Abstract
Ca2+ signalling is proposed to play an important role in skeletal muscle function during exercise. Here, we examined the expression of multifunctional Ca2+–calmodulin-dependent protein kinases (CaMK) in human skeletal muscle and show that CaMKII and CaMKK, but not CaMKI or CaMKIV, are expressed. Furthermore, the effect of exercise duration and intensity on skeletal muscle CaMKII activity and phosphorylation of downstream targets was examined. Eight healthy men exercised at ∼67% of peak pulmonary O2 uptake (V̇o2peak) with muscle samples taken at rest and after 1, 10, 30, 60 and 90 min of exercise. Ten other men exercised for three consecutive 10 min bouts at 35%, 60% and 85% V̇o2peak with muscle samples taken at rest, at the end of each interval and 30 min post-exercise. There was a rapid and transient increase in autonomous CaMKII activity and CaMKII phosphorylation at Thr287 in skeletal muscle during exercise. Furthermore, the phosphorylation of phospholamban (PLN) at Thr17, which was identified as a CaMKII substrate in skeletal muscle, was rapidly (< 1 min) increased by exercise, and remained phosphorylated 5-fold above basal level during 90 min of exercise. The phosphorylation of serum response factor at Ser103, a putative CaMKII substrate, was higher after 30 min of exercise. PLN phosphorylation at Thr17 was higher with increasing exercise intensities. These data indicate that CaMKII is the major multifunctional CaMK in skeletal muscle and its activation occurs rapidly and is sustained during continuous exercise, with the activation being greater during intense exercise.
The rise in myoplasmic Ca2+ in skeletal myocytes following surface membrane depolarization is a critical second messenger event involved in excitation–contraction coupling (Melzer et al. 1995). Along with this, Ca2+ signalling is believed to be involved in changes in carbohydrate metabolism (Hargreaves & Richter, 1988; Spriet & Heigenhauser, 2002; Rose & Richter, 2005), gene transcription (Freyssenet et al. 2004; Chin, 2005), protein synthesis (Rose et al. 2005) and modulating ion homeostasis (Sacchetto et al. 2005a) in skeletal muscle during exercise/contractions. Calmodulin (CaM) is a Ca2+ receptor protein that can bind to and alter the activity of several downstream targets (Hook & Means, 2001). One group of proteins that bind Ca2+–CaM is the Ca2+–CaM-regulated protein kinases (CaMK) which include the unifunctional glycogen phosphorylase kinase, eukaryotic elongation factor 2 kinase, and myosin light chain kinase; and multifunctional CaMK I, II, IV, as well as CaMK kinases (Hook & Means, 2001).
Given that protein phosphorylation is a rapid, convenient and flexible way to modulate the function of proteins (Cohen, 2000), the covalent regulation of proteins by Ser/Thr phosphorylation via CaMK may be a potential mechanism by which Ca2+ signals to acutely and chronically modulate skeletal muscle function in response to exercise. While it is established that CaMKII is expressed in mammalian skeletal muscle (Woodgett et al. 1983; Bayer et al. 1998; Damiani et al. 2000; Rose & Hargreaves, 2003), the extent to which other multifunctional CaMKs and upstream kinases (i.e. CaMKK) are expressed in human skeletal muscle has not been rigorously determined. Furthermore, knowledge of the activation of multifunctional CaMKs during exercise is rudimentary. It was recently shown that CaMKII autophosphorylation and Ca2+–CaM-independent CaMKII in vitro activity is higher in contracting skeletal muscle after 40 min of high intensity exercise when compared with rest (Rose & Hargreaves, 2003). Still, no definitive information exists on the rapidity of CaMKII activation during exercise or whether the magnitude of activation differs at different exercise intensities. Given that the main mode of activation of CaMK is allosteric in that the binding of Ca2+–CaM relieves autoinhibition allowing a potent increase in activity during a rise in intracellular Ca2+ (Hudmon & Schulman, 2002), perhaps a better method of detecting higher CaMKII activity is to examine a downstream target of CaMKII which would reflect an overall in vivo activation of CaMKII during repeated stimulation. Such substrates might include phospholamban (Sacchetto et al. 2005a) and serum response factor (Flück et al. 2000a). Thus, the purpose of these studies was to further characterize the expression of multifunctional CaMKs and CaMKK and potential activation in human skeletal muscle during exercise of differing duration and intensity. An additional aim of these studies was to define downstream substrates of CaMKII.
Methods
Experimental protocol
Two separate studies were conducted to investigate the effects of exercise duration and intensity on skeletal muscle CaMK and putative CaMKII substrates. The subject characteristics, experimental protocols and basic physiological responses for these studies have been reported in detail previously (Richter et al. 2004; Rose et al. 2005). The subject characteristics were not different between the studies (Table 1). The present data are published in this separate publication for reason of clarity of discussion, and because the methods to examine CaMK expression and signalling were not established in the present laboratory at the time of publication of the other studies.
Table 1.
Subject characteristics from the exercise duration and intensity studies
| Study type | Duration | Intensity |
|---|---|---|
| n | 8 | 10 |
| Age (years) | 25 ± 1 | 25 ± 2 |
| Height (m) | 1.83 ± 0.02 | 1.86 ± 0.05 |
| Weight (kg) | 77 ± 3 | 82 ± 5 |
| V̇o2peak (ml kg−1 min−1) | 55 ± 1 | 53 ± 1 |
V̇o2peak, peak rate of pulmonary O2 uptake. Data are mean ± S.E.M.
All subjects gave their informed consent to participate in these studies, which conformed to the Declaration of Helsinki. One study (Rose et al. 2005) was conducted where eight young men exercised on a cycle ergometer for 90 min at a constant workload corresponding to 67 ± 2% of their peak pulmonary oxygen consumption (V̇o2peak) with biopsies taken from vastus lateralis muscle before and after 1, 10, 30, 60 and 90 min of exercise. In another study (Richter et al. 2004), 10 young men exercised continuously for 30 min, with the exercise intensity being approximately 35%, 60% and 85% V̇o2peak for three consecutive 10 min bouts with muscle biopsies taken before exercise, after each 10 min bout, and 30 min post-exercise. Subjects fasted overnight prior to experimentation and resting biopsies were obtained after 1 h of inactivity in the supine position. Muscle samples taken during exercise were frozen in liquid nitrogen between 15 and 30 s after cessation of activity, and the tissue was stored at −80°C until required.
Analytical techniques
All materials were purchased from Sigma-Aldrich (USA) unless otherwise stated. The sample preparation prior to analyses has been reported in detail previously (Richter et al. 2004; Rose et al. 2005). To measure protein expression and phosphorylation, equal amounts of muscle lysate proteins were resolved by SDS-PAGE, transferred to polyvinyl-difluoride filter membrane (Immobilon, Millipore, USA), after which membranes were incubated in a blocking buffer (2% skim milk or 3% BSA, pH 7.4) to reduce background signal. The membranes were then incubated with primary and secondary antibodies for optimized times and concentrations, and washed with Tris-buffered saline (0.01% Tween-20). Proteins were visualized with chemiluminescence (ECL plus, Amersham Pharmacia Biotech, UK) and light detection under conditions of negligible ambient light (Kodak Image Station 2000MM, USA). The primary antibodies used were anti-CaMKII (BD Biosciences-Pharmingen, USA; 612624), anti-CaMKII (Santa Cruz Biotech., USA; sc-9035), anti-phospho-Thr287-CaMKII (Cell Signalling Technology, inc., USA; 3361), anti-phospholamban (Cyclacel, UK; 010-14), anti-phospho-Thr17-phospholamban (Cyclacel, UK; 010-13), anti-serum response factor (Santa Cruz Biotech., USA; sc-335), and anti-phospho-Ser103-serum response factor (Cell Signalling Technology, inc., USA; 4261). Secondary antibodies were from DakoCytomation (Denmark). Band intensity was quantified by Kodak imaging software (Kodak 1D 3.5, USA). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown).
To measure kinase activity muscle extracts were analysed according to Rose & Hargreaves (2003). In brief, CaMKII activity of muscle extracts was measured in the presence (maximal activity) or absence (autonomous activity) of Ca2+–calmodulin with autocamtide-2 (Upstate Biotechnology, USA) as the peptide substrate. To verify that the activities from muscle extracts were CaMKII, CaMKII was immunoprecipitated from samples of select time points using polyclonal antibodies (3 μg; Santa-Cruz Biotech., USA; sc-9035) from 50 μg of muscle extract proteins, and CaMKII activity was measured from the immunoprecipitates (Rose & Hargreaves, 2003).
To determine whether the antibodies used were detecting CaM-regulated proteins, pooled human skeletal muscle and rat brain lysate proteins were subjected to CaM affinity precipitation (CaM-AP). To do this, rat brain (0.1 μg μl−1) and human skeletal muscle (1.0 μg μl−1) samples were diluted with immunoprecipitation (IP) buffer (50 mm Tris (pH 7.4), 1mm EGTA, 5 mm Na4P207) and 500 μl of the diluted samples were mixed with 20 μl of calmodulin–agarose slurry (Upstate Biotech., USA; 14-426) in two conditions: in the presence or absence of 2 mm CaCl2 (∼1 mm free Ca2+). An untreated aliquot of the diluted samples was also taken. After gentle mixing at 4°C overnight, samples were spun (1000 g, 1 min) and aliquots of the supernatants were taken (post-AP). The remaining supernantants were aspirated and the pellets were washed 5 × with IP buffer containing 2 mm CaCl2 depending on the condition. A 30 μl aliquot of 2 × sample buffer was added to each pellet and other samples were mixed with appropriate amounts of 6 × sample buffer, and the samples were denatured by boiling and immunoblotted for CaM kinases using isoform-specific antibodies. The primary antibodies used were anti-CaMKI (provided by D. G. Hardie, Dundee, UK), anti-CaMKII (BD Biosciences-Pharmingen, USA; 612624), anti-CaMKIV (Calbiochem, USA; 208709), and anti-CaMKK (BD Biosciences-Pharmingen, USA; 610544).
In vitro experiments
All phospho-specific antibodies used were tested for phosphospecificity. This was done by powdering human skeletal muscle samples and extracting aliquots of powdered muscle in a buffer with or without phosphatase inhibitors (i.e. sodium fluoride, sodium pyrophosphate, sodium orthovanadate). Equal amounts of proteins from these extracts were then subjected to incubation in a reaction buffer containing 10 mm Hepes (pH 7.2), 5 mm MgCl2, 1 mm EGTA, 5 mm MnCl2 with or without 2 U μg−1λ-protein phosphatase (New England Biolabs, UK) at 30°C for 1 h. The incubation was stopped by addition of sample buffer and samples were subjected to immunoblotting procedures. Extraction without phosphatase inhibitors and incubation with phosphatase completely removed or greatly reduced signal obtained when using the phospho-specific antibodies used in this study (Fig. 1). Aside from the phospho-specific band at 67 kDa, the anti-pS103-SRF antibody also gave a strong band at about 52 kDa that was not a phosphoprotein, and was cut away prior to transfer with subsequent phospho-SRF analysis. Importantly, when the samples were immunoblotted using an anti-actin antibody there was no difference in signal between samples (data not shown).
Figure 1. Validation of phosphospecific antibodies.
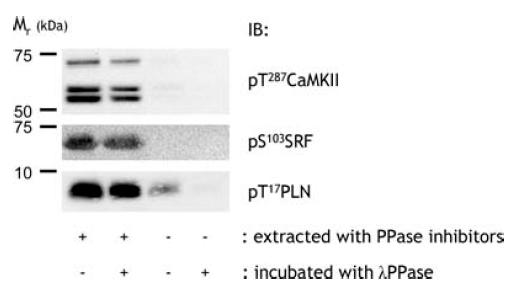
Human skeletal muscle extract proteins were subject to dephosphorylation and were immunoblotted using various phosphospecific antibodies as described in Methods. IB, immunoblot; CaMKII, Ca2+–calmodulin-dependent protein kinase II; PLN, phospholamban; SRF, serum response factor; T, threonine; S, serine; PPase, protein phosphatase.
To further examine the relationship between CaMKII autophosphorylation and autonomous activity, an in vitro experiment was performed to manipulate CaMKII autophosphorylation levels. This was achieved by taking aliquots of four separate basal human skeletal muscle extracts (final concentration: 2.4 μg μl−1) and incubating them with differing amounts of Ca2+ and CaM (Upstate Biotechnology, USA). All samples were reacted in duplicate in a buffer containing 10 mm Hepes (pH 7.2), 1 mm EGTA, 0.5 mm Na3VO4 and 400 μm ATP under four different conditions. One condition was without added Ca2+ or CaM, another was with 2 mm CaCl2, another with 2 mm CaCl2+ 0.12 μm CaM, and another with 2 mm CaCl2+ 1.2 μm CaM. These reaction mixes were preheated to 30°C, and the reaction was initiated by the addition of the sample. The reaction proceeded for 1 min at 30°C and was stopped by placing in an ice slurry (0°C) and the addition of 5 mm EGTA (final concentration). An aliquot of each sample was then taken for immunoblot analysis of CaMKII autophosphorylation. Aliquots of the remaining sample were taken and CaMKII was immunoprecipitated in duplicate as described above and these immunopreciptates were analysed for CaMKII activity (Rose & Hargreaves, 2003). Immunoprecipitate CaMKII activity is lower than lysate CaMKII activity as there is an inability to completely precipitate CaMKII from lysates using this antibody, even at higher concentrations (data not shown).
To determine whether PLN phosphorylation at Thr17 is catalysed by CaMKII in human skeletal muscle an in vitro experiment was performed. To this end, biopsies (110–200 mg) were taken from the vastus lateralis muscle from three young men (26 ± 1 years, 1.88 ± 0.04 m, 83 ± 11 kg) at rest. These samples were placed immediately in a buffer (10 μl mg−1) containing 50 mm Tris (pH 7.4), 150 mm NaCl, 250 mm sucrose, 1 mm EDTA, 1 mm EGTA, 0.2 mm PMSF, 1 mm benzamidine, 1% (vol/vol.) protease inhibitor cocktail and mechanically homogenized (Polytron, Kinematica, USA) and placed on ice for 15 min after which time they were spun at 90 000 g for 30 min at 4°C. The supernatants (soluble cytosolic proteins; C) were harvested, and the pellets were resuspended in the above buffer (5 μl mg−1 original mass) by gentle mechanical disruption by passing the pellet material through a 21 gauge needle and mixed well until no visible particles remained. This material was then spun at 500 g for 15 min at 4°C and the supernatants (soluble light membranes; P2) were taken. The remaining pellets were suspended in the above buffer (5 μl mg−1 original mass) containing 300 mm NaCl, 1% Nonidet-P40, and 0.1% SDS, mixed well, spun (500 g, 15 min, 4°C) and the supernatants (detergent-soluble heavy membranes; P1) were taken. Aliquots (25 μl) of P2 were incubated in a final volume of 50 μl in a buffer containing 10 mm Hepes (pH 7.2), 1 mm EGTA, 5 mm MgCl2, 0.5 mm Na3VO4 and 400 μm ATP under three different conditions. One condition was without added Ca2+ or CaM, another with 2 mm CaCl2+ 1.2 μm CaM, and another with 2 mm CaCl2+ 1.2 μm CaM + 1 μm autocamtide-2-related inhibitory peptide (AIP; Calbiochem, USA; 189480). AIP is a specific CaMKII inhibitor when compared with other multifunctional CaM kinases, cAMP-dependent protein kinase, and protein kinase C (Ishida et al. 1995) and inhibits the majority of CaM kinase activity in human skeletal muscle extracts (Rose & Hargreaves, 2003). These reaction mixes were preheated to 30°C, and the reaction was initiated by the addition of the sample. The reaction proceeded for 10 min at 30°C and was stopped by placing in an ice slurry (0°C) and the addition of 10 μl of sample buffer. These samples were then immunoblotted for phosphorylated and total PLN and CaMKII. In addition, the protein concentration of the tissue fractions was determined, and samples were prepared for immunoblotting to determine the enrichment of certain proteins in fractions (SERCA2 antibodies were from Santa Cruz Biotechnology, USA; 8094). Of note, 68 ± 3%, 16 ± 1% and 15 ± 2% of total protein extracted was in fractions C, P1 and P2, respectively. SRF was not examined because very little SRF was detected in P2 (data not shown).
Calculations and statistics
Relative mobility (Mr) values of bands were calculated by a plot of log Mr of molecular mass standards (Precision Plus, Bio-Rad, USA) versus electrophoretic mobility of the given band. Statistical testing was done with descriptive analyses, t tests (two-tailed), or one-way ANOVA for repeated measures with post hoc (Scheffe) testing performed when differences were significant as appropriate using SPSS v. 13.0. Correlations and regression analyses were performed using SigmaPlot v. 9.0. Data are expressed as mean ± s.e.m. and differences were considered to be significant when P was less than 0.05.
Results
Expression of multifunctional Ca2+–CaM-dependent kinases in human skeletal muscle
Figure 2 shows representative blots of Ca2+–CaM-dependent kinase (CaMK) isoforms in rat brain and human skeletal muscle. Isoforms of CaMKI, CaMKII, CaMKIV and CaMKK are expected to migrate at a relative mobility of 37–45 kDa (Naito et al. 1997), 50–73 kDa (Tombes et al. 2003), 65 kDa (Ohmstede et al. 1989), and 67–73 kDa (Edelman et al. 1996), respectively. Substantial signal was obtained in human skeletal muscle at the appropriate relative mobility's with antibodies for CaMKI (38 kDa), CaMKII (55–60 and 73 kDa) and CaMKK (66–70 kDa), albeit at much lower signal: protein when compared with rat brain. Studies of mammalian skeletal muscle also show expression of a non-kinase splice variant of the CaMKIIα gene called α-CaMKII kinase anchoring protein (αKAP) that migrates at 21–23 kDa (Bayer et al. 1998; Damiani et al. 2003). As has been shown before (Damiani et al. 2003), αKAP (23 kDa) was also detected in human skeletal muscle, but not in rat brain, when using an antibody (i.e. sc-9035) directed against an epitope within the association domain of CaMKII. As indicated in Fig. 2, for skeletal muscle CaMKII the bands at 55–60 kDa probably correspond to γ/δ isoforms, whereas the 73 kDa band is likely to be the muscle specific β isoform splice variant termed βM (Bayer et al. 1998; Damiani et al. 2000). However, when blotting for CaMKIV, only trace amounts of reactivity was detected which is in line with other studies showing a lack of CaMKIV expression in skeletal muscle (Ohmstede et al. 1989; Rose & Hargreaves, 2003; Akimoto et al. 2004; Chin, 2004).
Figure 2. Detection of multifunctional Ca2+–calmodulin-dependent kinase isoforms in human skeletal muscle.
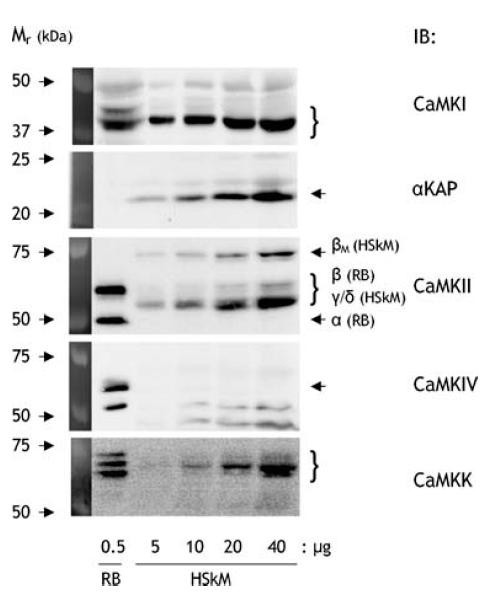
Rat brain (RB) and human skeletal muscle (HSkM) extract proteins were immunoblotted using isoform-specific CaM kinase antibodies. The expected mobility of the isoforms is indicated (see Results for details). IB, immunoblot; CaMK, Ca2+–calmodulin-dependent protein kinase.
To determine whether these proteins detected were Ca2+–CaM binding proteins, lysates of rat brain and human skeletal muscle were incubated with CaM agarose in the presence or absence of free Ca2+. As shown in Fig. 3 (left panel), although there was incomplete precipitation, all antibodies used detected an enrichment of signal when CaM binding proteins were affinity precipitated from rat brain lysates with calmodulin–agarose in the presence of Ca2+ (Fig. 3, left panel). An additional band with lower mobility was also detected in the rat brain CaM-AP when blotting using CaMKI antibodies, and may be a lowly expressed isoform of CaMKI. However, when Ca2+–CaM binding proteins were affinity precipitated from human skeletal muscle lysates (Fig. 3, right panel), only CaMKII and CaMKK could be detected. Surprisingly, despite detecting substantial signal in human skeletal muscle using CaMKI antibodies as has also been shown in murine skeletal muscle (Akimoto et al. 2004), this protein was not enriched by Ca2+–CaM affinity precipitation, indicating that it is not a CaM kinase. Taken together, these data indicate that CaMKII is the major multifunctional CaMK in human skeletal muscle, with some CaMKK also expressed, whereas CaMKI and CaMKIV are not expressed at detectable levels.
Figure 3. CaMKII and CaMKK, but not CaMKI or CaMKIV, are expressed in human skeletal muscle.
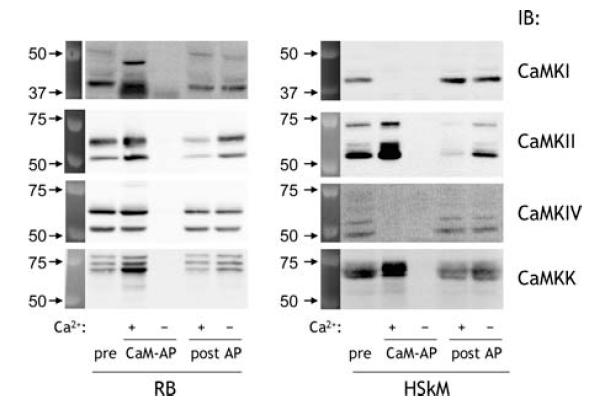
Rat brain (RB) and human skeletal muscle (HSkM) extract proteins were subject to calmodulin affinity precipitation (CaM-AP) in the presence or absence of free Ca2+ as described in Methods. Pre-AP, CaM-AP and post-AP samples were immunoblotted using isoform-specific CaM kinase antibodies. IB, immunoblot; CaMK, Ca2+–calmodulin-dependent protein kinase.
CaMKII and exercise duration
In response to exercise, there were no significant changes in CaMKII isoform expression or maximal CaMKII activity (Figs 4–6). However, in response to exercise there was a transient increase in autonomous CaMKII activity and phosphorylation. In particular, when measured from skeletal muscle lysates, autonomous CaMKII activity was 1.3 ± 0.2% of maximal at rest, and increased to 11.3 ± 1.4% (12 ± 3-fold) of maximal after 1 min of exercise but then decreased to 2–4% (1- to 3-fold higher than basal) of maximal by 10 min of exercise and remained at this level for the remaining 90 min of exercise (Fig. 4). To validate that CaMKII activity obtained from assaying lysates was specific for CaMKII, activity from CaMKII immunoprecipitates was also measured. Analogous to activity measured from lysates, skeletal muscle autonomous CaMKII activity measured from immunoprecipitates was increased from 0.9 ± 0.3% maximal at rest to 16.7 ± 2.3% maximal at 1 min with a decrease to 2.0 ± 0.4% maximal at 60 min of exercise (Fig. 5). Importantly, autonomous CaMKII activity was higher at all times during exercise when compared with the resting state (Figs 4 and 5).
Figure 4. Effect of exercise duration on skeletal muscle lysate Ca2+–calmodulin-dependent protein kinase II activity.
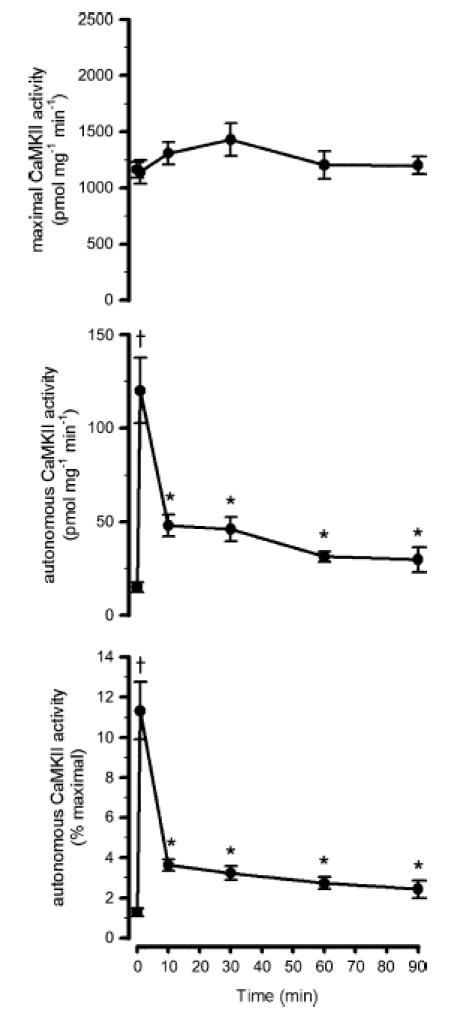
Skeletal muscle extracts were assayed in vitro for Ca2+–calmodulin-dependent protein kinase II (CaMKII) activity in the presence (i.e. maximal activity; top panel) or absence (i.e. autonomous activity; middle panel) of Ca2+ and calmodulin. The bottom panel shows the autonomous activity expressed relative to the maximal activity (i.e.% maximal). Data are mean ± s.e.m., n = 8. †P < 0.01 versus time 0, 10, 30, 60 and 90 min; *P < 0.05 versus time 0.
Figure 6. Effect of exercise duration on skeletal muscle Ca2+–calmodulin-dependent protein kinase II expression and phosphorylation.
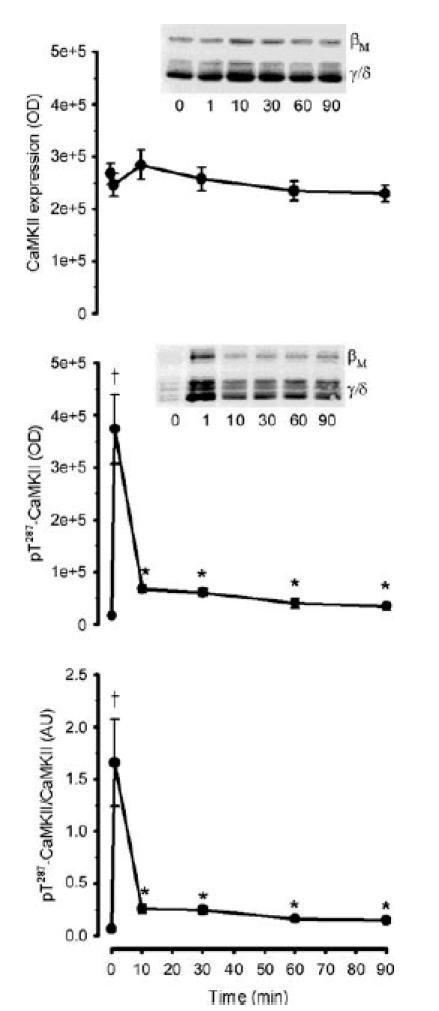
Skeletal muscle extract proteins were immunoblotted for Ca2+–calmodulin-dependent protein kinase II expression (CaMKII; top panel) and phospho-Thr287 CaMKII (middle panel). The bottom panel is signal intensity for phospho-Thr287 CaMKII relative to CaMKII expression. Representative immunoblots are shown and CaMKII isoforms are indicated. Data are mean ± s.e.m., n = 8. †P < 0.01 versus time 0, 10, 30, 60 and 90 min; *P < 0.05 versus time 0.
Figure 5. Effect of exercise duration on skeletal muscle immunoprecipitate Ca2+–calmodulin-dependent protein kinase II activity.
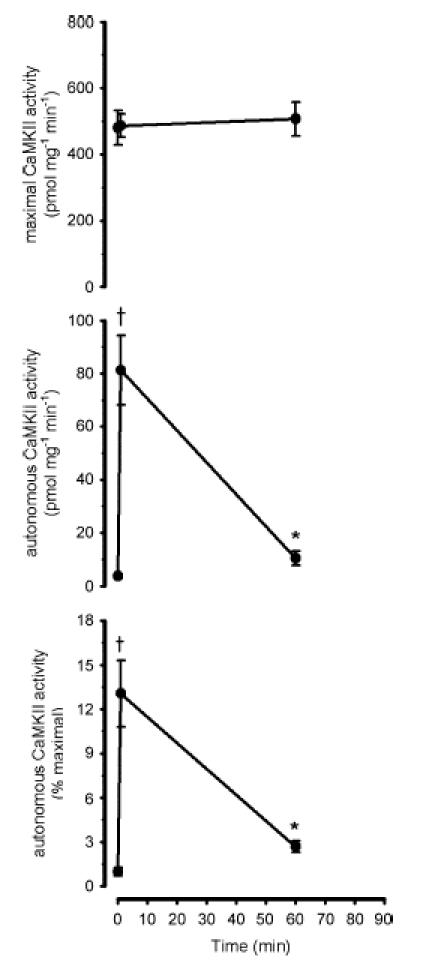
Ca2+–calmodulin-dependent protein kinase II (CaMKII) was immunoprecipitated from skeletal muscle extracts and immunoprecipitates were assayed in vitro for CaMKII activity in the presence (i.e. maximal activity; top panel) or absence (i.e. autonomous activity; middle panel) of Ca2+ and calmodulin. The bottom panel shows the autonomous activity expressed relative to the maximal activity (i.e.% maximal). Data are mean ± s.e.m., n = 8. †P < 0.01 versus time 0 and 60 min; *P < 0.05 versus time 0.
There was a positive linear correlation between the changes in phospho-Thr287 CaMKIIβM and CaMKIIγ/δ in individual samples (r2 = 0.932, P < 0.01; data not shown), so the phosphorylation of each of these isoforms of individual samples was summed. CaMKII phosphorylation at Thr287 was 35 ± 9-fold higher than rest after 1 min of exercise, but decreased to levels 2- to 3-fold higher than basal after 10 min of exercise and remained at this level thereafter (Fig. 6). There was a positive-linear correlation between CaMKII phosphorylation at Thr287 and autonomous CaMKII activity (r2 = 0.884, P < 0.01; Fig. 7A), even when the 1 min data points were removed (r2 = 0.330, P < 0.05; Fig. 7A, inset). Furthermore, when the level of autophosphorylation was manipulated by prior incubation of skeletal muscle lysates with varying concentrations of free Ca2+ and CaM, there was also a positive-linear correlation between CaMKII phosphorylation and activity (r2 = 0.996, P < 0.01; Fig. 7B).
Figure 7. In vitro autonomous Ca2+–calmodulin-dependent protein kinase II activity is positively related to phosphorylation at Thr287.
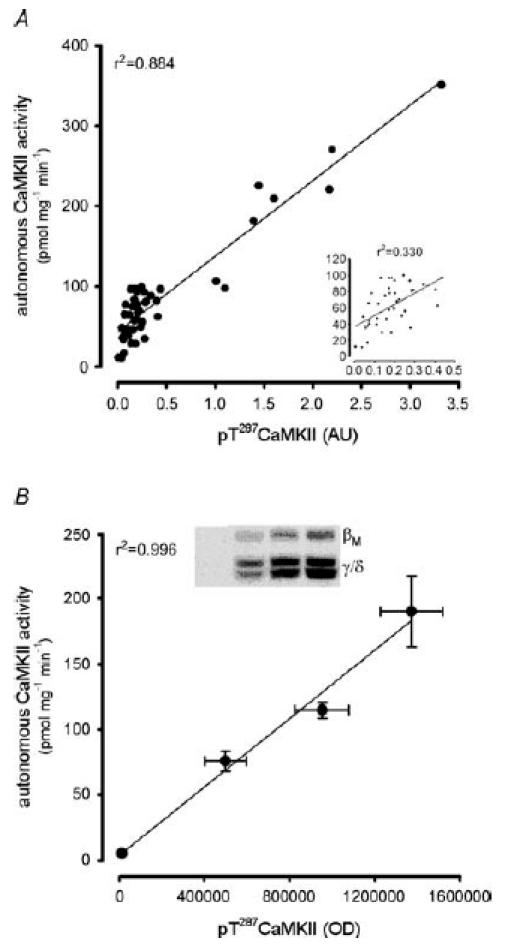
Correlation between Ca2+–calmodulin-dependent protein kinase II (CaMKII) phosphorylation at Thr287 and autonomous CaMKII activity from skeletal muscle samples taken before and during exercise (A; r2 = 0.884, P < 0.01) and from basal skeletal muscle extracts (n = 4) manipulated to differing levels of phosphorylation by incubation with differing amounts of Ca2+ and calmodulin (B; r2 = 0.996, P < 0.01). A, inset shows the correlation without the 1 min data points (r2 = 0.330, P < 0.05). B, inset shows a representative immunoblot of differing levels of CaMKII phosphorylation at Thr287 with different incubation conditions.
To examine the in vivo activation of CaMKII, the phosphorylation of putative CaMKII substrates was examined. As phospholamban (PLN) was the only identified substrate of CaMKII in human skeletal muscle (Margreth et al. 2000), the phosphorylation of this protein was examined using phosphospecific antibodies. In response to exercise, there was a 3.5 ± 0.6-fold increase in PLN phosphorylation at Thr17 after 1 min, and was 5- to 7-fold higher than basal at 10 min and remained at this level thereafter (Fig. 8). There was no change in PLN expression with exercise (Fig. 8). Serum response factor (SRF) phosphorylation at Ser103 was not different at 1 or 10 min, but was approximately 1-fold higher than basal between 30 and 90 min of exercise (Fig. 9). There was no change in SRF expression with exercise (Fig. 9).
Figure 8. Effect of exercise duration on skeletal muscle phospholamban expression and phosphorylation.
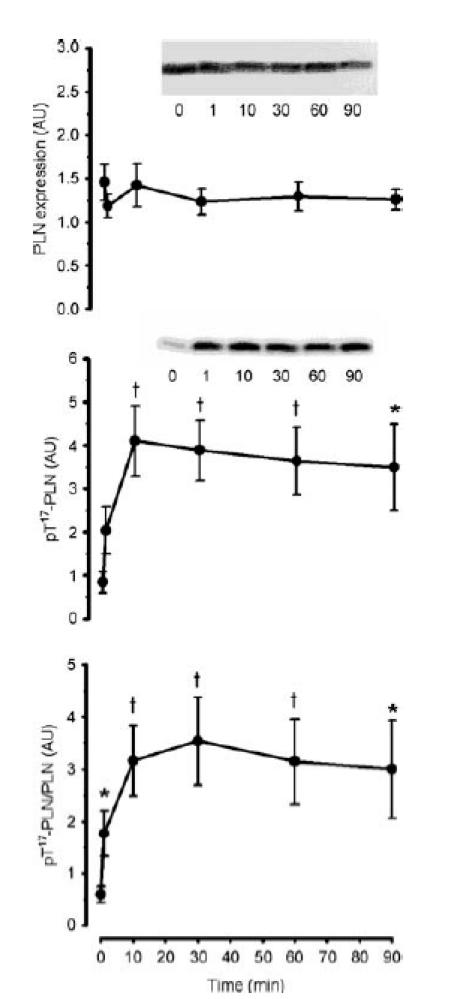
Skeletal muscle extract proteins were immunoblotted for phospholamban expression (PLN; top panel) and phospho-Thr17 PLN (middle panel). The bottom panel is signal intensity for phospho-Thr17 PLN relative to PLN expression. Representative immunoblots are shown. Data are mean ± s.e.m., n = 8. †P < 0.01 versus time 0; *P < 0.05 versus time 0.
Figure 9. Effect of exercise duration on skeletal muscle serum response factor expression and phosphorylation.
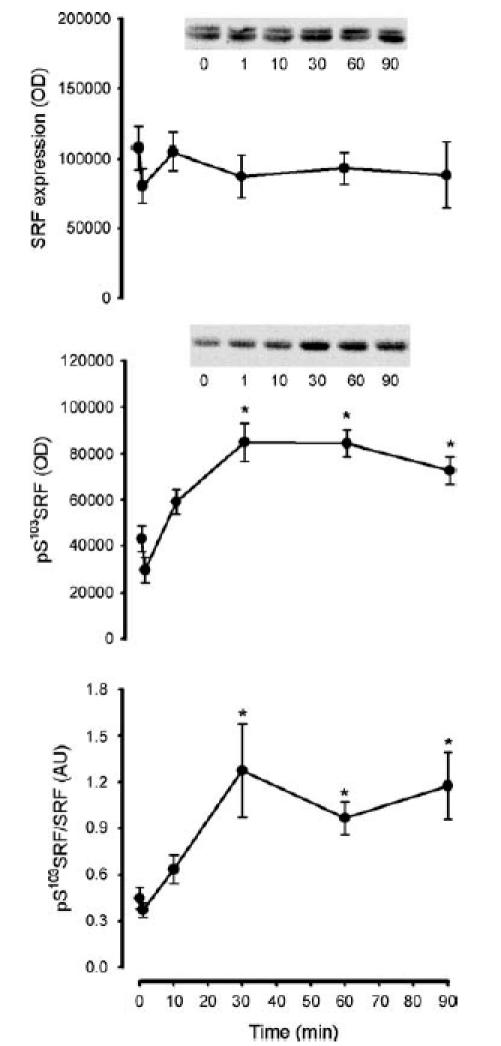
Skeletal muscle extract proteins were immunoblotted for serum response factor (SRF) expression (top panel) and phospho-Ser103SRF (middle panel). The bottom panel is signal intensity for phospho-Ser103SRF relative to SRF expression. Representative immunoblots are shown. Data are mean ± s.e.m., n = 8. *P < 0.05 versus time 0.
CaMKII and exercise intensity
As PLN phosphorylation at Thr17 may be representative of CaMKII activity in vivo (see below) and plateaus after 10 min of exercise (Fig. 8), the effect of exercise intensity was examined using an exercise bout with 10 min stages of incrementally graded exercise intensity. Skeletal muscle PLN phosphorylation at Thr17 was approximately 1- to 3-fold higher with exercise intensities of 35% and 60% V̇o2peak, but was higher still (3- to 5-fold) at 85% V̇o2peak when compared with rest (Fig. 10). PLN phosphorylation at Thr17 had returned to resting levels at 30 min post-exercise, and there were no differences in PLN expression at any time (Fig. 10). Of note, as samples were denatured, only the PLN monomer (6 kDa) was detected.
Figure 10. Effect of exercise intensity on skeletal muscle phospholamban expression and phosphorylation.
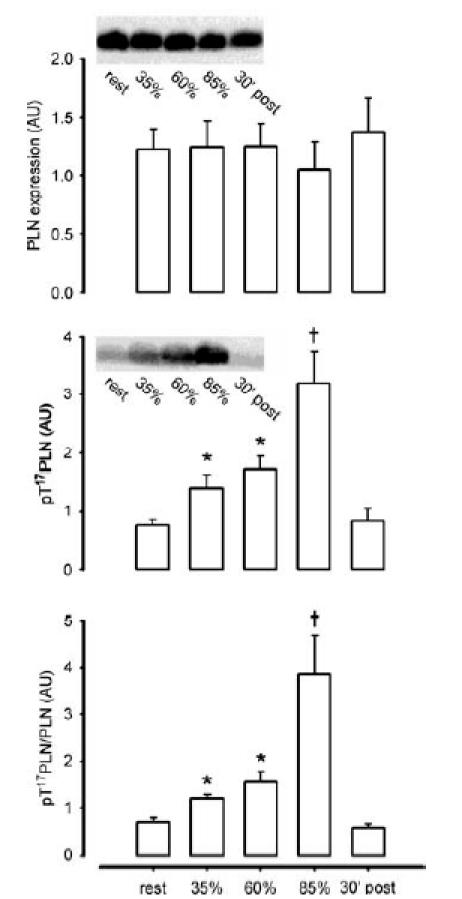
Skeletal muscle extract proteins were immunoblotted for phospholamban expression (PLN; top panel) and phospho-Thr17PLN (middle panel). The bottom panel is signal intensity for phospho-Thr17PLN relative to PLN expression. Representative immunoblots are shown. Data are mean ± s.e.m., n = 8. †P < 0.05 versus rest, 35%, 60% and 30 min post-exercise; *P < 0.05 versus rest.
PLN is a CaMKII substrate in human skeletal muscle
To investigate whether PLN phosphorylation at Thr17 is a CaMKII substrate, an in vitro phosphorylation experiment was performed. To do this, endogenous CaMKII was activated in intact membranes from human skeletal muscle samples (see Methods). Although the extraction of membranes using this procedure was poor as indexed by the low amount of SERCA2 (110 kDa) detected in P2, CaMKII and PLN were detected in this fraction, with the majority (i.e. ∼80%) of CaMKII expressed in the soluble, cytosolic fraction (Fig. 11A). Furthermore, PLN phosphorylation at Thr17 was markedly induced by combined incubation with free Ca2+, CaM and ATP versus ATP alone and could be largely prevented by the addition of 1 μm of autocamtide-2-related inhibitory peptide (AIP; Fig. 11B), a specific CaMKII inhibitor (Ishida et al. 1995). Importantly, CaMKII autophosphorylation was also largely blocked by AIP (Fig. 11B). Taken together, these data strongly indicate that PLN is a substrate of endogenous CaMKII in human skeletal muscle.
Figure 11. Phosphorylation of PLN at Thr17 is CaMKII dependent.
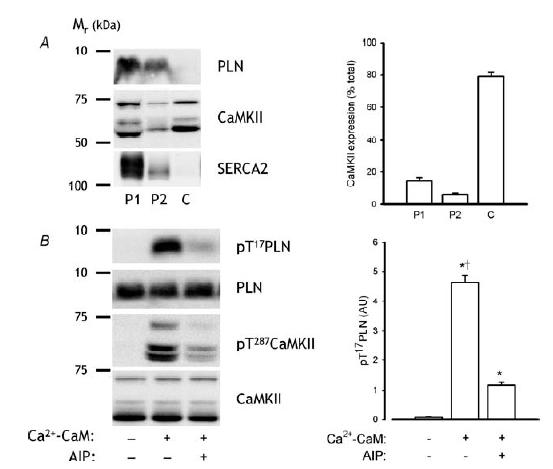
Skeletal muscle membrane proteins were extracted and subject to phosphorylation assays as described in Methods. A, expression of PLN, CaMKII and SERCA2 in different fractions. B, PLN and CaMKII phospho-Thr level and expression after different incubation conditions. Representative immunoblots are shown. Data are mean ± s.e.m., n = 3. †P < 0.01 versus −/−; *P < 0.001 versus +/+.
Discussion
One major novel finding of these studies is that autonomous CaMKII activity and phosphorylation at Thr287 is rapidly and transiently induced by exercise in working skeletal muscle (Figs 4–6). Similar to a previous study (Rose & Hargreaves, 2003) there was no effect of exercise on skeletal muscle CaMKII expression or maximal activity (Figs 4–6). In contrast to this apparent transient activation of CaMKII during continuous exercise, the phosphorylation of PLN at Thr17, a CaMKII substrate, was rapid and sustained during exercise suggesting that in vivo CaMKII activity follows the same pattern.
In the study by Rose & Hargreaves (2003) there was a 50–100% increase in skeletal muscle autonomous CaMKII activity after 40 min, but not after 5 min of exercise. In the present study, there was a marked increase in autonomous CaMKII activity after 1 min of exercise, which then decreased to levels 2- to 3-fold higher than basal from 10 to 90 min of exercise (Figs 4 and 5). The differences between the two studies is difficult to explain, but may relate to the lack of increase in the phosphorylation of the βM isoform of CaMKII during exercise in the previous study (Rose & Hargreaves, 2003). Indeed, in the present study the changes in CaMKII phosphorylation at Thr287 appeared to mimic those of autonomous CaMKII activity with a positive linear correlation between changes in CaMKII autophosphorylation and autonomous activity with exercise (Fig. 7A). Furthermore, when CaMKII phosphorylation levels were manipulated in vitro, there was also a positive-linear relationship between CaMKII autophosphorylation and autonomous activity (Fig. 7B), as has been shown previously using rabbit skeletal muscle (Pelosi & Donella-Deana, 2000). Taken together, these data indicate that changes in CaMKII phosphorylation at Thr287 are causally related to changes in autonomous CaMKII activity in skeletal muscle with exercise. These findings concur with studies using mutational analysis of autophosphorylation sites of neuronal CaMKIIα that phosphorylation of this residue is obligatory for the increase in autonomous activity in response to increases in Ca2+ (Hanson et al. 1989).
The mechanism by which skeletal muscle CaMKII becomes phosphorylated during exercise is probably an autophosphorylation mechanism as discussed by Rose & Hargreaves (2003). Indeed, similar to neuronal CaMKII (Hudmon & Schulman, 2002), skeletal muscle CaMKII probably exists as a multimeric complex of 12 individual CaMKII enzymes (Woodgett et al. 1983, 1984), and upon rises in cytosolic [Ca2+] and Ca2+–CaM binding, the CaMKII multimer undergoes intersubunit phosphorylation at Thr287 (Fig. 7B; Hudmon & Schulman, 2002). One functional consequence of CaMKII autophosphorylation is that the affinity for calmodulin is greatly enhanced (Meyer et al. 1992), and thus the rapid autophosphorylation may be a mechanism to accelerate the activation of CaMKII with contractions. It is also possible that autophosphorylation may allow CaMKII to remain partially active between Ca2+ transients by disrupting autoinhibition (Hudmon & Schulman, 2002), thereby allowing persistent phosphorylation of downstream substrates. However, it should be noted that the increase in autonomous activity was only up to 2–4% of Ca2+–CaM-stimulated activity during the majority of exercise time (Figs 2 and 3), meaning that the rise in activity during the Ca2+‘spike’ may be quantitatively more important in the overall increase in CaMKII activity. Indeed, the patterns of CaMKII phosphorylation (Fig. 6) and phosphorylation of the putative CaMKII substrate PLN (Fig. 8), which may be indicative of in vivo CaMKII activity (Fig. 11) during exercise, were remarkably different. The transient nature of the increase in autophosphorylation with exercise (Fig. 4) is difficult to explain, but may be related to differences in Ca2+ uptake and release kinetics by the sarcoplasmic reticulum and thus Ca2+–CaM binding, or a differential action of CaMKII phosphatases, on CaMKII over time. Indeed, skeletal muscle CaMKII may indirectly regulate its own dephosphorylation by increasing protein phosphatase-1c (PP1c) activity through phosphorylation of the PP1c-targeting subunit GM (Sacchetto et al. 2005b).
To gain further insight into the regulation of skeletal CaMKII activity with exercise as well as functional consequences of CaMKII activation, an examination of the phosphorylation of putative downstream targets was conducted. It is known that CaMKII is enriched in the sarcoplasmic reticulum (SR) in skeletal muscle and can phosphorylate various SR proteins, and thus may be involved in regulating Ca2+ homeostasis (reviewed by Sacchetto et al. 2005a). Indeed, studies using murine fast-twitch muscle show that CaMKII inhibition affects Ca2+ release, but not uptake rate, during twitches (Tavi et al. 2003), and thus may directly phosphorylate SR Ca2+-release channels or an associated protein (Sacchetto et al. 2005a). The evidence for using PLN phosphorylation at Thr17 as an index of in vivo CaMKII activity is rather strong. When purified human skeletal muscle SR was used, the only detected substrate of Ca2+–CaM-induced phosphorylation appeared to be phospholamban (Margreth et al. 2000), probably at Thr17 (Damiani et al. 2000) and CaMKII inhibition decreases PLN phosphorylation at Thr17 in human skeletal muscle in vitro (Fig. 11) as well as cardiac muscle in vivo (Zhang et al. 2005). In the present study, the phosphorylation of PLN at Thr17 was higher after 1 min of exercise and remained elevated throughout 90 min of exercise (Fig. 6), indicating that in vivo skeletal muscle CaMKII activity is rapidly increased and is sustained during exercise. This intuitively seems likely because increases in myoplasmic Ca2+ are a requirement for excitation–contraction coupling (Melzer et al. 1995), and thus skeletal muscle Ca2+ signalling should occur during each muscle contraction during exercise. Indeed, other studies show that skeletal muscle eukaryotic elongation factor 2 (eEF2) phosphorylation by the CaM dependent eEF2 kinase is rapid and sustained during exercise (Rose et al. 2005) and electrically induced cardiac muscle contraction increases PLN phosphorylation at Thr17 (Hagemann et al. 2000). However, it cannot be discounted that CaMKII signals though an intermediary kinase/phosphatase to increase PLN phosphorylation in skeletal muscle with exercise.
With exercise intensity, PLN phosphorylation at Thr17 was 1- to 3-fold higher at 35 and 60% V̇o2peak, but was higher still (3- to 5-fold) at 85% V̇o2peak when compared with samples taken at rest, and was reversed to levels similar to resting 30 min after the cessation of activity (Fig. 10). These findings suggest that the increase in skeletal muscle CaMKII activity in vivo is greater at high compared with low or moderate intensity exercise. This may be explained by a greater recruitment of individual skeletal muscle fibres (Sale, 1987), differential levels of activation in fast versus slow twitch fibres (Baylor & Hollingworth, 2003), or greater Ca2+–CaM signalling in recruited fibres (Hennig & Lømo, 1985; Grottel & Celichowski, 1999), at hard exercise intensities. Importantly, unlike some other mammalian species, PLN is expressed in both type I and some type II skeletal muscle fibres of humans (Margreth et al. 2000).
When expressed, PLN binds to and inhibits sarco/endo-plasmic reticulum ATPases (SERCA; reviewed by (Simmerman & Jones, 1998), which actively transport Ca2+ from the myoplasm into the SR. Phosphorylation of PLN (Ser16 by cAMP-dependent protein kinase and Thr17 by CaMKII) relieves this inhibition allowing higher affinity of SERCA for Ca2+ and thus higher SR Ca2+ uptake rates (for review see Simmerman & Jones, 1998; Maclennan & Kranias, 2003). Along with the increase in Thr17 phosphorylation, a 1-fold increase in skeletal muscle PLN phosphorylation at Ser16 during exercise was also observed, albeit with a slower time course (A. J. Rose, J. F. P. Wojtaszewski, B. Kiens & E. A. Richter, preliminary data). Thus, it may be that skeletal muscle PLN phosphorylation during exercise is a mechanism to accelerate SR Ca2+-uptake rates during contraction to maintain normal Ca2+ homeostasis (Fig. 12), a process that consumes a substantial proportion of ATP turnover in contracting skeletal muscle (Szentesi et al. 2001; Zhang et al. 2006). While the role of PLN in skeletal muscle function of non-human mammals is controversial (Briggs et al. 1992; Movsesian et al. 1992; Liu et al. 1997; Slack et al. 1997; Ferrington et al. 2002; Song et al. 2004; Vangheluwe et al. 2005), it is possible that PLN may play a more important role in humans (Margreth et al. 2000; Haghighi et al. 2003), although this remains to be formally tested.
Figure 12. Schematic illustration of CaMKII signalling in human skeletal muscle during exercise.
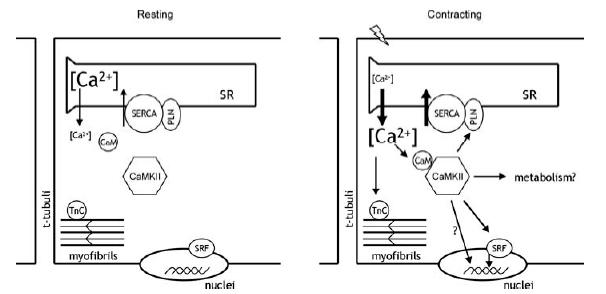
During contraction, myoplasmic free Ca2+ rises and is a pivotal event in excitation–contraction coupling via binding to myofibrillar troponin-C (TnC). Ca2+ also binds to calmodulin (CaM) which, in turn, binds to and regulates many proteins including Ca2+–calmdulin-dependent protein kinase II (CaMKII). Allosteric activation of CaMKII leads to phosphorylation of putative downstream targets such as phospholamban (PLN) and serum response factor (SRF), leading to modulation of Ca2+ homeostasis, gene transcription, and possibly metabolism in skeletal muscle during exercise (see Discussion for details). SR, sarcoplasmic reticulum; SERCA, sarco/endo-plasmic reticulum Ca2+-ATPase.
Another putative substrate of skeletal muscle CaMKII is the transcription factor serum response factor (SRF). Flück et al. (2000a) showed that SRF is a substrate of skeletal muscle CaMKII in vitro, and was phosphorylated at Ser103 and Thr160. The present study shows that, in contrast to the relatively rapid increase in phosphorylation of PLN, there was a more gradual increase in phosphorylation of SRF at Ser103 being approximately 1-fold higher than basal after 30 min of exercise (Fig. 9). This relatively slow phosphorylation of skeletal muscle SRF with exercise is consistent with findings by Irrcher & Hood (2004) who showed that SRF phosphorylation at Ser103 and SRF DNA-binding activity was increased after 30 and 60 min of electrical stimulation of C2C12 skeletal muscle cells. Furthermore, Ca2+–CaM-independent SRF kinase activity has been shown to be higher in skeletal muscle of rats in the hours after running exercise (Flück et al. 2000b). Given that SRF is a transcription factor that is phosphorylated by CaMKII (Flück et al. 2000a) and that CaMKII expressed in nuclei of muscle fibres may be activated during stimulation (Liu et al. 2005), these data suggest that CaMKII may also signal to alter gene transcription with exercise through SRF (Fig. 12). Alternatively, CaMKII might signal to other proteins such as mitogen-activated protein kinases, which are activated in skeletal muscle during exercise (Sakamoto & Goodyear, 2002), and can phosphorylate SRF (Heidenreich et al. 1999). Furthermore, CaMKII may also act via removal of class II histone deacetylase-mediated repression of myocyte enhancer factor 2 DNA-binding activity to increase gene transcription with exercise (Liu et al. 2005). However, since it has been questioned as to whether CaMKII is expressed in skeletal muscle nuclei (Sacchetto et al. 2005a), it is clear that further work is required to examine the potential role of CaMKII in the adaptation of skeletal muscle to the demands of exercise.
The activation of CaMKII during exercise may also be involved in alterations in carbohydrate metabolism. Indeed, inhibition of multifunctional CaM kinases reduces contraction-stimulated glucose transport in rodent skeletal muscles (Wright et al. 2004, 2005). It has also been shown that skeletal muscle CaMKII can phosphorylate glyceraldehyde-3-phosphate dehydrogenase (Sacchetto et al. 2005b; Singh et al. 2004), glycogen synthase (Woodgett et al. 1983; Sacchetto et al. 2005b) and the glycogen and protein phosphatase-1c targeting subunit GM (Sacchetto et al. 2005b) in a Ca2+–CaM-dependent manner. Thus along with phosphorylase kinase (Hargreaves & Richter, 1988) pyruvate dehydrogenase phosphatase (Spriet & Heigenhauser, 2002), and possibly fructose-1,6-bisphosphatase–aldolase complex (Mamczur et al. 2005), activation of CaMKII by Ca2+ may be a mechanism by which ATP provision via glycogenolysis and glycolysis is accelerated during contractions.
Finally, it is apparent that the majority (i.e. ∼80%) of CaMKII that is expressed in human skeletal muscle is localized to the soluble cytosolic fraction (Fig. 11). The majority of work that has been conducted has examined the functional properties and substrates of membrane-associated CaMKII (present study; Sacchetto et al. 2005a). Thus, it is clear that future work should focus on examining the potential substrates and functions of cytosolic CaMKII.
In summary, the findings of this study indicate that CaMKII activation in working skeletal muscle occurs rapidly and is sustained during continuous exercise, with the activation being greater during high-intensity exercise. While CaMKII phosphorylation at Thr287 probably does not reflect in vivo CaMKII activity, it may be an important mechanism for CaMKII activation during exercise.
Acknowledgments
The authors acknowledge the skilled technical assistance of Irene Beck Nielsen and Betina Bolmgren. Christa Broholm, Kristian Kiillerich and Morten Olsen are also acknowledged for assistance in conducting the studies. D. Grahame Hardie is acknowledged for kindly providing anti-CaMKI antibodies. Gratitude is extended to Thomas Jensen, Bjarke Kobberø and Thomas Alsted for helpful discussion during the preparation of the manuscript. The financial support from the Copenhagen Muscle Research Centre, the Danish Medical and Natural Science Research Council, an Integrated Project (contract number LSHM-CT-2004-005272) from the European Union, as well as the Novo-Nordisk Research and Lundbeck Foundations, is also acknowledged. A.J.R. was supported by a postdoctoral fellowship from the Carlsberg Foundation and from the European Union.
References
- Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, Schulman H. αKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs FN, Lee KF, Wechsler AW, Jones LR. Phospholamban expressed in slow-twitch and chronically stimulated fast-twitch muscles minimally affects calcium affinity of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1992;267:26056–26061. [PubMed] [Google Scholar]
- Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc. 2004;63:279–286. doi: 10.1079/PNS2004335. [DOI] [PubMed] [Google Scholar]
- Chin ER. Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol. 2005;99:414–423. doi: 10.1152/japplphysiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Cohen P. The regulation of protein function by multisite phosphorylation – a 25 year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Margreth A. Variation of phospholamban in slow-twitch muscle sarcoplasmic reticulum between mammalian species and a link to the substrate specificity of endogenous Ca2+-calmodulin-dependent protein kinase. Biochim Biophys Acta. 2000;1464:231–241. doi: 10.1016/s0005-2736(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Salviati L, Margreth A. Two splice variants of CaMKII-anchoring protein are present in the sarcoplasmic reticulum of rabbit fast-twitch muscle. Biochem Biophys Res Commun. 2003;302:73–83. doi: 10.1016/s0006-291x(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Edelman AM, Mitchelhill KI, Selbert MA, Anderson KA, Hook SS, Stapleton D, Goldstein EG, Means AR, Kemp BE. Multiple Ca2+-calmodulin-dependent protein kinase kinases from rat brain. Purification, regulation by Ca2+-calmodulin, and partial amino acid sequence. J Biol Chem. 1996;271:10806–10810. doi: 10.1074/jbc.271.18.10806. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Yao Q, Squier TC, Bigelow DJ. Comparable levels of Ca-ATPase inhibition by phospholamban in slow-twitch skeletal and cardiac sarcoplasmic reticulum. Biochemistry. 2002;41:13289–13296. doi: 10.1021/bi026407t. [DOI] [PubMed] [Google Scholar]
- Flück M, Booth FW, Waxham MN. Skeletal muscle CaMKII enriches in nuclei and phosphorylates myogenic factor SRF at multiple sites. Biochem Biophys Res Commun. 2000a;270:488–494. doi: 10.1006/bbrc.2000.2457. [DOI] [PubMed] [Google Scholar]
- Flück M, Waxham MN, Hamilton MT, Booth FW. Skeletal muscle Ca2+-independent kinase activity increases during either hypertrophy or running. J Appl Physiol. 2000b;88:352–358. doi: 10.1152/jappl.2000.88.1.352. [DOI] [PubMed] [Google Scholar]
- Freyssenet D, Irrcher I, Di Connor MK, Hood DA. Calcium-regulated changes in mitochondrial phenotype in skeletal muscle cells. Am J Physiol Cell Physiol. 2004;286:C1053–C1061. doi: 10.1152/ajpcell.00418.2003. CM &. [DOI] [PubMed] [Google Scholar]
- Grottel K, Celichowski J. The influence of changes in the stimulation pattern on force and fusion in motor units of the rat medial gastrocnemius muscle. Exp Brain Res. 1999;127:298–306. doi: 10.1007/s002210050799. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Richter EA. Regulation of skeletal muscle glycogenolysis during exercise. Can J Sport Sci. 1988;13:197–203. [PubMed] [Google Scholar]
- Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, et al. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrcher I, Hood DA. Regulation of Egr-1, SRF, and Sp1 mRNA expression in contracting skeletal muscle cells. J Appl Physiol. 2004;97:2207–2213. doi: 10.1152/japplphysiol.00388.2004. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kranias EG, Schneider MF. Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am J Physiol. 1997;273:C1915–C1924. doi: 10.1152/ajpcell.1997.273.6.C1915. [DOI] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Mamczur P, Rakus D, Gizak A, Dus D, Dzugaj A. The effect of calcium ions on subcellular localization of aldolase-FBPase complex in skeletal muscle. FEBS Lett. 2005;579:1607–1612. doi: 10.1016/j.febslet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Margreth A, Pallanca A, Damiani E. Calmodulin kinase-mediated phosphorylation of phospholamban in skeletal muscle sarcoplasmic reticulum. A critical reappraisal of the state of the problem at the light of new findings with human normal and diseased muscle. Basic Appl Myol. 2000;10:151–157. [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Movsesian MA, Morris GL, Wang JH, Krall J. Phospholamban-modulated Ca2+ transport in cardiac and slow twitch skeletal muscle sarcoplasmic reticulum. Second Messengers Phosphoproteins. 1992;14:151–161. [PubMed] [Google Scholar]
- Naito Y, Watanabe Y, Yokokura H, Sugita R, Nishio M, Hidaka H. Isoform-specific activation and structural diversity of calmodulin kinase I. J Biol Chem. 1997;272:32704–32708. doi: 10.1074/jbc.272.51.32704. [DOI] [PubMed] [Google Scholar]
- Ohmstede CA, Jensen KF, Sahyoun NE. Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. J Biol Chem. 1989;264:5866–5875. [PubMed] [Google Scholar]
- Pelosi M, Donella-Deana A. Localization, purification, and characterization of the rabbit sarcoplasmic reticulum associated calmodulin-dependent protein kinase. Biochemistry Mosc. 2000;65:259–268. [PubMed] [Google Scholar]
- Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol. 2004;560:909–918. doi: 10.1113/jphysiol.2004.071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+–calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Bovo E, Damiani E. The Ca2+-calmodulin dependent protein kinase II system of skeletal muscle sarcoplasmic reticulum. Basic Appl Myol. 2005a;15:5–17. [Google Scholar]
- Sacchetto R, Bovo E, Donella-Deana A, Damiani E. Glycogen- and PP1c-targeting subunit GM is phosphorylated at Ser48 by sarcoplasmic reticulum-bound Ca2+-calmodulin protein kinase in rabbit fast twitch skeletal muscle. J Biol Chem. 2005b;280:7147–7155. doi: 10.1074/jbc.M413574200. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev. 1987;15:95–151. [PubMed] [Google Scholar]
- Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- Singh P, Salih M, Leddy JJ, Tuana BS. The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme complex at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J Biol Chem. 2004;279:35176–35182. doi: 10.1074/jbc.M402282200. [DOI] [PubMed] [Google Scholar]
- Slack JP, Grupp IL, Luo W, Kranias EG. Phospholamban ablation enhances relaxation in the murine soleus. Am J Physiol. 1997;273:C1–C6. doi: 10.1152/ajpcell.1997.273.1.C1. [DOI] [PubMed] [Google Scholar]
- Song Q, Young KB, Chu G, Gulick J, Gerst M, Grupp IL, Robbins J, Kranias EG. Overexpression of phospholamban in slow-twitch skeletal muscle is associated with depressed contractile function and muscle remodeling. FASEB J. 2004;18:974–976. doi: 10.1096/fj.03-1058fje. [DOI] [PubMed] [Google Scholar]
- Spriet LL, Heigenhauser GJ. Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev. 2002;30:91–95. doi: 10.1097/00003677-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Szentesi P, van Zaremba R, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531:393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. MW &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Allen DG, Niemela P, Vuolteenaho O, Weckstrom M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. J Physiol. 2003;551:5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR, Cohen P, Yamauchi T, Fujisawa H. Comparison of calmodulin-dependent glycogen synthase kinase from skeletal muscle and calmodulin-dependent protein kinase-II from brain. FEBS Lett. 1984;170:49–54. doi: 10.1016/0014-5793(84)81366-6. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Davison MT, Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983;136:481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab. 2005;288:E1062–E1066. doi: 10.1152/ajpendo.00561.2004. [DOI] [PubMed] [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Andersson DC, Sandstrom ME, Westerblad H, Katz A. Cross-bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00578.2005. 10.1152/ajpcell.00578.2005. [DOI] [PubMed] [Google Scholar]
- Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]


