Abstract
Full expression of reflex cutaneous vasodilatation is dependent on nitric oxide (NO) and vasodilatation is attenuated in healthy older humans. NO bioavailability in aged skin may be decreased by an age-related upregulation of arginase, which reciprocally regulates the NO-synthase (NOS) substrate l-arginine (l-Arg). We hypothesized that increased arginase activity contributes to attenuated vasodilatation in aged skin by limiting l-Arg for NOS-mediated NO synthesis. Five microdialysis fibres were placed in forearm skin of 10 young (Y, 23 ± 1 years) and 9 older (O, 68 ± 1 years) human subjects, serving as control (C, Ringer solution), NOS-inhibited (10.0 mmNG-nitro-l-arginine), arginase-inhibited (5.0 mm (S)-(2-boronoethyl)-l-cysteine + 5.0 mmNω-hydroxy-nor-l-arginine), l-arg supplemented (l-Arg; 10.0 mml-arginine) and combined arginase-inhibited + l-Arg sites. After 20 min thermoneutral baseline, cutaneous vasodilatation was induced by passive whole-body heating to increase oral temperature (Tor) by 1.0°C. Red blood cell flux was measured by laser-Doppler flowmetry over each microdialysis site. Cutaneous vascular conductance was calculated (CVC = flux/mean arterial pressure) and normalized to maximal CVC (CVCmax, 28.0 mm sodium nitroprusside + local heating to 43°C). Cutaneous vasodilatation during heating was attenuated in O (Y, 42 ± 1, versus O, 30 ± 1%CVCmax, P < 0.001) at control sites. NOS inhibition decreased vasodilatation in both age groups compared to C (Y, 22 ± 2; O, 18 ± 2%CVCmax; P < 0.001). Arginase inhibition, l-Arg supplementation, and arginase inhibition + l-Arg supplementation augmented vasodilatation in O (arginase-inhibited, 46 ± 4; l-Arg, 44 ± 4; arginase-inhibited + l-arg, 46 ± 5%CVCmax; P < 0.001 versus C) but not in Y (arginase-inhibited, 46 ± 4; l-Arg, 38 ± 4; arginase-inhibited + l-Arg, 44 ± 4%CVCmax; P > 0.05 versus C). Increasing l-Arg for NO synthesis by either arginase inhibition or direct l-Arg supplementation restores the age-related deficit in reflex cutaneous vasodilatation.
Skin blood flow is neurally controlled by two distinct branches of the sympathetic nervous system: an adrenergic vasoconstrictor system and an active vasodilator system (Grant & Holling, 1938). Under normothermic conditions, the cutaneous vasculature is under tonic adrenergic control. However, with rising body core temperature, skin blood flow initially increases through a withdrawal of tonic vasoconstriction, and upon reaching a specific threshold skin blood flow further increases by the active vasodilator system (Roddie et al. 1957). Cutaneous active vasodilatation is mediated by sympathetic cotransmission of acetylcholine and an unknown neurotransmitter (Kellogg et al. 1995). Vasoactive intestinal peptide (VIP) and histamine 1 (H1) receptor activation contribute to reflex cutaneous vasodilatation (Bennett et al. 2003; Wilkins et al. 2004; Wong et al. 2004). Moreover, these vasodilator pathways mediate downstream nitric oxide (NO)-dependent vasodilatation, which is required for full expression of the reflex vasodilatory response (Kellogg et al. 1998a; Shastry et al. 1998).
Aged humans exhibit attenuated cutaneous vasodilatory responses during hyperthermia (Kenney et al. 1997), resulting from a diminished neurogenic cotransmitter contribution and an increased reliance on impaired NO-dependent vasodilatation (Holowatz et al. 2003). Attenuated NO-dependent vasodilatation is associated with decreased NO bioavailability. In aged skin, decreased NO bioavailability is probably multifaceted, involving several potential signalling pathways, including dysregulated utilization of the NO substrate l-arginine (l-Arg).
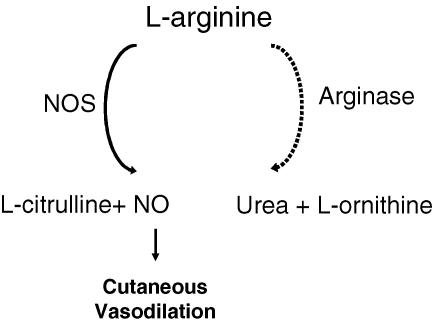
One potential mechanism that has been implicated in reduced l-Arg availability for NOS is augmented vascular arginase activity (Hecker et al. 1995; Berkowitz et al. 2003). Arginase is constitutively expressed in two isoforms (I and II), which catalyse the conversion of l-Arg to l-ornithine and urea during the final step of the urea cycle. Arginase I is most likely the predominant isoform in the vasculature and is capable of reciprocally regulating endothelial NOS by competing for the common substrate l-Arg (Berkowitz et al. 2003; White et al. 2006) (Fig. 1).
Figure 1. Schematic representation of the l-arginine pathway in the cutaneous vasculature.
l-Arginine (l-Arg) is the common substrate for both nitric oxide synthase (NOS) and arginase. Arginase catalyses the conversion of l-Arg to urea and l-ornithine, which is the precursor to proline and is important in cell growth and repair. NOS catalyses the conversion of l-Arg to l-citrulline and nitric oxide (NO). NO is required for full expression of reflex cutaneous vasodilatation.
In aged and hypertensive animal models of microvasculature dysfunction, augmented arginase activity contributes to impaired NO bioavailability (Berkowitz et al. 2003; John & Schmieder, 2003; White et al. 2006), and both acute and chronic inhibition of arginase restore endothelial NO-dependent vasodilatation. Furthermore, augmented arginase activity may serve as a potential explanation for the ‘l-arginine paradox’, which states that despite the high intracellular concentrations of l-Arg that far exceed the Michaelis constant for endothelial NOS (2 × 10−6m) (Grody et al. 1987; Griffith & Stuehr, 1995), NO-dependent vasodilatation in vivo can be further increased by the administration of exogenous l-Arg. Taken together, these findings suggest that increased arginase activity may limit the available pool of l-Arg for NOS and therefore the bioavailability of NO. However, the role of arginase in regulating intracellular stores of l-Arg and subsequent NO-dependent vasodilatation in humans remains unclear.
Therefore, the purpose of this study was to determine the role of arginase in reflex cutaneous vasodilatation in aged humans. We hypothesized that arginase inhibition alone and with concurrent l-Arg supplementation would augment cutaneous vasodilatation during passive whole-body heat stress by increasing l-Arg availability for NO synthesis.
Methods
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University, and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent was voluntarily obtained from all subjects prior to participation. Studies were performed on 10 young (18–27 years) and 9 older (65–72 years) men and women. Each subject underwent a complete medical screening, including blood chemistry, lipid profile evaluation (Quest Diagnostics, Nichol Institute, Chantilly, VA, USA), physical examination, and an assessment of maximal oxygen uptake (V̇O2max) (SensorMedics Corporation, Yorba Linda, CA, USA). All subjects were screened for the presence of cardiovascular, dermatological and neurological disease. Subjects were normally active, normotensive, nondiabetic, healthy nonsmokers who were currently not taking medications, including aspirin therapy, hormone replacement therapy or oral contraceptives. All young female subjects were studied on days 2–7 of the early follicular phase of their menstrual cycle.
Instrumentation and measurements
All protocols were performed in a thermoneutral laboratory, with the subject in the supine position and with the experimental arm at heart level. Upon arrival at the laboratory between 07.00 and 09.00 h, subjects were instrumented with five intradermal microdialysis fibres (MD 2000; Bioanalytical Systems, IN, USA) (10 mm, 20 kDa cutoff membrane) in the skin on the right ventral forearm. Microdialysis sites were at least 4.0 cm apart to insure no cross-reactivity of pharmacological agents being delivered to the skin. Microdialysis fibres were placed at each site by first inserting a 25 gauge needle through unanaesthetized skin using sterile technique. The entry and exit points were ∼2.5 cm apart. The microdialysis fibre was then threaded through the needle, and the needle was withdrawn, leaving the fibre in place. The microdialysis fibres were taped in place and perfused with lactated Ringer solution during the insertion trauma resolution period at a rate of 2.0 µl min−1 (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems) for 60–90 min.
To obtain an index of skin blood flow, cutaneous red blood cell (RBC) flux was measured with an integrated laser-Doppler flowmeter probe placed in a local heater (MoorLAB, Temperature Monitor SH02; Moor Instruments, Devon, UK) on the skin directly above each microdialysis membrane. All laser-Doppler probes were calibrated using Brownian standard solution. Cutaneous vascular conductance (CVC) was calculated as RBC flux divided by mean arterial pressure.
To control whole-body temperature, subjects wore a water-perfused suit that covered the entire body except head, hands and experimental arm, and a water-impermeable rain suit to minimize evaporative heat loss. The subject's electrocardiogram was monitored throughout the protocol, and blood pressure was measured via brachial auscultation every 5 min. Oral temperature (Tor) was continuously monitored during baseline and throughout whole-body heating with a thermister placed in the sublingual sulcas as an index of body core temperature. The subjects were instructed to keep the thermister in the same location in the sublingual sulcus and not to open their mouths or speak during the protocol. Mean skin temperature was calculated as the unweighted average of six copper–constantan thermocouples placed on the chest, middle back, abdomen, upper arm, thigh and calf. During the insertion trauma resolution and baseline periods, thermoneutral water (34°C) was perfused through the suit to clamp whole-body temperature. During whole-body heating, 50°C water was perfused through the suit to raise the Tor of the subject by 1.0°C above baseline body temperature.
Experimental protocol
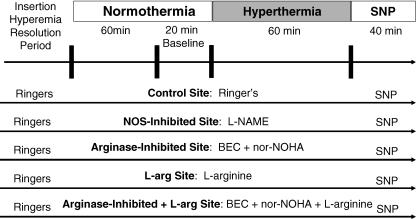
A schematic representation of the protocol is illustrated in Fig. 2. RBC flux over each microdialysis site was monitored during the insertion trauma resolution period. Following this period, microdialysis sites were randomly assigned to receive the following: (1) 10.0 mmNG-nitro-l-arginine methylester (l-NAME) to inhibit NO production by NOS, (2) a combination of 5.0 mm (S)-(2-boronoethyl)-l-cysteine-HCl (BEC) and 5.0 mmNω-hydroxy-nor-l-arginine (nor-NOHA) to inhibit arginase (Calbiochem, San Diego, CA, USA), (3) 10.0 mml-Arg (Sigma) to supplement the substrate for NOS and arginase, and (4) 5.0 mm BEC + 5.0 mm nor-NOHA + 10.0 mml-Arg to inhibit arginase and supplement the substrate for NOS and arginase. All pharmacological agents were dissolved in lactated Ringer solution. A fifth microdialysis site was perfused with only lactated Ringer solution to serve as a control.
Figure 2. A schematic illustration of the protocol.
Each arrow represents a microdialysis treatment site. BEC, (S)-(2-boronoethyl)-l-cysteine; nor-NOHA, Nω-hydroxy-nor-l-arginine; SNP, sodium nitroprusside.
Our laboratory previously showed that a 10.0 mm dose of l-NAME was sufficient to maximally inhibit NOS in both subject groups (Minson et al. 2002). Extensive pilot work was conducted to ensure that the concentrations of arginase inhibitors maximally inhibited the arginase pathway. Briefly, varying concentrations (0.1, 1.0, 2.5, 5.0 and 10.0 mm) of each BEC + nor-NOHA were delivered to different skin microdialysis sites during a standardized local heating protocol described elsewhere (Minson et al. 2001). Similar pilot testing was conduced using (2.5, 5.0, 10.0 and 20.0 mm) l-Arg. Increasing concentrations above 2.5 mm BEC + 2.5 mm nor-NOHA and 5.0 mml-Arg did not further increase the NO-dependent plateau phase of the local heating response.
All microdialysis sites were perfused with assigned pharmacological agents continuously for at least 60 min prior to the start of the baseline, and during the baseline and heating periods, at a rate of 2.0 µl min−1. Baseline data were collected for 20 min prior to the start of whole-body heating. After the baseline data collection period, whole-body heating was conducted to raise Tor by 1.0°C. At the end of the heating protocol, each microdialysis site was perfused with 28.0 mm sodium nitroprusside (SNP; Nitropress; Abbot Laboratories, Chicago, IL, USA) at a rate of 4.0 µl min−1 to achieve maximal CVC. Local heating of the skin to 43°C was conducted simultaneously with SNP infusion to ensure maximal CVC had been obtained.
Data acquisition and analysis
Data were acquired using Labview software and the National Instruments data acquisition system (Austin, TX, USA). The data were collected at 40 Hz, digitized, recorded and stored on a personal computer for further analysis. CVC data were averaged over 3 min periods for baseline and every 0.1°C rise in Tor, and are presented as a percentage of maximal CVC (%CVCmax). Two reviewers blinded to the age of the subjects and to the pharmacological treatment of the microdialysis sites visually identified the absolute Tor and delta Tor (ΔTor) at which the threshold for reflex cutaneous vasodilatation was initiated in each microdialysis site.
Student's t tests were used to determine significant differences between the young and older groups for physical characteristics and baseline Tor. A two-way repeated measures analysis of variance (ANOVA) was conducted to detect age and pharmacological treatment effects on the threshold Tor (absolute Tor and ΔTor) for reflex cutaneous vasodilatation. A three-way repeated measures ANOVA was conducted to detect differences between subject groups at the pharmacological treatment sites over the rise in Tor (SAS, version 8.01). Planned comparison tests, including Tukey post hoc tests, were performed when appropriate to determine where differences between groups and drug treatments occurred. The level of significance was set at α = 0.05. Values are presented as means ± s.e.m.
Results
The physical characteristics of the subjects are presented in Table 1. There were no differences between the groups for body mass index or mean arterial pressure, but the older group had a significantly lower V̇O2max (P = 0.002). Total cholesterol and low density lipoprotein levels were significantly higher in the older subject group (P = 0.001 for both), but there was no difference in high density lipoproteins between the age groups.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Sex (M, F) | 5, 5 | 4, 5 |
| Age (years) | 23 ± 1 | 69 ± 1* |
| BMI (kg m−2) | 23 ± 1 | 25 ± 1 |
| V̇O2max(ml kg−1 min−1) | 40 ± 2 | 28 ± 2* |
| Total cholesterol (mg dl−1) | 158 ± 9 | 209 ± 10* |
| HDL (mg dl−1) | 55 ± 3 | 57 ± 5 |
| LDL (mg dl−1) | 87 ± 9 | 120 ± 7* |
| MAP (mmHg) | 89 ± 2 | 93 ± 2 |
Values are means ± s.e.m.; HDL, high density lipoprotein; LDL, low density lipoprotein; MAP, mean arterial pressure.
Significant difference versus young subject group (P < 0.05).
Table 2 shows the threshold values for reflex cutaneous vasodilatation in both age groups. There was no difference between groups in baseline Tor (P = 0.12). The threshold for reflex vasodilatation at the control site and the NOS-inhibited site was significantly lower in the young subject group versus the aged subject group (P < 0.05). There was no difference between the control site, the arginase-inhibited, the l-Arg supplemented, or the arginase-inhibited + l-Arg supplemented, within each age group (P > 0.05).
Table 2.
Thresholds for cutaneous vasodilatation: absolute Tor and ΔTor
| Young | Older | |||
|---|---|---|---|---|
| Tor (°C) | ΔTor (°C) | Tor (°C) | ΔTor (°C) | |
| Control | 36.74 ± 0.10 | 0.41 ± 0.07 | 36.76 ± 0.09 | 0.57 ± 0.06* |
| NOS inhibited | 36.89 ± 0.08‡ | 0.56 ± 0.04‡ | 36.94 ± 0.06† | 0.75 ± 0.05*† |
| Arginase inhibited | 36.73 ± 0.11 | 0.40 ± 0.06 | 36.69 ± 0.07 | 0.50 ± 0.05 |
| l-Arg supplemented | 36.95 ± 0.13 | 0.52 ± 0.05 | 36.70 ± 0.09 | 0.52 ± 0.05 |
| Arginase inhibited + | ||||
| l-Arg supplemented | 36.75 ± 0.10 | 0.42 ± 0.06 | 36.73 ± 0.09 | 0.54 ± 0.07* |
Values are means ± s.e.m.; Tor, oral temperature; ΔTor, change in oral temperature from baseline; NOS, nitric oxide synthase; l-Arg, l-arginine.
Significant difference versus young subject group (P < 0.05).
Significant difference versus control site within the older subject group (P < 0.05).
Significant difference versus control site within the young subject group (P < 0.05).
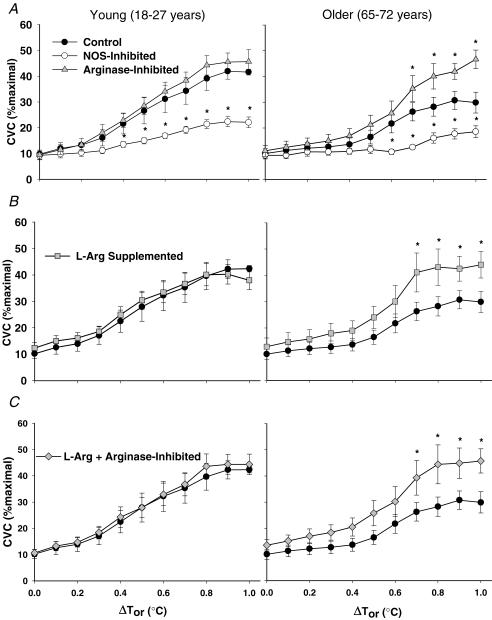
Figure 3 illustrates the %CVCmax responses across the rise in body core temperature in both age groups. In the young subject group the differences between the control site and the NOS-inhibited site started at ΔTor 0.4°C; this difference was observed in the aged subject group at ΔTor 0.6°C (P < 0.001 between groups). There was no difference in the %CVCmax responses in the arginase-inhibited compared with the control site in the young subject group (Fig. 3A). However, in the older subject group, arginase inhibition significantly increased %CVCmax above the level of the control site at ΔTor 0.7°C. Similarly, CVC in the l-Arg-supplemented (Fig. 3B) and the arginase-inhibited + l-Arg-supplemented (Fig. 3C) site was not significantly different from the control site in young subjects, but was significantly augmented compared with the control site in the older subject group starting at ΔTor 0.7°C. Moreover, arginase inhibition, l-Arg supplementation, and arginase inhibition + l-Arg supplementation in the older subject group increased cutaneous vasodilatation such that there was no difference between these microdialysis sites compared with the young subject group's control site (P > 0.05).
Figure 3. Group cutaneous vascular conductance as a percentage of maximal response during passive whole-body heating.
Left panels, young subjects; right panels, older subjects. A, the arginase-inhibited site and the NOS-inhibited site. B, the l-Arg-supplemented site. C, the combined l-Arg + arginase-inhibited site. Cutaneous vascular conductance (CVC) (%maximal) during the rise in oral temperature (ΔTor,°C) in the control site is shown for comparison. Arginase inhibition, l-Arg supplementation, and combined treatments augmented CVC in old but not in young subjects. Values are means ± s.e.m. *P < 0.05 significant difference versus the control site within subject groups.
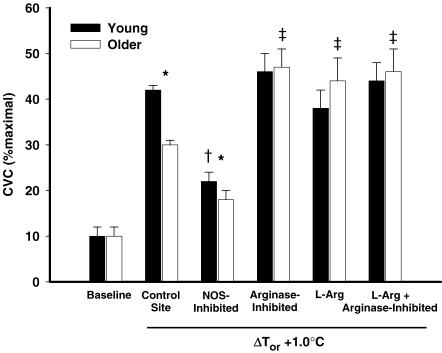
Figure 4 summarizes the %CVCmax responses in each microdialysis treatment site with a 1.0°C rise in body core temperature. Baseline %CVCmax is included for visual comparison. Older subjects had significantly attenuated responses in the control and the NOS-inhibited sites compared with young subjects (P > 0.05). There were no differences between young and older subjects in the arginase-inhibited, l-Arg-supplemented, and arginase-inhibited + l-Arg-supplemented sites. Arginase inhibition, l-Arg supplementation, and arginase inhibition + l-Arg supplementation significantly increased %CVCmax above the level of the control site in the older subjects but not in young subjects.
Figure 4. Group CVC as a percentage of maximal vasodilatation with a 1.0°C increase in oral temperature (ΔTor) in all drug treatment sites.
Baseline CVC (%maximal) is illustrated for visual comparison. Filled bars, young subjects; open bars, older subjects. Arginase inhibition, l-arginine supplementation, and combined treatments augmented CVC in old but not young subjects. *P < 0.05, significant difference between age groups; †P < 0.001, significant versus control site young subject group; ‡P < 0.001, significant versus control site older subject group.
Discussion
The principle finding of the present study was that acute arginase inhibition, l-Arg supplementation, or both, in the cutaneous vasculature, selectively augments reflex cutaneous vasodilatation in aged human subjects. In young subjects, these treatments did not significantly alter the cutaneous vasodilatory response during whole-body heat stress. Furthermore, the effects of combined arginase inhibition and l-Arg supplementation in aged skin were not additive. These results suggest that arginase activity may be increased in aged human skin and may limit the intracellular availability of l-Arg for NOS. Cumulatively, these data demonstrate that the age-related deficit in reflex cutaneous vasodilatation can be restored by either (1) inhibiting arginase to replenish the available pool of l-Arg for NOS, or (2) directly supplementing superphysiological concentrations of l-Arg to effectively saturate the arginase and NOS pathways (Fig. 1).
Our results indicate that NO bioavailability is compromised in aged human skin, and that pharmacological interventions specifically targeting the l-Arg–NO pathway can increase cutaneous blood flow during hyperthermia. In young human subjects, NO is required for full expression and contributes approximately 40–50% to the total reflex cutaneous vasodilatory response (Kellogg et al. 1998a; Shastry et al. 1998); additionally both VIP and H1 receptor activation mediate vasodilatation through NO-dependent mechanisms (Wilkins et al. 2004; Wong et al. 2004). Furthermore, NO is capable of mediating cutaneous vasodilatation synergistically with sympathetic cotransmitters, resulting in a combined vasodilatation that is greater than the sum of the individual contributions (Wilkins et al. 2003). In the context of human ageing, we have previously shown that older subjects have an impaired cotransmitter contribution to cutaneous vasodilatation with significant increases in body core temperature. Instead, the aged rely on an impaired NO-dependent mechanism to increase blood flow to the skin during thermal stress. Our current findings suggest that in aged human skin, the intracellular stores of l-Arg available for NOS and NO-dependent vasodilatation are insufficient to support the full expression of the increase in skin blood flow during hyperthermia. Alternatively, the interventions employed in the present investigation affecting the l-Arg–NO pathway may have capitalized on the synergistic relationship between NO and the cotransmitter(s) mediating reflex cutaneous vasodilatation; by increasing the amount of bioavailable NO in the cutaneous vasculature, the total vasodilatory response of both NO- and non-NO-mediated vasodilatation may have been augmented.
Our data show that the cutaneous vasodilatation in the combined treatment site was not significantly different compared with the arginase-inhibited or l-Arg-supplemented treatments alone, indicating that the effects of the individual treatments were not additive. One potential explanation for this finding is that the individual drug treatments were sufficient to replenish intracellular l-Arg for NO synthesis through NOS, such that the l-Arg–NOS pathway was operating near Vmax. Another possibility is that we maximized the capacity of the cutaneous vessels to vasodilate at this level of hyperthermic stress (1.0°C), approaching a ‘ceiling effect’ with our individual drug treatments, and that increasing the degree of hyperthermic stress may have unmasked further vasodilatation in the combined treatment site.
There are several putative mechanisms affecting the l-Arg–NO pathway and subsequent NO-dependent vasodilatation that may be impaired in aged skin. The most plausible mechanisms directly alter the intracellular availability of l-Arg for NOS and include augmented arginase activity, the subcellular distribution of l-Arg in relation to NOS, and age-related increases in endogenous NOS inhibitors. Additionally, these mechanisms have also been suggested as possible explanations for the l-Arg paradox where the intracellular concentration of l-Arg far exceeds the Km for NOS, but the addition of exogenous l-Arg augments NO-dependent vasodilatation.
Our in vivo findings implicate a role for augmented arginase activity limiting the availability of l-Arg for NOS and subsequent NO-dependent cutaneous vasodilatation in humans. These results are in agreement with in vitro isolated vessel investigations where arginase I is capable of reciprocal regulation of endothelial NOS, and its activity is upregulated in aged vessels (Berkowitz et al. 2003; White et al. 2006). These authors found that pretreatment with arginase inhibitors directly restored NO signalling and l-Arg responsiveness in aged vessels. Similarly, our data also demonstrate that the age-associated decline in cutaneous vasodilatory function was restored by arginase inhibition. However, in contrast to the study by Berkowitz et al. (2003) we were able to induce augmented cutaneous vasodilatation with l-Arg supplementation alone in the absence of concurrent arginase inhibition. These divergent findings may be due to species, tissue, and vascular tree differences, and methodological differences including the dose of l-Arg delivered to the vasculature. Alternatively, another explanation involves the intracellular compartmentalization of l-Arg available for NO synthesis in relation to NOS localization. In young endothelial cells, intracellular l-Arg is sequestered in several pools including (1) a pool associated with the cationic amino acid transporter (CAT) that is freely exchangeable with the extracellular space and associated with calveolar NOS, and (2) a pool in the cytosolic fraction where arginase I is localized that is non-freely exchangeable (McDonald et al. 1997; Flam et al. 2001). Localized cutaneous l-Arg supplementation most probably affected the calveolar associated pool of l-Arg, whereas arginase inhibition probably increased the availability of l-Arg in the cytosolic pool.
Another putative explanation for the l-Arg paradox is the accumulation of endogenous NOS inhibitors. Asymmetric dimethylarginine (ADMA) is the most abundant of the endogenous NOS inhibitors in humans, and both hyperlipidaemia and age-related impairments in microvascular function and subsequent NO bioavailability have been linked to increases in ADMA (Miyazaki et al. 1999; Kielstein et al. 2003). The mechanisms mediating increased ADMA are twofold, and include an increase in its formation and a decrease in degradation via oxidative stress (Fliser, 2005). In vivo human studies have demonstrated that ADMA-mediated NOS inhibition in the forearm circulation is reversible by the administration of l-Arg (Kielstein et al. 2005). In the context of the current study, an increase in endogenous NOS inhibitors in the aged subjects could help explain why cutaneous vasodilatation was significantly augmented with l-Arg supplementation in the absence of arginase inhibition.
Our findings show that direct l-Arg supplementation to the cutaneous vasculature through intradermal microdialysis significantly improves cutaneous vasodilatory function during hyperthermia in aged humans. Other investigations examining the effects of l-Arg supplementation on vasodilatory responsiveness have reported mixed results depending on the dose, duration and route of administration. Consistent with our data, l-Arg infusion into forearm and coronary vascular beds has more consistently demonstrated an increase in NO bioavailability and improved endothelium-dependent vasodilatation (Chauhan et al. 1996; Pernow et al. 2003; Perticone et al. 2005). In contrast, recent evidence from a clinical trail examining the effects of oral l-Arg supplementation (9 mg day−1) on non-invasive measures of resting vascular function (pulse pressure, arterial compliance, pulse wave velocity and arterial elastance) in patients following acute myocardial infarction failed to demonstrate a significant effect of l-Arg treatment (Schulman et al. 2006). These non-specific tests of arterial stiffness during resting conditions may have not been sufficient to observe significant differences between treatment and placebo groups. Both, in vitro and in vivo human data in the forearm circulation suggest that potent vasodilatory stimuli enhance l-Arg transport through CAT-1, significantly increasing NO production (Parnell et al. 2004). In the present study we stimulated the cutaneous microvasculature to induce pronounced vasodilatation through whole-body hyperthermia; in this construct it is feasible that a sufficient vasodilatory stimulus to the vasculature is necessary to observe increased vasodilatation with l-Arg supplementation.
Limitations
Our aim in the present study was to investigate the role of arginase and l-Arg availability in the regulation of cutaneous blood flow during systemic hyperthermia. We chose to use five separate microdialysis treatment sites in each subject during passive whole-body heat stress and compare between sites. Furthermore, our chosen pharmacological treatments specifically target the arginase pathway and are therefore instrumental in studying the interplay between arginase and NO (Cox et al. 1999; Tenu et al. 1999). However, we did not choose to inhibit NOS subsequent to the established plateau in skin blood flow to quantify the NO contribution within each microdialysis treatment site due to the longer duration of whole-body heating and associated increased cardiovascular risk and discomfort for the aged subjects. This additional data would have helped to clarify the pharmacological action of our chosen arginase inhibitors and the synergistic role between NO and cotransmitter-mediated vasodilatation during reflex cutaneous vasodilatation.
Our findings that the aged subjects had attenuated cutaneous vasodilatation in the control site and rely on impaired NO-dependent vasodilatation are consistent with our previous investigations. However, in both the aged and young subjects, we observed systematically attenuated levels of cutaneous vasodilatation (∼30%CVCmax) with increasing core body temperature in comparison with our previous study (Holowatz et al. 2003). In our previous studies, we induced maximal vasodilatation by infusion of 28 mm SNP, whereas in the present study we induced maximal vasodilatation by both infusion of this dose of SNP and by simultaneously locally heating the skin to 43°C. The most likely explanation for this discrepancy between studies is that we obtained a truer level of maximal vasodilatation with our current protocol, accounting for the consistent difference in cutaneous vascular conductance across age groups. It is likely that our higher maximal CVC values attained in this study account for the lower normalized CVC values.
Summary
In conclusion, this study demonstrated that both arginase inhibition and direct l-Arg supplementation through intradermal microdialysis in the cutaneous vasculature selectively augment reflex cutaneous vasodilatation in aged humans. Moreover, these data suggest that NO bioavailability is decreased in aged skin due to limited l-Arg availability by an age-related upregulation of vascular arginase activity. The age-related impairment in cutaneous vasodilatation during whole-body heat stress can be restored by replenishing the available pool of l-Arg for NO synthesis through NOS.
Acknowledgments
We are grateful for the outstanding technical and data collection assistance of Jane Pierzga and David DeGroot. This research was supported by NIH R01-AG-07004-15 (W.L.K.); American Heart Association predoctoral fellowship 0515392 U, the American College of Sports Medicine Carl V. Gisolfi memorial student research grant (L.A.H.); and NIH M01-RR-10732 (General Clinical Research Center).
References
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Chauhan A, More RS, Mullins PA, Taylor G, Petch C, Schofield PM. Aging-associated endothelial dysfunction in humans is reversed by L-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- Cox JD, Kim NN, Traish AM, Christianson DW. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat Struct Biol. 1999;6:1043–1047. doi: 10.1038/14929. [DOI] [PubMed] [Google Scholar]
- Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- Fliser D. Asymmetric dimethylarginine (ADMA): the silent transition from an ‘uraemic toxin’ to a global cardiovascular risk molecule. Eur J Clin Invest. 2005;35:71–79. doi: 10.1111/j.1365-2362.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- Grant R, Holling H. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci (Lond) 1938;3:273–285. [Google Scholar]
- Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Grody WW, Dizikes GJ, Cederbaum SD. Human arginase isozymes. Isozymes Curr Top Biol Med Res. 1987;13:181–214. [PubMed] [Google Scholar]
- Hecker M, Nematollahi H, Hey C, Busse R, Racke K. Inhibition of arginase by NG-hydroxy-L-arginine in alveolar macrophages: implications for the utilization of L-arginine for nitric oxide synthesis. FEBS Lett. 1995;359:251–254. doi: 10.1016/0014-5793(95)00039-c. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, et al. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- John S, Schmieder RE. Potential mechanisms of impaired endothelial function in arterial hypertension and hypercholesterolemia. Curr Hypertens Rep. 2003;5:199–207. doi: 10.1007/s11906-003-0021-1. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998a;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol. 1997;272:H1609–H1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Bode-Boger SM, Frolich JC, Ritz E, Haller H, Fliser D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107:1891–1895. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Tsikas D, Fliser D. Effects of asymmetric dimethylarginine (ADMA) infusion in humans. Eur J Clin Pharmacol. 2005:1–6. [Google Scholar]
- McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the ‘arginine paradox’. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, et al. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99:1141–1146. doi: 10.1161/01.cir.99.9.1141. [DOI] [PubMed] [Google Scholar]
- Parnell MM, Chin-Dusting JP, Starr J, Kaye DM. In vivo and in vitro evidence for ACh-stimulated L-arginine uptake. Am J Physiol Heart Circ Physiol. 2004;287:H395–H400. doi: 10.1152/ajpheart.01094.2003. [DOI] [PubMed] [Google Scholar]
- Pernow J, Bohm F, Beltran E, Gonon A. 1-arginine protects from ischemia-reperfusion-induced endothelial dysfunction in humans in vivo. J Appl Physiol. 2003;95:2218–2222. doi: 10.1152/japplphysiol.00515.2003. [DOI] [PubMed] [Google Scholar]
- Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, et al. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–523. doi: 10.1016/j.jacc.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol. 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. 1-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. Jama. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N (omega) -hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291–1298. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol. 2003;548:963–969. doi: 10.1113/jphysiol.2002.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]