the rate of heat storage. Nybo & Nielsen (2001b) have shown a close correlation between changes in brain electrical rhythms, RPE and increases in body temperature indicating that sensations of fatigue can be explained by changes occurring in the brain. It is known that exercise in the heat at a predetermined and fixed work rate is accompanied by elevated ratings of perceived exertion compared to exercise in cool conditions (Galloway & Maughan, 1997; Nybo & Nielsen, 2001a), and that increases in the rating of perceived exertion are closely correlated with core temperature during passive heating (Gonzalez-Alonso et al. 1999; Armada-da-Silva et al. 2004). Head cooling during exercise reduces thermal strain (Nunneley et al. 1982) and RPE (Armada-da-Silva et al. 2004) without altering rectal or brain temperatures or hypothalamic function (Nybo et al. 2002). It has been suggested that this effect is mediated by sensory information from skin thermoreceptors (Armada-da-Silva et al. 2004).
Marino et al. (2004) found that African and Caucasian runners ran an 8-km time-trial in hot conditions at similar ratings of perceived exertion, but the African runners maintained a faster running speed at a given RPE. This was attributed to the smaller body size of the Africans, which resulted in a reduced rate of heat storage at a given running speed (Dennis & Noakes, 1999). This identifies the relationship between the RPE and heat storage as an important component in the regulation of exercise intensity during self-paced exercise, as excessively high RPE values associated with inappropriately high rates of heat storage would have developed in the larger Caucasian runners had they attempted to run faster. We have previously observed the same phenomenon during exercise in hot and cool conditions (Tucker et al. 2004). Furthermore, changes in running speed in the HOT trial occurred from the onset of exercise, confirming the presence of an anticipatory (feedforward) component of this regulation.
Further support for the hypothesis that the RPE is an important mediator of exercise in the heat comes from the study of Watson et al. (2005), showing that the administration of a dopamine/noradrenaline reuptake inhibitor improved cycling performance in the heat. It was suggested that the administration of the drug acted on dopamine and noradrenaline neurotransmission to maintain arousal, motivation and reward (Watson et al. 2005), which may be reflected in a reduced RPE for a given exercise intensity. However, in that study it was found that the RPE during exercise was similar whether or not the re-uptake inhibitor had been ingested. Because the exercise in that study was self-paced, subjects clearly chose to increase their power output when using the drug, thereby exercising at a higher intensity but at the same RPE. Thus, the drug-induced ‘down-regulation’ of RPE allowed the power output to be increased at the level of discomfort (RPE) that the subjects were voluntarily prepared to sustain during exercise. However, the drug did not increase the level of discomfort subjects were prepared to accept during exercise. The undesirable consequence was that temperature rose more rapidly in the drug trial, indicating that (i) homeostatic regulation had been over-ridden, and (ii) the rating of perceived exertion plays a role in the homeostatic regulation.
In summary, this study provides evidence that the regulation of exercise intensity in the heat is achieved through a combination of afferent feedback from skin and perhaps blood thermoreceptors and the rate of heat storage and a feedforward calculation of the rate of heat storage, which, together, maintain thermal homeostasis by reducing the exercise work rate specifically to ensure that excessive heat accumulation does not occur. This shows that exercise is regulated in anticipation by a complex, intelligent system (St Clair Gibson & Noakes, 2004).
Acknowledgments
Funding for this research was provided by the Medical Research Council of South Africa, the University of Cape Town Harry Crossley and Nellie Atkinson Staff Research Funds, Discovery Health and the National Research Foundation of South Africa through the Technology and Human Resources Industry Programmes (THRIP) initiative.
References
- Armada-da-Silva PAS, Woods J, Jones DA. The effect of passive heating and face cooling on perceived exertion during exercise in the heat. Eur J Appl Physiol. 2004;91:563–571. doi: 10.1007/s00421-003-1006-0. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Bruck K, Olschewski H. Body temperature related factors diminishing the drive to exercise. Can J Physiol Pharmacol. 1987;65:1274–1280. doi: 10.1139/y87-203. [DOI] [PubMed] [Google Scholar]
- Cheung SS, Sleivert GG. Lowering of skin temperature decreases isokinetic maximal force production independent of core temperature. Eur J Appl Physiol. 2004;91:723–728. doi: 10.1007/s00421-004-1062-0. [DOI] [PubMed] [Google Scholar]
- Colin J, Timbal J, Houdas Y, Boutelier C, Guieu JD. computation of mean body temperature from rectal and skin temperatures. J Appl Physiol. 1971;31:484–489. doi: 10.1152/jappl.1971.31.3.484. [DOI] [PubMed] [Google Scholar]
- Dennis SC, Noakes TD. Advantages of a smaller body mass in humans when distance-running in warm, humid conditions. Eur J Appl Physiol. 1999;79:280–284. doi: 10.1007/s004210050507. [DOI] [PubMed] [Google Scholar]
- Drust B, Rasmussen P, Mohr M, Nielsen B, Nybo L. Elevations in core and muscle temperature impairs repeated sprint performance. Acta Physiol Scand. 2005;183:181–190. doi: 10.1111/j.1365-201X.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29:1240–1249. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Noakes TD. Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol. 1992;65:79–83. doi: 10.1007/BF01466278. [DOI] [PubMed] [Google Scholar]
- Hunter AM, St Clair Gibson A, Lambert M, Noakes TD. Electromyographic (EMG) normalization method for cycle fatigue protocols. Med Sci Sports Exerc. 2002;34:857–861. doi: 10.1097/00005768-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Hunter AM, St Clair Gibson A, Lambert DL, Nobbs L, Noakes TD. Effects of supramaximal exercise on the electromyographic signal. Br J Sports Med. 2003;37:296–299. doi: 10.1136/bjsm.37.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D, Marino FE, Cannon J, St Clair GA, Lambert MI, Noakes TD. Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur J Appl Physiol. 2001;84:115–121. doi: 10.1007/s004210000340. [DOI] [PubMed] [Google Scholar]
- Kayser B. Exercise starts and ends in the brain. Eur J Appl Physiol. 2003;90:411–419. doi: 10.1007/s00421-003-0902-7. [DOI] [PubMed] [Google Scholar]
- Kayser B, Narici M, Binzoni T, Grassi B, Cerretelli P. Fatigue and exhaustion in chronic hypobaric hypoxia: influence of exercising muscle mass. J Appl Physiol. 1994;76:634–640. doi: 10.1152/jappl.1994.76.2.634. [DOI] [PubMed] [Google Scholar]
- Marino FE. Anticipatory regulation and avoidance of catastrophe during exercise-induced hyperthermia. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:561–569. doi: 10.1016/j.cbpc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Marino FE, Lambert MI, Noakes TD. Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol. 2004;96:124–130. doi: 10.1152/japplphysiol.00582.2003. [DOI] [PubMed] [Google Scholar]
- Marino FE, Mbambo Z, Kortekaas E, Wilson G, Lambert MI, Noakes TD, Dennis SC. Advantages of smaller body mass during distance running in warm, humid environments. Pflugers Arch. 2000;441:359–367. doi: 10.1007/s004240000432. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Wyndham CH. Comparison of weighting formulas for calculating mean skin temperature. J Appl Physiol. 1969;26:616–621. doi: 10.1152/jappl.1969.26.5.616. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. Influence of graded dehydration on cardiovascular drift and hyperthermia during exercise. J Appl Physiol. 1992;73:1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol. 2004;91:729–736. doi: 10.1007/s00421-004-1063-z. [DOI] [PubMed] [Google Scholar]
- Nielsen B. Olympics in Atlanta: a fight against physics. Med Sci Sports Exerc. 1996;28:665–668. doi: 10.1097/00005768-199606000-00004. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hyldig T, Bidstrup F, Gonzalez-Alonso J, Christoffersen GR. Brain activity and fatigue during prolonged exercise in the heat. Pflugers Arch. 2001;442:41–48. doi: 10.1007/s004240100515. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Rowell LB, Bonde-Petersen F. Cardiovascular responses to heat stress and blood volume displacements during exercise in man. Eur J Appl Physiol Occup Physiol. 1984;52:370–374. doi: 10.1007/BF00943365. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Nunneley SA, Reader DC, Maldonado RJ. Head-temperature effects on physiology, comfort, and performance during hyperthermia. Aviat Space Environ Med. 1982;53:623–628. [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001a;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol. 2001b;91:2017–2023. doi: 10.1152/jappl.2001.91.5.2017. [DOI] [PubMed] [Google Scholar]
- Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A, Schabort EJ, Noakes TD. Reduced neuromuscular activity and force generation during prolonged cycling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R187–R196. doi: 10.1152/ajpregu.2001.281.1.R187. [DOI] [PubMed] [Google Scholar]
- St Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med. 2004;38:797–806. doi: 10.1136/bjsm.2003.009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AG, Dugas JP, Tucker R, Lambert MI, Noakes TD. The effects of different air velocities on heat storage and body temperature in humans cycling in a hot, humid environment. Acta Physiol Scand. 2004;183:241–255. doi: 10.1111/j.1365-201X.2004.01400.x. [DOI] [PubMed] [Google Scholar]
- Tatterson AJ, Hahn AG, Martin DT, Febbraio MA. Effects of heat stress on physiological responses and exercise performance in elite cyclists. J Sci Med Sport. 2000;3:186–193. doi: 10.1016/s1440-2440(00)80080-8. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R, Rauch L, Harley YXR, Noakes TD. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch. 2004;448:422–430. doi: 10.1007/s00424-004-1267-4. [DOI] [PubMed] [Google Scholar]
- Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. 2005;565:873–883. doi: 10.1113/jphysiol.2004.079202. [DOI] [PMC free article] [PubMed] [Google Scholar]


