Abstract
An important prerequisite for effective motor action is the discrimination between active and passive body movements. Passive movements often require immediate reflexes, whereas active movements may demand suppression of the latter. The vestibular system maintains correct body and head posture in space through reflexes. Since vestibular inputs have been reported to be largely suppressed in the vestibular nuclei during active head movements, we investigated whether head movement-related signals in the primate parietal cortex, a brain region involved in self-motion perception, could support both reflex functions and self-movement behaviour. We employed a paradigm that made available direct comparison of neuronal discharge under active and passive movement conditions. In this study, we demonstrate that a population of intraparietal (VIP (ventral) and MIP (medial)) cortex neurons change their preferred directions during horizontal head rotations depending on whether animals have performed active movements, or if they were moved passively. In other neurons no such change occurred. A combination of these signals would provide differential information about the active or passive nature of an ongoing movement. Moreover, some neurons' responses clearly anticipated the upcoming active head movement, providing a possible basis for vestibular-related reflex suppression. Intraparietal vestibular neurons thus distinguish between active and passive head movements, and their responses differ substantially from those reported in brainstem vestibular neurons, regarding strength, timing, and direction selectivity. We suggest that the contextual firing characteristics of these neurons have far-reaching implications for the suppression of reflex movements during active movement, and for the representation of space during self-movement.
The posterior parietal cortex has been thought for a long time to be involved in space perception, self-motion detection and navigation (Colby & Goldberg, 1999; Swan, 2001). In the present study, we examined the recently discovered vestibular signals in macaque intraparietal cortex neurons (Bremmer et al. 2002b; Klam & Graf, 2003a), in particular in the ventral intraparietal area (VIP), with reference to their responses to actively generated and passively imposed head movements. A distinction between active and passive movements is an important function for goal-directed movements without interference by reflex mechanisms, and to allow vital reflexes to happen when necessary. Furthermore, perceiving the external world as stable requires compensating for visual motion, and – more generally – sensory stimulation induced by self-movement.
Recent studies of vestibular nuclei neurons during passive and active head movements in monkeys showed that vestibular signals were strongly attenuated by self-generated movements as early as the first vestibular projection neurons (Gdowski & McCrea, 1999; McCrea et al. 1999; Roy & Cullen, 2001a; Cullen & Roy, 2004; but see also Khalsa et al. 1987). For instance, neurons could stop firing during active head movements, while during passive movements, they would transmit a head velocity signal. Furthermore, in the vestibular nuclei, neurons carrying eye and head movement-related signals have been found in a similar proportion as neurons that only signal head velocity, with no eye movement signals present (Gdowski & McCrea, 1999). The latter are thought to be part of the vestibulo-cortical relay. Vestibular thalamic and cortical units have been reported not to carry eye movement signals (Büttner et al. 1977; Magnin & Fuchs, 1977; Grüsser et al. 1990). We were therefore interested in the functional properties of cortical neurons that carry signals about head movements. This work extends our previous reports on vestibular responses in VIP (Bremmer et al. 2002b; Klam & Graf, 2003a), with the principal aim to analyse head movement-related signals in intraparietal vestibular neurons in relation to passive and active movements, in particular, in light of the known large differences between active and passive movement responses as early as second-order vestibular neurons, and also with respect to the involvement of the parietal cortex in self-motion perception, heading detection, and representation of extrapersonal space (Duhamel et al. 1991; Bremmer et al. 2000; Zhang et al. 2004).
Preliminary accounts have been presented in brief, earlier (Klam & Graf, 2003b,c).
Methods
General
Extracellular recordings were made in the left hemispheres (Fig. 1A) of two macaque monkeys, one male rhesus (Macaca mulatta), and one female fascicularis monkey (Macaca fascicularis). The animals were purpose-bred and purchased from authorized suppliers. Animal care (housing, nourishment, veterinary consultations, surgical procedures, postoperative care, daily care) conformed to French government regulations (Ministries of Agriculture and Research, CNRS: approval no. 75–546) and European Union standards (European Communities Council Directive 86/609/EEC).
Figure 1. Recording sites and active–passive replay paradigm.
A, location and reconstruction of recording sites. Overall lateral view of the left hemisphere of a macaque monkey indicating the main cortical landmarks: intraparietal sulcus, ips; central sulcus, cs; lateral fissure, lf. The broken line indicates the placement of the coronal section shown below, with reconstructed typical electrode tracks and recording sites (MIP, medial intraparietal area; VIP, ventral intraparietal area) in the intraparietal sulcus. Area VIP is highlighted in light grey, and area MIP in dark grey. (LIP, lateral intraparietal area; sts, superior temporal sulcus). B, the animal was allowed spontaneous horizontal head movements in darkness while neuronal discharges and head movement trajectories were recorded. C, subsequently, the animal's head was fixed, and the previously recorded head movement was replayed with a horizontal turntable, again monitoring neuronal firing.
Animals were in experimental sessions, usually on a daily basis during work days, for a duration of 3–4 h per day. No experiments were scheduled during weekends. The animal facilities were adjacent to the laboratory, thus minimizing travel time for bringing the animals into the experimental set-up. Monkey 1 was used for 14 months and monkey 2 for 8 months for these particular experiments. Naturally, there was some down-time in the experimental schedule for the animals to recover for more than just the weekends.
Surgical procedures
All surgical procedures were performed aseptically under general anaesthesia initiated with ketamine (20 mg kg−1i.m.) and acepromazine (0.5 mg kg−1i.m.). Valium (diazepam 10 mg i.m.), atropine (0.5 mg subcutaneous) and corticosteroids (methylprednisolone 40 mg i.m.) were administered as premedication. Subsequently, venous access was established. Deep general anaesthesia was maintained via continuous infusion of Rapinovet (propofol 10 mg kg−1 for induction; 25 mg kg−1 h−1 for maintenance) with a syringe pump for the duration of the procedure. The animals were intubated, but typically respired spontaneously. An ECG was recorded routinely.
For the surgical procedures, the monkeys' heads were fixed in a stereotaxic head-holder via ear bars and a mouth clamp. At first, sclera-concentric coils made of three turns of Teflon-coated silver wire were implanted in each eye. The ends were led under the skin to the top of the head and soldered to previously manufactured plugs. These were later anchored with dental acrylic to the skull.
The skin over the skull was opened and small self-tapping stainless-steel screws were threaded into the bone. Onto these, an earth-vertical head-holding device was anchored with dental acrylic. At the coordinates of the intraparietal sulcus (centred at P3.5/L12), a trepan hole was made into the skull without opening the underlying dura. A stainless steel cylinder was mounted over the opening. Into this cylinder, a Teflon grid for electrode placement was inserted, and a hydraulic motor-driven microdrive (Narishige) was mounted during recording sessions. The grid allowed reproducible electrode penetrations with a 500-μm resolution.
Postoperative care involved local application of neomycin/hydrocortisone ophthalmic cream, administration of antibiotics (penicillin 75 mg kg−1, i.m., daily) and analgesics (aspirin 60 mg kg−1, i.m. twice a day), and monitoring of the behaviour of the animal several times a day.
During subsequent routine surgical procedures (e.g. dura scrape), the animals' heads were fixed by the head-holding device.
Animal training
Head-fixed animals were initially trained to fixate a small spot of light within a narrow target window (2 deg × 2 deg) for a minimum of 3.5 s, to receive a liquid reward. The light spot could be kept stationary in darkness and in light to monitor a given neuron's resting activity. To determine a neuron's eye position sensitivity, the spot was moved in random order into nine different locations on a tangent screen (Bremmer et al. 1999). The spot could also be moved to test smooth pursuit sensitivity. Pursuit eye movements were only tested in a subset of neurons with a step-ramp paradigm (step size 10 deg), where target excursions could be one of eight cardinal directions at velocities of 10 deg s−1 or 20 deg s−1 for 2000 ms and 1000 ms, respectively.
The animals' heads were fastened in a specialized head-holder system that allowed free head movements in the stereotaxic frame of reference (Fig. 1B and C). The head-holder was constructed to allow horizontal head movements (vertical axis rotations) once a set screw was released, but no vertical excursions. The set screw had a very short grading, and was operated from behind the animal. Thus, there was minimal to no disturbance for the animal when switching from head-fixed to head-free, and vice versa. Once the head was freed, the animals were allowed to make spontaneous head movements. From the combined signals, head, eye and gaze information could be derived.
Each animal was allowed an adjustment period to find its ‘comfort point’ for horizontal head rotation on the holder's horizontal arm along the naso-occipital axis. Such a procedure was necessary since the head post needed to be placed over the frontal bone in order not to interfere with the chamber implant over the parietal cortex. Our previous biomechanical studies had indicated the exact rotation axis for horizontal head movements within the atlanto-axial joint (C1/C2) (Vidal et al. 1986; Graf et al. 1995). Smooth and friction-free operation of the moving parts of the head-holder was ensured by enclosed ball bearings.
Recordings
Single cells were recorded extracellularly with glass-coated tungsten microelectrodes (F. Haer) in areas VIP and MIP (medial intraparietal area) of the two left hemispheres of the two monkeys (for details see Bremmer et al. 2002b; and Klam & Graf, 2003a). The animals were awake, and performed several oculomotor tasks. Neurons were classified as being located in area VIP on the basis of the recording sites and depth within the intraparietal sulcus, and with respect to their response properties (Colby & Duhamel, 1991; Colby et al. 1993; Schaafsma & Duysens, 1996; Schaafsma et al. 1997; Duhamel et al. 1998). Neurons in MIP were characterized by absent or low visual sensitivity and strong somatosensory responses located on the fingers, hands and forearms (Klam & Graf, 2003a). In a typical recording session, the passage of the electrode from the MIP into the VIP was marked by a distinct change in background and resting activity of the recorded neuronal elements (Klam & Graf, 2003a).
Eye movements were recorded with the magnetic search coil method, head movements with a head-holder-mounted potentiometer. Previous tests had shown that the eye movement signal remained linear within the physiological working range of the monkey's eye–head movements (see Figs 2 and 3). Neuronal signals were sampled at 1000 Hz, and eye and head position at 250 Hz.
Figure 2. Comparison of neuronal firing characteristics during active and passive head movements: quantitative differences (firing intensity differences).
Left-hand columns (Aa, Ba, Ca) show firing behaviour (Rate) during active and passive (replay) horizontal head movements (H Head). Head movement trajectories are depicted as position traces (black lines), and as velocity profiles (red shading). Upward deflections symbolize movement to the right, and vice versa. Velocity traces were divided by a factor of 10 for graphical scaling purposes. Right-hand columns (Ab, Bb, Cb) depict mean firing rates as a function of head velocity for the same neurons. Small vertical bars represent standard error. Vertical dotted lines indicate change-over points between leftward (negative) and rightward (positive) velocities. Aa, Type II neuron only active during passive rotation. Ab, the velocity traces emphasize that this neuron does not respond during active movement, but that it shows a clear preference for rightward head movements (i.e. positive velocities) under passive conditions (Type II). Ba, Type II neuron only active during active head movement. Bb, velocity traces indicate that the neuron responds during rightward active head movements (Type II), but is silent during passive head rotation. Ca, Type III neuron whose activity is greater during passive stimulation. Cb, velocity traces show that the neuron responds to head movements in both directions (Type III) under active as well as passive movement conditions, but its response intensity is much greater in the passive condition.
Figure 3. Comparison of neuronal firing characteristics during active and passive head movements: qualitative differences (preferred direction and timing differences).
Left-hand columns (Aa, Ba, Ca) show firing behaviour (Rate) during active and passive (replay) horizontal head movements (H Head). Head movement trajectories are depicted as position traces (black lines), and as velocity profiles (red shading). Upward deflections symbolize movement to the right, and vice versa. Velocity traces were divided by a factor of 10 for graphical scaling purposes. Vertical dotted lines in Ba and Ca indicate the sampling periods used for quantitative data analysis. Right-hand columns (Ab, Bb, Cb) depict mean firing rates as a function of head velocity for the same neurons. Small vertical bars represent standard error. Vertical dotted lines indicate change-over points between leftward (negative) and rightward (positive) velocities. Aa, change of directional selectivity: under active movement conditions, the neuron shows Type II behaviour, under passive stimulation Type I behaviour. In addition, neuronal activity is greater during passive head movements, than during active stimulation. Ab, velocity traces clearly show the change of preferred direction: the neuron shows a Type II behaviour (increase of firing towards positive velocity or rotation to the right) during active head movement, and Type I behaviour (increase of firing towards negative velocity or rotation to the left) during passive stimulation. Ba, change of directional selectivity: this neuron shows Type I behaviour during active head movements (responding clearly more strongly to leftward then to rightward head velocities), and a Type III response following passive rotation. In this case, response strength remains about the same. Bb, velocity traces show Type I behaviour (dominant increase of firing towards negative velocity or rotation to the left) during active head movement, and change to a Type III response (equivalent increase of firing towards negative and positive velocities) during passive stimulation. Ca, anticipatory response to active movement: Type II neuron with a response build-up starting before the active movement; response to passive movement, naturally, begins with rotation onset. Cb, velocity traces show that the neuron responds to rightward velocities (Type II) during active and passive movements, but the response is greater in the passive condition.
Experimental paradigm
We employed a direct testing paradigm to compare vestibular signals during active and passive head movements, the ‘replay method’ (see also Robinson & Tomko, 1987). To that end, the animal was first allowed to make spontaneous horizontal head rotations in the dark. Neuronal activity and the head movement profile were recorded for various periods. After that, the animal's head was fixed, and the previously recorded active head movement trajectory was reproduced by the turntable, again recording neuronal activity. Thus, a direct comparison during active and passive neuron discharge became available (see Figs. 2 and 3). Data acquisition was performed with 32 s trials. Each neuron included in this study was recorded for at least 96 s, and up to 256 s. In the active condition, the animals were encouraged to make spontaneous head movements through liquid reward delivery by the experimenter after each head rotation. The liquid volume was adjusted so that slower head movements were more rewarded, and rapid or ‘jerky’ movements discouraged. In the passive condition, the head movement was replayed by the turntable, and the reward given randomly. The animal then had no specific task to perform.
In some neurons we tested eventual neck input to parietal vestibular neurons. To that end, the animal's head was held fixed in space, and the body was rotated underneath the animal's head (so-called ‘passive neck rotation’, PNR) in a sinusoidal fashion. Neuronal activity was compared to recordings of sinusoidal whole-body rotations (WBR) in the head-fixed animal (the ‘classical’ paradigm), and to sinusoidal passive head-on-trunk rotations (HTR) with the animal's body held stationary (see also McCrea et al. 1999).
Stimulation parameters
Since the turntable control system had an acceleration limit of 500 deg s−2, the recorded head traces were modified as follows: accelerations above 500 deg s−2 were changed to 500 deg s−2, which slightly modified the velocity profiles of some isolated head movements. Typically, about 90% of head movements remained unchanged. Moreover, we tested the frequency distribution of the head velocity across sessions, between active and passive movements, and found no significant differences for both animals (Kolmogorov–Smirnov P > 0.9). Therefore, we conclude that our active–passive comparison was valid. In order to eliminate further possible effects of velocity differences due to stimulation conditions, which could influence the active–passive neuronal strength of response analysis, we also re-computed this analysis by normalizing the firing rate by the mean velocity of the head movement. The results were not different.
Stimulation and characterization of neuronal responsiveness
Vestibular stimulation was delivered via a vertical axis turntable (horizontal rotation) that could be moved manually or via a servo-controller. In order to exclude any visual influence on vestibular responses during purely vestibular testing, the animals' eyes were covered with opaque pads, in addition to darkening the laboratory. During vestibular testing, the animals, naturally, had to be left free to make compensatory eye movements (vestibulo-ocular reflex, VOR). Fixation and smooth pursuit targets were back-projected onto a translucent tangent screen. Directional selectivity was assessed as previously described (Bremmer et al. 2002a).
Once we were convinced, on the basis of visual and somatosensory responses, that the electrode was located either in VIP or MIP, we used sinusoidal vertical-axis vestibular stimulation (0.25 Hz/30 deg amplitude) as a search stimulus. Neurons which had an appreciable modulation of their firing rates with the turntable motion were tested further. In addition, when in a location where such vestibular responses had been identified, we tested neurons directly under active head movement conditions. Nevertheless, this search method could have led us to underestimate the proportion of neurons which respond to active movement, but not at all or only weakly to passive movement.
Data analysis
Vestibular responses were evaluated according to their preferred, or on-directions (Type I, II and III: Duensing & Schaefer, 1958), with regard to the response strength and the response latencies under the two movement conditions, i.e. active or passive. Vestibular on-directions are referred to according to the recording sites in the left hemisphere, i.e. a neuron that reacts with excitation during leftward (ipsilateral) rotation is defined as a Type I neuron, etc. For instance, when the animal is rotated to the left, a given neuron may increase its discharge, and decrease its discharge when the animal is rotated to the right; such a neuron would be classified as a Type I neuron. By contrast, if a neuron reacts with a firing rate increase when the animal is rotated to the right (i.e. contraversive) and shows a decrease in discharge during rotation to the left, then the neuron would be classified as a Type II. A Type III neuron would react with discharge increase to both leftward and rightward rotation, i.e. to ipsiversive and contraversive. For completeness' sake it should be mentioned that we never encountered so-called Type IV neurons, which react with a decrease of firing rate in both rotation directions.
Since reflex compensatory eye movements (VOR) had to be allowed in our experimental tasks, eye movement sensitivities were evaluated separately. The sensitivities to eye position were usually smaller than the vestibular sensitivities by an order of magnitude. Smooth pursuit sensitivities were typically negligible as well, and there is no saccade-related activity in VIP or MIP. We thus proceeded with our analysis without taking eye movement effects further into account. Preferred directions of visual stimulus motion were determined using the weighted average method. The weighted average method simply calculates the average vector direction based on a temporal sliding window of 500 ms width used to determine the preferred directions of neurons to time-varying directional visual stimuli (for details see Bremmer et al. 2002a,b). All analyses were performed using either the SAS statistical package, or programs in MATLAB and in visual C++. Details of the analysis protocols are given in the following sections.
Determination of latencies of neuronal responses
Selection of head movement parameters
Episodes of head movements, or head ‘saccades’, were selected by an automated procedure in order to avoid any manual selection-induced variation in the analysis. The beginning of a movement was set as the bin-time where head-velocity was equal or above 10 deg s−1 and head acceleration was equal or above 0.4 times its standard deviation. The end of a head movement was the following point in time where the head-velocity dropped below 10 deg s−1. These criteria allowed determination of the beginning and end of movements very close to those obtained by manual selection.
For a neuron to be counted in, its activity had to be recorded during at least 17 active head movement episodes. However, on average, 132 (from 30 to 230) head movements were available for analysis for each neuron and in each stimulation condition.
Computation of firing rates
We used a standard spike density function, computed by convolving the spike–time temporal series with a Gaussian function (m = 0, σ = 50 ms).
Computation of latencies
The average latency of neuronal responses was determined by a cross-correlation. For each cell, we computed the cross-correlation between the head velocity trace and the neuronal response, shifted in time by one time bin (4 ms) at a time. The estimate of the latency was the time shift for which the cross-correlation was maximized. By convention, negative latencies indicated neural responses anticipating head movements.
Determination of vestibular response types (Duensing & Schaefer, 1958)
Response to head movement
To determine if a neuron responded significantly to head movement, a linear regression analysis of the neuronal response as a function of head velocity was computed, binned in 5 deg s−1-wide bins (see also Figs 2Ab, Bb, Cb and 3Ab, Bb, Cb). In cases where the neurons responded to both positive (rightward) and negative (leftward) velocities (see, e.g. Fig. 3Ab), linear regressions were performed separately for positive and negative velocities. t tests on the regression coefficients were performed, slopes and P values are given in the text when appropriate: P values below 0.05 were considered as significant.
Vestibular response type
A neuron was classified as a Type I (or Type II), if it responded significantly only to ipsi- or contralateral head movement, or if responses to one side clearly dominated the response to the other movement direction, as evidenced by the linear regression slopes, i.e. if (||Sipsi|| – ||Scontra||)/(||Sipsi|| + ||Scontra||) > 0.1. Those neurons which responded to both movement directions with similar intensities were classified as Type III.
Change of preferred direction
When a different vestibular response type was encountered in a neuron under active and passive movement conditions, it qualified as a neuron that changed its preferred direction according to the nature of the movement.
In order to give a comprehensive graphical representation of the changes of preferred direction as a function of the movement condition (and of the other types of response changes), we plotted the neuronal response as a function of head velocity (Fig. 2Ab, Bb and Cb and 3Ab, Bb and Cb). Velocity traces were binned in 5 deg s−1 bins, and the neuronal response was averaged in each bin.
Comparison of response intensities between active and passive movement conditions
We used the previously described head movement epochs to average neural responses for each movement, and grouped these average responses separately for leftward and rightward movements. The two groups of average responses corresponding to the preferred direction of a neuron under active and passive movement conditions were subsequently compared with a Mann–Whitney rank test. Significant differences between responses in the active (A) and passive (P) conditions were interpreted as A > P, A < P groups, and non-significant differences as A ∼ P (Fig. 4).
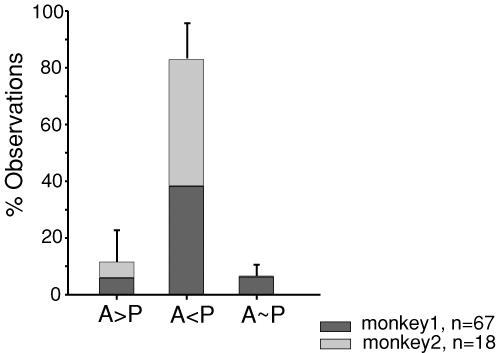
Figure 4. Quantitative comparison of response strengths of intraparietal neurons between active and passive movement conditions.
Mean percentages of observations, i.e. (P1 + P2)/2 are given for the three classes, with grey shading indicating the relative distribution of these values for the two animals. Error bars indicate the 95% confidence intervals of the percentages. Most cells had a weaker response to active movement (51/67 and 16/18 for monkeys 1 and 2, respectively, i.e. 79% of the total population). However, a small but sizeable proportion of neurons responded with equal strength under both movement conditions (8/67 and 0/18 for monkeys 1 and 2, respectively, i.e. 9%) or more vigorously to active movements (8/67 and 2/18 for monkeys 1 and 2, respectively, i.e. 12%).
Since the turntable servo control limited accelerations to 500 deg s−2, somewhat lower head velocities than the actual active head movements were reproduced in some instances under passive movement conditions (see also ‘Stimulation parameters’). Therefore, we also performed a response intensity test with averaged neural responses normalized by average head-movement velocity, i.e. using average firing rate (E[Frate]) and average head velocity (E[Hvel]) instead of E(Frate). The classification obtained following the Mann–Whitney test results using this variable did not differ from the previous analysis, thus ruling out a significant effect of occasional head-movement reproduction velocity differences.
Neck input testing: WBR and HTR
For neck input testing, we used sinusoidal stimulus profiles. Responsiveness to these conditions was explored as described in Klam & Graf (2003a) in detail. In brief, we used a Mann–Whitney test on a 300-ms averaging window around the peak and the minimum of a given neuron's response.
Eye position effects
Evaluation of responses to eye position
Protocol and data analysis were essentially the same as described by Bremmer et al. (1999). In brief, eye position effects were measured by averaging the neuronal activity during a 2000-ms fixation period, at nine fixation positions of eight cardinal directions at 15 deg excentricity, and one central fixation. The significance of the responses was tested with a Kruskal–Wallis non-parametric ANOVA.
Direction of the eye position effects
Directions of eye position effects were further evaluated by a 2D-linear regression, expressing the firing rate as a function of horizontal and vertical eye positions. As in Bremmer et al. (1999), we found that with this protocol, eye position responses were fairly well described with this linear model. Since in our experiments head movements were limited to the horizontal plane, we further analysed only those neurons which had a significant horizontal eye position regression coefficient (t test on the regression coefficients).
Verification of recording sites
After the electrophysiological recordings were finished, the animals were used for anatomical tract tracing experiments. To that end, a tracer was injected into the area of recording to determine the input sources of vestibular signals to the VIP/MIP region. However, in this publication, we will not elaborate on these forthcoming neuroanatomical data (see Graf et al. 2005). After the appropriate survival time, the animals were deeply anaesthetized as described above, except that aseptic conditions were not maintained in this case. A lethal dose of pentobarbital (30 mg kg−1i.v.) was then administered, and when respiration had ceased, the thorax was opened and the animals were perfused transcardially with phosphate buffer and paraformaldehyde. The brains were subsequently removed for histological processing. All procedures have been published in detail previously (Graf et al. 2002; Grantyn et al. 2002; Moschovakis et al. 2004; Ugolini et al. in press). From the processed histological material, electrode traces could be recovered within the topography around the intraparietal sulcus, which was reconstructed in three dimensions via light microscopy and the Neurolucida program of MicroBrightfield.
Results
A total of 304 cells was recorded in areas the VIP and MIP areas in the intraparietal sulcus (Fig. 1A) of two left hemispheres of two macaque monkeys in response to various visual, vestibular and oculomotor paradigms, and active–passive head movement comparisons restricted to the horizontal plane. In monkey 1, 230 cells were recorded, and 74 cells were recorded in monkey 2. Of these, 92 cells in monkey 1, and 32 cells in monkey 2 responded significantly to vestibular stimulation. In total, 82 VIP and 30 MIP cells were available for the active head movement analysis (i.e. were recorded during more than 17 head movements), and 64 VIP and 22 MIP cells for active–passive movement comparison. Those of the VIP (and some MIP cells) were clearly visually direction selective, as reported previously (Bremmer et al. 2002a,b). While all vestibular-responsive intraparietal neurons were reported to carry eye position sensitivity in a previous study (Bremmer et al. 2002b), we found that only about 80% (n = 18) of the tested neurons of the present study had eye position signals (see below: Possible eye position effects). This difference to the previous study may be due to our sample of VIP as well as MIP neurons. Smooth pursuit activity was almost negligible at the velocities tested in our experiments (up to 20 deg s−1) (Colby et al. 1993; Schaafsma & Duysens, 1996; Schlack et al. 2003). As already mentioned, there was no saccade-related activity.
Classes of responses to active versus passive head movement
To ensure the most direct comparison possible, response characteristics of intraparietal vestibular neurons during active and passive head movements were studied by comparing the neuronal firing rate during an active horizontal head rotation to that of the replay of the same head movement profile under a passive, head-fixed condition (Figs. 1B and C). The active condition consisted of head-on-trunk rotations, and the passive condition involved whole-body-rotation.
The spike density functions of the recorded neurons show a wide variety of responses (Figs. 2Aa, Ba and Ca and 3Aa, Ba and Ca), confirmed in the average behaviour (Figs. 2Ab, Bb and Cb and 3Ab, Bb and Cb). The illustrated examples thus range from total extinction of the vestibular signal during active movement (Fig. 2Aa and b: test on the head velocity regression coefficient, Pact > 0.3 Ppas < 0.0001, and Mann–Whitney on mean velocity per head movement for significant difference A < P: P < 0.0002), to the presence of activation only during active movement with absence of any neuronal reaction during passive stimulation (Fig. 2Ba, and 4b: regression coefficient Pact = 0 Ppas > 0.08, Mann–Whitney A > P, P < 0.001). Most often, neuronal signals were seen to be diminished in the active movement condition compared to passive stimulation (Fig. 2Ca and b: regression coefficients pact-right = 0, Pact-left = 0, Ppas-right = 0, Ppas-left = 0, Mann–Whitney A < P, Pright = 0, Pleft = 0). So far, all these changes only involved quantitative differences (differences in firing intensity) between active and passive movement distinction. However, there were also changes that signalled a truly qualitative difference (differences in preferred direction and timing) in active–passive mode distinction in addition to some qualitative differences. Most surprisingly, quite frequently we also found a change of directional selectivity with respect to the vestibular on-directions of the recorded neurons (Fig. 3Aa and b and Ba and b). The neuron illustrated in Fig. 3Aa and b responded with activation to rightward movements (contraversive response, a so-called Type II of the classical nomenclature of Duensing & Schaefer, 1958) under active head movement conditions, but showed activation to leftward rotation (ipsiversive response, a so-called Type I response) under passive vestibular stimulation (see Fig. 3Ab: regression coefficient sact = 0.11sp (spikes) deg−1, Pact = 0, spas = −0.13 sp deg−1, Ppas = 0). The neuron illustrated in Fig. 3Ba and b responded to both leftward and rightward movements under active and passive conditions. However, under active movement conditions, it responded with a clear dominance towards leftward movements (Fig. 3Bb: sact-left = −0.52 sp deg−1, Pact-left = 0 and sact-right = 0.18 sp deg−1, Pact-right = 0) and was thus classified as a Type I neuron. In the passive condition, this dominance disappeared (Fig. 3Bb: sact-left = −0.50 sp deg−1, Pact-left = 0 and sact-right = 0.44 sp deg−1, Pact-right = 0), and the neuron was now classified as a Type III.
Another remarkable feature of the responses to active movements was that some neurons started responding to an upcoming head rotation before the actual head movement began (Fig. 3Ca: average latency = −124 ms). Some neurons thus anticipated the movement under active movement conditions, behaving as motor command or movement intention units, but naturally they did not fire similarly under passive movement situations. The illustrated neuron (Fig. 3Ca and b) showed a Type II response, and had no change in directional selectivity (regression coefficient, Pact = 0, Ppas = 0).
Relation of neuronal responses to the direction of the ongoing movement
In accordance with our previous observations (Bremmer et al. 2002b; Klam & Graf, 2003a), the majority of the recorded population of parietal vestibular neurons responded to head rotations directed towards the contralateral side relative to the recording site for both active and passive movement (Type II neurons, 61/107, i.e. 57%, and 50/86, i.e. 58% for active and passive movement, respectively). Substantially fewer neurons responded to ipsiversive movement (Type I, 33% in the active and 30% in the passive condition) and even less to both directions of head rotation (Type III, 10% during active and 12% during passive stimulation).
Quantification of changes of directional selectivity as a function of the nature (active or passive) of the movement was provided by plotting firing rates as a function of head velocity of selected neurons under both movement conditions (Fig. 3Ab and Bb; see also Methods, Data analysis, Section 2). In the illustrated examples, one neuron's firing behaviour changed from Type II (contraversive) in the active condition, to Type I (ipsiversive) under passive stimulation (Fig. 3Ab). In the other case (Fig. 3Bb), neuronal firing showed a predominantly Type I (ipsiversive) directional selectivity during active head movements, which then changed to Type III (both ipsi- and contraversive) behaviour under passive stimulation conditions. The full complement of our neuron sample is illustrated in Table 1. Clearly, a large number of neurons change their on-directions depending on the movement condition, involving all possible combinations of the encountered vestibular response types, i.e. Type I, Type II and Type III. Of a total of 86 neurons that were thus tested, 34 (26 and 8, or 38% and 44% for monkeys 1 and 2, respectively), i.e. more than one-third (40%), showed the described change in vestibular on-direction depending on whether the ongoing head movement was active or passive. From active to passive movement, pure directional changes between Types I and II were more frequently observed (19/34) than changes towards bidirectionality (Types I or II to Type III: 8/34) or loss of bidirectionality (Type III to Types I or II: 7/34).
Table 1.
Change of vestibular direction selectivity of neurons tested with active and passive movements
| I → II | I → III | II → I | II → III | III → II | III → I | Total | |
|---|---|---|---|---|---|---|---|
| Monkeys 1 and 2 | 9 | 2 | 10 | 6 | 5 | 2 | 34 (n = 86) |
Neurons changed from Type I to Type II, from Type I to Type III, etc. This observation was made in 34 of a total of 86 neurons tested in this way.
Response intensities
Quantification of the strength of neuronal responses under active versus passive movement conditions also demonstrated that in the majority of cases (79%), neuronal responses were diminished under active movement conditions compared to passive stimulation (Fig. 4). In about equal proportions (9% and 12%), response strengths either stayed the same, or were even stronger in the active condition (Fig. 4).
In addition, we computed neuron sensitivity to movement velocity as the average increment of discharge rate per degree per second of head motion. Such measurement takes into account any slight difference between the head velocity produced by the animal and its reproduction by the turntable in the passive condition. On average, sensitivities were fairly large, with a substantial proportion of neurons gaining more than 1 sp s−1 with an increase of 1 deg s−1 of head velocity (34/67 and 16/18 for monkeys 1 and 2, respectively). In line with the preceding results, the average sensitivity was significantly larger for passive relative to active movements (mpass = 1.1 ± 0.65 (sp s−1)/(deg s−1) and mact = 0.55 ± 0.41 (sp s−1)/(deg s−1), n = 67, P = 0; and mpass = 1.99 ± 1.06 (sp s−1)/(deg s−1) and mact = 1.21 ± 0.64 (sp s−1)/(deg s−1), n = 18, P < 0.03, for monkeys 1 and 2, respectively), whatever the preferred direction of the cells. Hence, decreases of response strength to active movements were generally accompanied by a reduced neuronal sensitivity to head velocity.
Timing aspects
When determining response delays to vestibular stimulation, neurons clearly had earlier reaction times under active movement conditions (Fig. 5A). Naturally, under passive stimulation conditions, a given neuron only reacted after the onset of turntable movement (Fig. 5B; see also Fig. 3Ca). Under active head movement conditions, neurons could fire as early as 300 ms before the ensuing head movement, although such cases were rare exceptions (n = 1; three cells fired 150 ms before movement onset). A sizeable minority (19%) was observed to be activated already up to 50 ms before the actual head movement occurred (Fig. 5A). The vast majority of neurons started firing within 150 ms after movement onset (94% of cells under active, and 93% of cells under passive conditions).
Figure 5. Response latencies of firing rates relative to the onset of head movements.
A, distribution for active movements. B, passive movement condition. Mean percentages of observations, i.e. (P1 + P2)/2 are given for each class with grey shading indicating the relative distribution of values for the two animals. Bin width was set to 100 ms. Under both conditions, most neurons started firing within 150 ms after the beginning of head movements (102/108 and 80/86 for active and passive movements, respectively). For active movements, a fair number of cells started firing at least 50 ms before the onset of head movements (21/108), and one cell had already fired about 350 ms before the movement.
When averaged across the entire examined neuron population, latencies under active (latact = 10 ± 86 ms) versus passive (latpass = 70 ± 66 ms) movement conditions were also clearly shorter (Mann–Whitney test, P = 0).
In order to appreciate fully the anticipatory characteristics of these neurons, future experiments will have to be conducted in tandem with neck muscle EMG recordings. However, we do not expect a significant reduction in the population of these anticipatory neurons, since the majority of neurons do not show anticipatory behaviour.
Possible eye position effects
Intraparietal neurons, and VIP neurons in particular, carry eye position signals (Andersen et al. 1985; Bremmer et al. 1999; Bremmer et al. 2002b). Clearly, eye movements, and thus the distribution of eye positions are different between the two head movement conditions employed. In the active condition, the animals perform spontaneous eye plus head saccades, and in the passive condition, vestibulo-ocular reflex (VOR) eye movements take place. Quantification of eye position sensitivity was performed on a subset of neurons (n = 18), to verify that this sensitivity could not account for the active–passive differences we report. Fifteen neurons (15/18; i.e. 83%) were significantly modulated by static eye position, as tested with a distribution-free ANOVA (Kruskal–Wallis; see also Bremmer et al. 1999). For those neurons, a corrected firing frequency was computed in order to compensate for potential eye position effects:
where F is the original response, Ehor and Ever the horizontal and vertical eye positions and σeyehor, σeyever the eye position sensitivities. The corrected responses for active and passive conditions were then used, and all 15 neurons showing significant modulation of eye position still showed significant differences between the active and passive movement condition. Thus, in all of the tested population (18 cells), differences between active and passive movement responses could not be explained by eye position sensitivities.
Neck rotation
One difference between the active and passive movement paradigms to be taken into consideration is the presence of a head rotation on the body (head-on-trunk rotation, HTR) during active movement which was absent during the subsequent passive whole-body rotation (WBR). In order to verify whether active–passive differences can be at least be explained partly by neck proprioceptive input, we tested a subset of neurons (n = 24) under two additional passive movement conditions: one was a passive body rotation under the head with the head held stationary (passive neck rotation; PNR; n = 20). The other was a passive head-on-trunk rotation (HTR; n = 4). Only those cases were used where the monkey did not resist the forced head rotation. In all cases, neurons were also tested with sinusoidal whole-body rotations (WBR) for comparison.
Only about one-third (7/20) of the neurons tested with PNR showed a significant response (Fig. 6), thus leaving 65% of cells unaffected by passive neck inputs. The neuron shown in Fig. 6 had a weak neck-proprioceptive modulation of the same polarity as the vestibular response, i.e. a synergistic response.
Figure 6. Effect of neck input on parietal vestibular neurons.
A, Passive sinusoidal rotation (whole-body rotation, WBR). B, trunk-under-head rotation (passive neck rotation, PNR). The two top rows show neuronal firing rates as histograms (bin width 100 ms) and as raster plots, the two bottom rows show relative neck rotation (PNR) and whole-passive-body rotation (WBR). The vertical broken line indicates the change from rightward (up) to leftward (down) rotation. In this case of a Type II neuron, 10 rotations were superimposed/averaged. In A, only passive sinusoidal rotation was used with the animal's head fixed to the turntable, i.e. head and body were rotated together (classical paradigm), and no PNR occurred as symbolized by the straight line. In B, the animal's head was held fixed in space, while its body was rotated passively underneath. The bottom two rows indicate the direction of trunk rotation (WBR) and the direction of relative head rotation with respect to the trunk (PNR), although in this case, no vestibular stimulation occurred. Equivalent relative neuronal firing rates are indicated by grey shading. In such cases, vestibular and neck inputs would work synergistically, although the neck component is considerably smaller than the vestibular contribution.
All neurons tested with HTR (4/4) responded significantly. The majority (3/4) was less modulated by HTR as compared to the WBR, suggesting a possible attenuating role of the putative neck input. In any case, the change in preferred vestibular direction would remain unaffected by neck input.
All in all, the majority of the subset of tested neurons showed no significant modulation from neck inputs: thus we suggest that the overall differences between neuronal responses following active and passive head movements in parietal vestibular neurons cannot be due to neck proprioceptive input (see also Roy & Cullen, 2002).
Anatomical localization
In our experiments, neurons were recorded along microelectrode tracks determined by a grid that allowed reproducible positionings across experimental sessions. While descending in the intraparietal sulcus from the surface, vestibular testing was performed regularly. Besides VIP, we recorded also from MIP, a second intraparietal vestibular zone that was quite distinct from VIP with respect to anatomical location and physiological characteristics. The recording sites have been verified in both monkeys to be located in the medial bank and in the fundus of the intraparietal sulcus (Fig. 1A) (for details see Klam & Graf, 2003a; their Fig. 9; see also Bremmer et al. 2002a; their Fig. 1).
Vestibular responses in the VIP and MIP
Regarding basic vestibular response types, there were no differences between VIP and MIP neurons. Under the classical vestibular classification using passive rotations, VIP and MIP showed similar proportions of Type I, Type II and Type III responses. Significant differences in proportions between Types I and II were found only for the active population (Table 2). Regarding response strength, no real significant differences between VIP and MIP neurons were observed (Table 3).
Table 2.
VIP/MIP vestibular response types
| Active VIP | Active MIP | Passive VIP | Passive MIP | |
|---|---|---|---|---|
| Type I | 23.2% (19/82)* | 66.7% (20/30)* | 25% (16/64) | 45.5% (10/22) |
| Type II | 65.8% (54/82)* | 26.7% (8/30)* | 62.5% (40/64) | 45.5% (10/22) |
| Type III | 11% (9/82) | 6.6% (2/30) | 12.5 (8/64) | 9% (2/22) |
Significant proportion comparisons are indicated by asterisks, and are only present in the active movement condition.
Table 3.
VIP/MIP vestibular response strengths between active and passive movement conditions
| VIP | MIP | |
|---|---|---|
| Active > passive | 13% (8/63) | 9% (2/22) |
| Active < passive | 74% (47/63) | 91% (20/22) |
| Active ∼ passive | 13% (8/63)* | 0% (0/22)* |
Note, that the proportion comparison in the third row (Active ∼ passive) only appears significant (*) because of the absence of any such recorded neuron in MIP.
The proportions of neurons that changed their preferred directions between active and passive movement conditions were not significantly different between the two areas either, i.e. 39% (25/64) and 41% (9/22) for VIP and MIP, respectively.
Discussion
Posterior parietal cortex and efference copy
The variety of vestibular responses in posterior parietal cortex neurons points to a complex processing pattern that is not readily accessible to traditional methods of vestibular analysis. Since our present and previous testing (Bremmer et al. 2002b) had shown little influence of eye movement signals on the neuronal firing of intraparietal vestibular neurons, we assume that these neurons are fundamentally different from brainstem vestibular neurons. Their role has to be sought in sensory space representation rather than reflex behaviour and motor control contexts. Clearly, each time we perform a head movement, vestibular receptors become activated, and central processing between commands and reflexes takes place. Since the introduction of the concept of the reafference principle (von Holst & Mittelstaedt, 1950) to control and calibrate self-generated movements, numerous studies have sought to uncover the actual underlying neuronal elements and control signals for efference copy expressions and corollary discharges (Sperry, 1950).
Since vestibular receptors per se do not distinguish between active and passive movements (Cullen & Minor, 2002), this distinction has to be furnished by central neurons. Second-order vestibular neurons, i.e. two synapses away from the receptor cells, already react differentially to active and passive head movements (Gdowski & McCrea, 1999; McCrea et al. 1999; Roy & Cullen, 2001a; Cullen & Roy, 2004). Roy & Cullen (2004) have recently addressed the question of the origin of this effect, by testing the responses of vestibular neurons to active movement, passive whole-body rotation, and various combinations of head-in-space and/or head-on-trunk movements. Because neurons were suppressed only when the intended active movement and the actual displacement of the head in space were matched, they were then able to propose that the signal subserving active–passive differences in the vestibular nuclei are of central origin, arising from a comparison of an efference copy – or an internal prediction – and the actual movement of the head. Intraparietal cortex neurons may perform this function, or at least play an important role in it, for the following reasons: (1) based on its sensory input, area VIP is thought to participate in self-motion perception (Duhamel et al. 1991; Colby et al. 1993; Bremmer et al. 2000); (2) recently, Gabel et al. (2002) have shown that VIP neurons distinguish between active and passive visual motion, i.e. neurons tend to be more active, and to have a narrower tuning curve, when the animal performs smooth pursuit eye movements across a static random dot pattern, than when the eyes stay still and the dots move to generate an equivalent flow field. Similarly as for the active–passive difference in the vestibular nuclei, these authors suggested the involvement of extra-retinal signals – such as an efference copy – for these visual motion effects; (3) the posterior parietal cortex sends direct projections to the vestibular nuclei (Ventre & Faugier-Grimaud, 1988; Fukushima, 1997); (4) in addition, ablation of the parietal cortex leads to impairment of VOR control (Ventre & Faugier-Grimaud, 1986), suggesting a strong functional cortico-brainstem interaction in relation to self-motion and body reference stabilization.
Intention and attention
VIP and the posterior parietal cortex also play a role in movement intention and awareness (see e.g. Snyder et al. 2000; Thoenissen et al. 2002; Sirigu et al. 2004), and neurons can be modulated by attention (Cook & Maunsell, 2002; see also Colby & Goldberg, 1999). While it was not possible to influence or monitor movement intention of the animals during the experiment, where they generated spontaneous head movements, attention cues were entirely absent in the completely dark laboratory environment. Thus, any external attention modulation of the observed responses of this study can be ruled out.
Role of neck proprioceptive input
Neck proprioceptive input has been discussed as one possible source of the attenuation or cancellation of vestibular responses observed in the vestibular nuclei (McCrea et al. 1999; Gdowski & McCrea, 2000; Roy & Cullen, 2001 a,b, 2002). In general, neck input is thought to be largely antagonistic to the vestibular on-direction in decerebrate (Wilson et al. 1990) and alert preparations (McCrea et al. 1999; Gdowski & McCrea, 2000), but some synergistic interactions have been reported as well, although in anaesthetized and paralysed animals (Anastasopoulos & Mergner, 1982). With regard to our findings, using alert macaque monkeys, we could still expect antagonistic interaction of neck proprioceptors with vestibular signals in light of the findings in squirrel monkeys by McCrea et al. (1999). However, similar experiments in macaque monkeys, the species we are using (Roy & Cullen, 2001a,b, 2002), failed to demonstrate any significant influence on vestibular nucleus neurons. In fact, their neck responses were in general smaller by a factor of 10 when compared to the vestibular responses. While some central processing certainly cannot be excluded, it seems unlikely that separate relay channels for neck proprioceptive input transmit such information to cortical units when convergence already occurs at the level of the vestibular nuclei. Furthermore, given the sign of the neck input, i.e. antagonistic, such input could explain only those of our neurons which show a reduced activity during active head movements, when compared to the passive rotation condition. It would not explain increased response strength, firing before the onset of the movement, and especially the change in preferred direction of a subpopulation of the neurons.
Indeed, our limited sample of neurons tested for neck proprioceptive input does not allow any definite conclusions as to its significance. The majority of our neuron sample showed no significant effect, and inputs to the rest of the population can either be synergistic or antagonistic. Clearly, our neuron population is a rhesus monkey sample (Roy & Cullen, 2001a,b, 2002), and not a squirrel monkey sample (McCrea et al. 1999; Gdowski & McCrea, 2000), showing little or no neck influence whatsoever.
Cortical vestibular neurons and brainstem vestibular neurons
In this context, some important differences between brainstem vestibular and cortical vestibular units also have to be addressed. Brainstem vestibular units have been very well studied over the past decades with respect to their signal content for head and eye movements, and their involvement in oculomotor and spinal motor functions. In this context (McCrea & Gdowski (2003), and Roy & Cullen (2001a, 2002; for a review see Cullen & Roy, 2004) studied their units with particular focus on eye movements and ‘gaze saccades’. One problem arising with some of these experiments thus appears that they were done in a more or less illuminated environment. Gdowski & McCrea (1999) even indicated this fact in the methods of their publication, i.e. those experiments were done ‘in dim light’. Their subsequent publications (e.g. McCrea et al. 1999; Gdowski & McCrea, 2000; McCrea & Gdowski, 2003) only refer to the methods of Gdowski & McCrea (1999). Thus, we have to assume that in these experiments, no pure vestibular responses were studied, as with our neurons, but only combined visual-vestibular signals. Roy & Cullen (2002) specified which experiments were done in darkness, and when visual cues were present.
The question of visual input, even in ‘dim light’, during vestibular stimulation cannot be underestimated or neglected because of the well-known visual–vestibular interaction taking place in vestibular nucleus neurons (e.g. Dichgans et al. 1973; Allum et al. 1976; Henn et al. 1974; Waespe & Henn, 1977; Keller & Precht, 1979), where visual input plays a significant role in the production of optokinetic nystagmus. Since our parietal vestibular neurons could also be driven by optokinetic stimuli (Bremmer et al. 2002a), and because of the involvement of VIP in optokinetic nystagmus (Galati et al. 1999; Konen et al. 2005), we ensured elimination of any possible visual stimulus contamination during the experiments presented in this study. Cleary, as soon as vision is allowed, a whole new set of parameters has to be considered that removes any interpretation from a purely ‘vestibular’ quality.
Change in preferred direction
A potential reversal of preferred direction, similar to that observed in 40% of our neurons might be construed from a population of eight so-called ‘cancelled’ vestibulo-spinal units reported in McCrea et al. (1999) (their Fig. 5), but has to be accepted with caution in light of the above-mentioned methodological shortcomings of this study. In any case, these neurons would change from Type I to Type IV (inhibited in both directions) during a ‘gaze saccade’. Hence, such a change would not be equivalent, for instance, to a change from a Type I into a Type III neuron, where an activation of the discharge has to take place.
A true change of preferred direction comparing active and passive movement conditions, by contrast, has been reported in cortical motor neurons (Murphy et al. 1978; Fetz et al. 1980), as well as in spinal interneurons (Fetz et al. 2002) involving limb and wrist movements. Furthermore, during active movement, evoked cutaneous input to spinal interneurons controlling the movement area is suppressed (Fetz et al. 2002). This finding is analogous to suppression of vestibular information in the vestibular nuclei during active head movements, vestibulo-spinal circuits being one of the archaic motor control circuits.
Vestibulo-cortical projection
When vestibular nucleus neuron behaviour during active–passive movement was categorized by Gdowski & McCrea (1999) and McCrea et al. (1999), units were globally divided into eye-movement-related and non-eye-movement-related neurons. Non-eye-movement-related neurons were shown to project either to the upper cervical cord, and thus controlled head movements, or they did not, and were interpreted to play a role in perceptive mechanisms relaying information to the cortex. No details on a specific projection pattern were offered. While there is global information available as to the general pathways transmitting vestibular information to cortical areas (Deecke et al. 1974; Büttner & Lang, 1979; Lang et al. 1979), details about specific neuron categories are almost absent. Only a few studies have unequivocally identified the neurons projecting to the thalamus (Matsuo et al. 1994, 1995a,b). Contrary to the assumption presented above, i.e. that non-eye-movement-related neurons that do not project to the cervical chord participate in cortical functions, these neurons are vestibulo-oculomotor neurons producing the vestibulo-ocular reflex. Thus, the absence of eye-movement-related signals in the thalamic and cortical vestibular pathways remains unresolved and awaits further study. Vestibular input to VIP seems to arrive via the classical route involving the ventro-posterior inferior nucleus of the thalamus, and area 2v in the intraparietal sulcus/parieto-insular vestibular cortex (PIVC) (Lewis & Van Essen, 2000). A second, more indirect route, may involve the fastigial and interpositus nuclei, the pulvinar and a direct projection from there to the VIP (Graf et al. 2005). In addition, neurons in area MST, a major input source to area VIP, are modulated by vestibular stimulation (Ilg et al. 2004; Gu et al. 2006). The role and weight of these various vestibular convergent and parallel inputs remains to be determined.
The possible role of change in preferred direction
While a small number of our posterior parietal vestibular neurons receive weak neck input (Fig. 6; see also Roy & Cullen, 2001a,b), the key to understanding the active–passive movement distinction processing may be the surprising finding that a number of intraparietal neurons change their vestibular preferred direction, depending on whether the movement is active or passive. This phenomenon, found in 40% of the neurons (Fig. 3Aa and b and Ba and b), has not been observed in the vestibular nuclei (Khalsa et al. 1987; McCrea et al. 1999; Cullen & Roy, 2004). Based on these response properties, the nervous system may simultaneously have access via VIP and MIP to information about the direction of an ongoing movement (leftward or rightward) as well as to its nature (active or passive). A possible mechanism is illustrated in Fig. 7. Our interpretation is based on a population of neurons that change their vestibular on-direction, and others that do not. If the populations of output neurons in our scheme (OUT1 – OUT4) that are selective to both movement direction (left or right) and type (active or passive) respond only when two input populations (IN1 – IN2) are active simultaneously, then the proposed mechanism is sufficient to obtain specific responses with regard to discriminating between active and passive movements. Such cortical signals, in turn, could be used to suppress reflex movements during active movement, by providing the neural basis for the observed extinction of, for instance, vestibular signals during active head rotation in the vestibular nuclei (Gdowski & McCrea, 1999; McCrea et al. 1999; Roy & Cullen, 2001a; Cullen & Roy, 2004), at least for the subpopulation of cells whose activity starts before the active head movement.
Figure 7. Discrimination between active and passive movements by intraparietal neurons, depending on selectivity to movement type (active A: blue; passive P: magenta) and direction (left or right: arrows).
In the illustrated examples, discrimination between an active (A) and a passive head rotation (B) to the left is elaborated. In the general scheme, a set of input neurons (IN1–IN4) is connected in a particular fashion to a set of output neurons (OUT1–OUT4). With regard to the preferred directions of vestibular responses, two types of input neuron populations are distinguished when comparing active and passive movement, i.e. neurons which change their preferred direction depending on active or passive mode (IN1, IN3), and those that do not (IN2, IN4). In case of discriminating an active head movement to the left (A), two neurons would be involved, the first (IN1) responding with activation during leftward active movement and rightward passive movement, and another (IN2) responding to both leftward active and passive movements. An output neuron (OUT1) receiving afferents (bold lines) from these two neurons, would signal only leftward active movements. In such case, the two components signalling leftward active movement would get reinforced, whereas the components signalling leftward and rightward passive movement would oppose each other and cancel out (neurons not involved are shaded in grey). In the case of a passive movement (B), a population of output neurons (OUT2) receiving afferents from neurons activated during active and passive leftward movement (IN2), and from those responding to leftward passive and rightward active movements (IN3), would signal leftward passive movement. In this case, the two components signalling leftward and rightward active movement would oppose each other and cancel out, whereas the two leftward passive movement-signalling components would reinforce each other (again, neurons not involved are shaded in grey). The two other distinct types for active and passive rightward movements (OUT3, OUT4) are constructed analogously.
The possible roles of parietal vestibular signals
On the other hand, these signals could be used in the representation of space, based on three lines of argument. First, vestibular stimulation leads to a remission of the parietal syndrome of visual spatial hemineglect (Karnath, 2001; Rorden et al. 2001; Swan, 2001; Karnath & Dieterich, 2006). Patients improve in visuo-spatial tasks (Rubens, 1985) as well as in mental spatial representations (Rode & Perenin, 1994), thus confirming a diverse and fundamental role of vestibular information in the representation of space. Second, Snyder et al. (1998) described effects of static head position after active or passive movements (gain fields) in parietal areas 7a and LIP, which could serve as a distributed coding of body-(egocentric) or space-based (allocentric) reference frames. Quite differently, the signals detailed here occur before and during head movements. We suggest that such neuronal responses could be a basis for an immediate, i.e. online change of reference frame from egocentric (during passive movement) to allocentric (during active movement), representation described in psychophysical experiments. Wexler (2003), using an ambiguous three-dimensional virtual visual object whose motion could appear to be minimal either in an egocentric or an allocentric reference frame, has shown that subjects tend to assume that the object is stable in space (allocentric judgement) when they move themselves, and that it is stable relative to their head when there are being moved (egocentric judgement). Such a change of reference frame requires no learning, and is immediate, occurring on a trial-by-trial basis. Likewise, in the neuron population of the present study, the active–passive switch is dependent on the presence of specific motor activities involved in moving the head in space. Third, the hippocampal formation is known to be involved in spatial navigation, and hippocampo-parietal interactions seem to be necessary for the build-up of allocentric space representations found in the hippocampus in rats (Save & Poucet, 2000) and humans (Maguire et al. 1998). In particular, in rats, the associative parietal cortex may perform the first step of the series of transformations towards allocentric coordinates, by combining visuo-spatial and self-motion information (Save & Poucet, 2000). Active action in space seems to be necessary for the build-up of an allocentric representation in monkeys: place cells have reliable responses only when the animal does active steering in a virtual environment (Nishijo et al. 1997). Furthermore, head direction cell responses in the rat's antero-dorsal nucleus of the thalamus seem to be influenced by the nature of the head movement (Basset et al. 2005). Given that the parietal and para-hippocampal regions are reciprocally connected in the monkey (Cavada & Goldman-Rakic, 1989), we suggest that the signals described in the present study could serve as a basis for those active–passive effects on spatial perception.
Alternatively, parietal vestibular responses could also participate in the preparation of movements of the whole or parts of the body in space, acting as an interface between sensory and motor cortices as proposed by Mountcastle et al. (1975), through its projections to the premotor cortex. Schaefer et al. (1975) have described active–passive direction changes in neurons in the reticular formation of rabbits similar to those we have observed. These authors proposed that such neurons have a motor role: during passive movement, they are activated because of compensatory muscle activation in the direction opposite to a given movement. During active movement, they would elicit a head rotation opposite to that of the passive movement. Similarly behaving parietal neurons of our study could therefore also have a motor role. Incidentally, Graziano et al. (1997) reported active–passive differences in ventral premotor cortex neurons in response to head movements. The ventral premotor cortex is reciprocally connected to VIP (Matelli & Luppino, 2001). In such cases, the parietal neurons signalling active movement and whose response begins before movement onset could participate in elaborating motor plans in space, whereas those that are insensitive to the nature of the movement could have a purely sensory role.
Conclusion and summary
All in all, here we demonstrate for the first time in the cortex of non-human primates a direct neural correlate to the nature – active or passive – of an ongoing head movement. These parietal responses differ from the ones described in the brainstem vestibular nuclei in three main respects. First, unlike the recent results on second-order vestibular neurons, some cortical cells respond to active movement with equal or increased strength. Second, a fair proportion of neurons respond before the beginning of active movements, and third, more than one-third of neurons change their preferred direction depending on whether the head movement is active or passive. We suggest that posterior parietal cortex neurons play an important role in discriminating between active and passive movements, at least for head movements. In addition, they may not have just one function, but may play manifold roles such as in self-motion perception, space–time-coordinated movements, attention, and spatial orientation.
Acknowledgments
This work was supported by European Union grants BIO4-CT98-0546 and QLK6-CT-2002-00151 (EUROKINESIS), the CNRS (UMR 9950/7124), and the ATER program of the Collège de France. The authors thank France Maloumian for help with the illustrations.
References
- Allum JHJ, Graf W, Dichgans J, Schmidt CL. Visual-vestibular interaction in the vestibular nuclei of the goldfish. Exp Brain Res. 1976;26:463–485. doi: 10.1007/BF00238821. [DOI] [PubMed] [Google Scholar]
- Anastasopoulos D, Mergner T. Canal–neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res. 1982;46:269–280. doi: 10.1007/BF00237185. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. doi: 10.1126/science.4048942. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Zugaro MB, Muir GM, Golob EJ, Muller RU, Taube JS. Passive movements of the head do not abolish anticipatory firing properties of head direction cells. J Neurophysiol. 2005;93:1304–1316. doi: 10.1152/jn.00490.2004. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Graf W, Ben Hamed S, Duhamel JR. Eye position encoding in the macaque ventral intraparietal area (VIP) Neuroreport. 1999;10:873–878. doi: 10.1097/00001756-199903170-00037. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Duhamel JR, Ben Hamed S, Graf W. Stages of self-motion processing in primate posterior parietal cortex. Int Rev Neurobiol. 2000;44:173–198. doi: 10.1016/s0074-7742(08)60742-4. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Ben Hamed S, Duhamel JR, Graf W. Optic flow processing in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002a;16:1554–1568. doi: 10.1046/j.1460-9568.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Klam F, Duhamel JR, Ben Hamed S, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002b;16:1569–1586. doi: 10.1046/j.1460-9568.2002.02206.x. [DOI] [PubMed] [Google Scholar]
- Büttner U, Henn V, Oswald HP. Vestibular-related neuronal activity in the thalamus of the alert monkey during sinusoidal rotation in the dark. Exp Brain Res. 1977;30:435–444. doi: 10.1007/BF00237267. [DOI] [PubMed] [Google Scholar]
- Büttner U, Lang W. The vestibulocortical pathway: neurophysiological and anatomical studies in the monkey. In: Granit R, Pompeiano O, editors. Reflex Control of Posture and Movement. Amsterdam: Elsevier; 1979. pp. 581–588. Progress in Brain Research, Vol 50. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory cortico-cortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;39:517–537. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. The ventral intraparietal area (VIP) of the macaque: anatomical location and visual properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Ann Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JH. Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey. J Neurosci. 2002;22:1994–2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 2002;22:RC226 (1–7). doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Deecke L, Schwarz DWF, Fredrickson JM. Nucleus ventroposterior inferior (VPI) as the vestibular thalamic relay in the rhesus monkey. I. Field potential investigation. Exp Brain Res. 1974;20:88–100. doi: 10.1007/BF00239019. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Schmidt CL, Graf W. Visual input improves the speedometer function of the vestibular nuclei in the goldfish. Exp Brain Res. 1973;18:319–322. doi: 10.1007/BF00234602. [DOI] [PubMed] [Google Scholar]
- Duensing F, Schaefer K-P. Die Aktivität einzelner Neurone im Bereich der Vestibulariskerne bei Horizontalbeschleunigungen unter besonderer Berücksichtigung des vestibulären Nystagmus. Arch Psychiat Nervenkrankh. 1958;196:265–290. doi: 10.1007/BF00941383. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Congruent representations of visual and somatosensory space in single neurons of monkey ventral intraparietal cortex (area VIP) In: Paillard J, editor. Brain and Space. Oxford: Oxford University Press; 1991. pp. 223–236. [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Finocchio DV, Baker MA, Soso MJ. Sensory and motor responses of precentral cortex cells during comparable passive and active joint movements. J Neurophysiol. 1980;43:1070–1089. doi: 10.1152/jn.1980.43.4.1070. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S. Roles of primate spinal interneurons in press and execution of voluntary hand movement. Brain Res Rev. 2002;40:53–65. doi: 10.1016/s0165-0173(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Fukushima K. Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res. 1997;117:1–16. doi: 10.1007/pl00005786. [DOI] [PubMed] [Google Scholar]
- Gabel SF, Misslich H, Gielen CCAM, Duysens J. Responses of neurons in area VIP to self-induced and external visual motion. Exp Brain Res. 2002;147:520–528. doi: 10.1007/s00221-002-1268-5. [DOI] [PubMed] [Google Scholar]
- Galati G, Pappata S, Pantano P, Lenzi GL, Samson Y, Pizzamiglio L. Cortical control of optokinetic nystagmus in humans: a positron emission tomography study. Exp Brain Res. 1999;126:149–159. doi: 10.1007/s002210050725. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Integration of vestibular and head movement signals in the vestibular nuclei during whole body rotation. J Neurophysiol. 1999;81:436–449. doi: 10.1152/jn.1999.82.1.436. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, McCrea RA. Neck proprioceptive inputs to primate vestibular nucleus neurons. Exp Brain Res. 2000;135:511–526. doi: 10.1007/s002210000542. [DOI] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP. Functional anatomy of the head–neck movement system of quadrupedal and bipedal mammals. J Anat. 1995;186:55–74. [PMC free article] [PubMed] [Google Scholar]
- Graf W, Gerrits NM, Yatim-Dhiba N, Ugolini G. Mapping the oculomotor systemml: the power of transneuronal labeling with rabies virus. Eur J Neurosci. 2002;15:1557–1562. doi: 10.1046/j.1460-9568.2002.01994.x. [DOI] [PubMed] [Google Scholar]
- Graf W, Klam F, Prevosto V, Ugolini G. Vestibular ascending input to posterior parietal areas VIP/MIP, revealed by retrograde transneuronal transfer of rabies virus. Abstr Soc Neurosci. 2005;31:932.1. [Google Scholar]
- Grantyn A, Brandi A, Dubayle D, Graf W, Ugolini G, Hadjidimitrakis K, Moschovakis A. Density gradients of trans-synaptically labeled collicular neurons after injection of rabies virus in the lateral rectus muscle of the rhesus monkey. J Comp Neurol. 2002;451:346–361. doi: 10.1002/cne.10353. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. J Neurophysiol. 1997;77:2268–2292. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- Grüsser O-J, Pause M, Schreiter U. Localization and responses of neurons in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis) J Physiol. 1990;430:537–557. doi: 10.1113/jphysiol.1990.sp018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Watkins PV, Angelaki D, DeAngelis GC. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci. 2006;26:73–85. doi: 10.1523/JNEUROSCI.2356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn V, Young LR, Finley C. Vestibular nucleus units in alert monkeys are also influenced by moving visual fields. Brain Res. 1974;71:144–149. doi: 10.1016/0006-8993(74)90198-x. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Ilg U, Schumann S, Thier P. Posterior parietal cortex neurons encode target motion in world-centered coordinates. Neuron. 2004;43:145–151. doi: 10.1016/j.neuron.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Karnath H-O. New insights into the functions of the superior temporal cortex. Nature Rev Neurosci. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Dieterich M. Spatial neglect-a vestibular disorder? Brain. 2006;129:293–305. doi: 10.1093/brain/awh698. [DOI] [PubMed] [Google Scholar]
- Keller EL, Precht W. Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience. 1979;4:1599–1613. doi: 10.1016/0306-4522(79)90023-x. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Tomlinson RD, Schwarz DW, Landolt JP. Vestibular nuclear neuron activity during active and passive head movement in the alert rhesus monkey. J Neurophysiol. 1987;57:1484–1497. doi: 10.1152/jn.1987.57.5.1484. [DOI] [PubMed] [Google Scholar]
- Klam F, Graf W. Vestibular response kinematics in posterior parietal cortex neurons of macaque monkeys. Eur J Neurosci. 2003a;18:995–1010. doi: 10.1046/j.1460-9568.2003.02813.x. [DOI] [PubMed] [Google Scholar]
- Klam F, Graf W. Vestibular signals of posterior parietal cortex neurons during active and passive head movements in macaque monkeys. Ann NY Acad Sci. 2003b;1004:271–282. doi: 10.1196/annals.1303.024. [DOI] [PubMed] [Google Scholar]
- Klam F, Graf W. Active and passive head movement encoding by posterior parietal cortex neurons of macaque monkeys: vestibular signals. Abstr Soc Neurosci. 2003c;29:268.3. [Google Scholar]
- Konen CS, Kleiser R, Seitz RJ, Bremmer F. An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans. Exp Brain Res. 2005;165:203–216. doi: 10.1007/s00221-005-2289-7. [DOI] [PubMed] [Google Scholar]
- Lang W, Büttner-Ennever JA, Büttner U. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain Res. 1979;177:3–17. doi: 10.1016/0006-8993(79)90914-4. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Magnin M, Fuchs A. Discharge properties of neurons in the monkey thalamus tested with angular acceleration, eye movement and visual stimuli. Exp Brain Res. 1977;28:293–299. doi: 10.1007/BF00235710. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14:S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Hosogai M, Nakao S. Ascending projections of posterior canal-activated excitatory and inhibitory secondary vestibular neurons to the mesodiencephalon in cats. Exp Brain Res. 1994;100:7–17. doi: 10.1007/BF00227274. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Hosogai M, Matsui H, Ikoma H. Posterior canal-activated vestibulocortical pathways in cats. Neurosci Lett. 1995a;183:131–134. doi: 10.1016/0304-3940(94)11132-3. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Hosogai M, Matsui H, Ikoma H. Posterior canal-activated excitatory vestibuloocular neurons participate in the vestibulocortical pathways in cats. Acta Otolaryngol Suppl. 1995b;520:97–100. doi: 10.3109/00016489509125200. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Gdowski G. Firing behaviour of squirrel monkey eye movement-related vestibular nucleus neurons during gaze saccades. J Physiol. 2003;546:207–224. doi: 10.1113/jphysiol.2002.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non eye- movement related neurons. J Neurophysiol. 1999;82:416–428. doi: 10.1152/jn.1999.82.1.416. [DOI] [PubMed] [Google Scholar]
- Moschovakis A, Gregoriou GG, Ugolini G, Doldán M, Graf W, Hadjidimitrakis K, Savaki HE. Oculomotor areas of the primate frontal lobes: a transneuronal transport of rabies virus and [14C]-2-deoxyglucose functional imaging study. J Neurosci. 2004;24:5726–5740. doi: 10.1523/JNEUROSCI.1223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Kwan HC, McKay WA, Wong YC. Spatial organization of precentral cortex in awake primates. III. Input–output coupling. J Neurophysiol. 1978;41:1132–1139. doi: 10.1152/jn.1978.41.5.1132. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Eifuku S, Tamura R. The relationship between monkey hippocampus place-related neural activity and action in space. Neurosci Lett. 1997;226:57–60. doi: 10.1016/s0304-3940(97)00255-3. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Tomko DL. Cat vestibular neurons that exhibit different responses to active and passive yaw head rotations. Aviat Space Environ Med. 1987;58:A247–A249. [PubMed] [Google Scholar]
- Rode G, Perenin MT. Temporary remission of representational hemineglect through vestibular stimulation. Neuroreport. 1994;14:869–872. doi: 10.1097/00001756-199404000-00004. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, Driver J. Do neck-proprioceptive and caloric-vestibular stimulation influence covert visual attention in normals, as they influence visual neglect? Neuropsychologia. 2001;39:364–375. doi: 10.1016/s0028-3932(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self generated head motion. J Neurosci. 2001a;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Passive activation of neck proprioceptive inputs does not influence the discharge patterns of vestibular nuclei neurons. Ann NY Acad Sci. 2001b;942:486–489. doi: 10.1111/j.1749-6632.2001.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens AB. Caloric stimulation and unilateral visual neglect. Neurology. 1985;35:1019–1024. doi: 10.1212/wnl.35.7.1019. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Hippocampal–parietal cortical interactions in spatial cognition. Hippocampus. 2000;10:491–499. doi: 10.1002/1098-1063(2000)10:4<491::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schaafsma SJ, Duysens J. Neurons in the ventral intraparietal area of awake macaque monkey closely resemble neurons in the dorsal part of the medial superior temporal area in their responses to optic flow patterns. J Neurophysiol. 1996;76:4056–4068. doi: 10.1152/jn.1996.76.6.4056. [DOI] [PubMed] [Google Scholar]
- Schaafsma SJ, Duysens J, Gielen CC. Responses in ventral intraparietal area of awake macaque monkey to optic flow patterns corresponding to rotation of planes in depth can be explained by translation and expansion effects. Vis Neurosci. 1997;14:633–646. doi: 10.1017/s0952523800012608. [DOI] [PubMed] [Google Scholar]
- Schaefer KP, Schott D, Meyer DL. On the organization of neuronal circuits involved in the generation of the orientation response (visual grasp reflex) Fortschr Zool. 1975;23:199–212. [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiol. 2003;551:551–561. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Daprati E, Ciancia S, Giraux P, Nighoghossian N, Posada A, Haggard P. Altered awareness of voluntary action after damage to the parietal cortex. Nat Neurosci. 2004;7:80–84. doi: 10.1038/nn1160. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body- and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–490. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Swan L. Unilateral spatial neglect. Phys Ther. 2001;81:1572–1580. doi: 10.1093/ptj/81.9.1572. [DOI] [PubMed] [Google Scholar]
- Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 2002;22:9024–9034. doi: 10.1523/JNEUROSCI.22-20-09024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Doldán Dans M, Dubayle D, Brandi A-M, Büttner-Ennever J, Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: differences in monosynaptic input to ‘slow’ and ‘fast’ abducens motoneurons. J Comp Neurol. 2006 doi: 10.1002/cne.21092. in press. [DOI] [PubMed] [Google Scholar]
- Ventre J, Faugier-Grimaud S. Effects of posterior parietal lesions (area 7) on VOR in monkeys. Exp Brain Res. 1986;62:654–658. doi: 10.1007/BF00236046. [DOI] [PubMed] [Google Scholar]
- Ventre J, Faugier-Grimaud S. Projections of the temporo-parietal cortex on vestibular complex in the macaque monkey (Macaca fascicularis) Exp Brain Res. 1988;72:653–658. doi: 10.1007/BF00250611. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Graf W, Berthoz A. The orientation of the cervical vertebral column in unrestrained awake animals. I. Resting position. Exp Brain Res. 1986;61:549–559. doi: 10.1007/BF00237580. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1977;27:523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Wexler M. Voluntary head movement and allocentric perception of space. Psychol Sci. 2003;14:340–346. doi: 10.1111/1467-9280.14491. [DOI] [PubMed] [Google Scholar]
- Wilson WJY, Yamagata BJ, Yates RH, Schor, Nonaka S. Response of vestibular neurons to head rotations in vertical planes. III. Respons of vestibulocollic neurons to vestibular and neck stimulation. J Neurophysiol. 1990;64:1695–1703. doi: 10.1152/jn.1990.64.6.1695. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hilary W, Britten KH. Parietal area VIP neuronal responses to heading stimuli are encoded in head-centered coordinates. Neuron. 2004;42:993–1001. doi: 10.1016/j.neuron.2004.06.008. [DOI] [PubMed] [Google Scholar]