Abstract
To investigate the phenotypic consequences of a deranged lymphangiogenesis in relation to tissue fluid accumulation and the possible role of inflammation in the pathogenesis of lymphoedema, we measured determinants of transcapillary fluid filtration and inflammatory mediators in the interstitial fluid in genetically engineered Chy mice, a model for primary congenital lymphoedema (Milroy's disease). Although initial lymphatics were not present in dermis in any of the areas studied (fore paw, hind paw, thigh and back skin) interstitial fluid pressure (Pif), measured with micropipettes, and tissue fluid volumes were significantly increased only in the areas with visible swelling – the fore and hind paw, whereas interstitial colloid osmotic pressure (COPif) was increased in all the skin areas examined. A volume load of 15% of body weight resulted in a more pronounced increase in Pif as well as a four-fold increase in interstitial fluid volume in Chy relative to wild-type (wt) mice, showing the quantitative importance of lymphatics for fluid homeostasis during acute perturbations. A similar level of proinflammatory markers in interstitial fluid in early established lymphoedema (3–4 months) in Chy and wt suggests that inflammation does not have a major pathogenetic role for the development of lymphoedema, whereas a reduced level of the immunomodulatory cytokine interleukin (IL)-4 may result in a reduced immunological defence ability and thus lead to the increase in inflammatory cytokines IL-2 and IL-6 observed at a later stage (11–13 months). Our data suggest that primary lymphoedema results in a high interstitial fluid protein concentration that does not induce an interstitial inflammatory reaction per se, and furthermore shows the paramount importance of the initial lymphatics in tissue fluid homeostasis, especially during perturbations of transcapillary fluid balance.
Oedema is a condition where there is an increase in interstitial fluid volume sufficient to produce a visible swelling, whereas in lymphoedema such swelling is due to failure of lymph drainage in situations where the capillary filtration is not increased (Mortimer, 1998). Lymphoedema can be primary if the cause is congenital or unknown, or secondary if the condition results from an obstruction of lymphatics due to, e.g. surgery or infection. Most of our previous knowledge of the pathophysiology of lymphoedema derives from studies in dogs with experimentally induced obstructive lymphoedema (Taylor et al. 1958; Olszewski et al. 1968; Knight et al. 1987; Chen et al. 1989). During the last decade, however, there has been a dramatic increase in the amount of data on growth factors and molecular markers specific for lymphatic vessels, and several mouse models for primary lymphoedema have been developed (for review see Alitalo et al. 2005), enabling us to generate new and more specific knowledge in this field.
Since all oedemas result from an imbalance between capillary filtration and lymph drainage, the factors governing capillary filtration have to be considered when studying the pathophysiology of lymphoedema (Mortimer, 1998). One of these is the interstitial fluid pressure (Pif) that also serves as filling pressure for the lymphatics, and therefore is important for lymphatic function (Aukland & Reed, 1993). In human secondary lymphoedema, Pif has been shown to increase significantly as measured by wick-in needle technique, and is considered a force opposing further fluid filtration into the lymphoedematous arm (Bates et al. 1992, 1994). Another determinant of capillary filtration that is likely to be altered due to the lack of lymph drainage is the interstitial fluid colloid osmotic pressure (COPif). Lymphoedema is characterized by an accumulation of protein and fluid in the interstitium, but whether the protein concentration and thereby COPif is higher than in normal interstitial fluid is still a matter of debate. In the classical view, the oedema fluid in lymphoedema has been considered to have a high protein concentration as a consequence of the oedema's lymphatic origin (Taylor et al. 1958; Knight et al. 1987), whereas more recent studies have reported decreased (Bates et al. 1993) as well as unaltered (Olszewski, 2003) protein concentration in interstitial fluid and lymph from humans subjected to secondary lymphoedema. In the latter study, Olszewski et al. (2003) also detected increased levels of cytokines in lymph from secondary lymphoedema patients, suggesting a role for inflammation in the pathogenesis of this condition. Whether an inflammatory reaction is an important part of the pathogenesis for this condition is another controversial issue that has not been resolved. While there is some evidence that the accumulation of interstitial water and protein results in a general inflammatory condition contributing to further fluid accumulation and generation of more extracellular matrix (e.g. Rockson, 2001), other reports assign a major pathogenic role to the increased outflow pressure (Mortimer, 1998).
Recently Karkkainen et al. (2001) described the Chy mouse, a model for Milroy's disease that in humans is a congenital, inherited disorder characterized by disfiguring swelling of the limbs. The lack of fluid drainage from the tissue is due to hypoplasia or aplasia of the cutaneous lymphatics, and the stagnant accumulated lymph fluid gives rise to pathophysiological changes of the skin (Mortimer, 1998). As for the human Milroy's disease, Chy mice express a heterozygous inactivating point mutation of the Vegfr3 gene resulting in inactivation of the VEGFR-3 signalling and subsequent swelling of the hind limbs (Karkkainen et al. 2000, 2001). Their name reflects on the development of visible chylous ascites after birth, which is reabsorbed within the first 2–3 days.
The aim of the present study was to use the Chy mouse model of lymphoedema to get new insight into the consequences of a deranged lymphangiogenesis, and try to resolve some of the controversial issues of this field discussed above. Here we used the Chy mouse model to determine the functional consequences of missing lymphatics in relation to transcapillary fluid balance parameters like interstitial fluid pressure, and whether the conditions resulted in interstitial protein accumulation and ensuing increase in interstitial fluid colloid osmotic pressure. We also asked how these mice respond to a volume load, given their lymphatic disability and if there was any sign of an ongoing inflammatory process in the interstitium, in an attempt to determine the potential role of inflammation in the pathogenesis of primary lymphoedema.
Methods
Animals
In the present study we used Chy mice as described in detail previously (Karkkainen et al. 2001). Chy males were bred with wild-type (wt) females to obtain heterozygous Chy offspring. The Chy mutant mice develop chylous ascites which is then spontaneously reabsorbed after the first 2–3 days after birth. Approximately 10% of the Chy mice develop gross swelling in the abdominal region within the three first weeks after birth (Karkkainen et al. 2001b). These mice were killed immediately with an overdose of CO2.
The mutation in Chy mice was verified by PCR analysis prior to experiments, using 5′-GAA GAC CTT GTA TGC TAC-3′ as the forward and 5′-AGG CCA AAG TCG CAG-AA-3′ as the reverse primer, where the 3′ nucleotide of the reverse primer matches with the Chy mutant allele but not the wt allele. The amplification used 40 cycles of denaturation at +94°C (60 s), annealing at +52°C (30 s), and extension at +72°C (40 s). The amplified PCR fragment was recognized as a 549 base pair band found only in the Chy mice when running the samples in 1% agarose gel electrophoresis.
Both Chy and wt mice received food and water ad libitum prior to experiments. The experiments were performed on both sexes of Chy mice, and their wt littermates were used as controls when they were between 12 and 16 weeks old (if not otherwise stated) having reached a body weight of 36 ± 6 g and 37 ± 5 g for Chy and wt, respectively. All mice were anaesthetized with a 1 : 1 mixture (0.1 ml (10 g body weight)−1) of midazolam (Dormicum; Roche) and fentanyl/fluanison (Hypnorm; Janssen) injected subcutaneously. The depth of anaesthesia during the experiments was controlled by testing the withdrawal reflex of the paws, by applying a pinch between the toes. Additional subcutaneous injection of anaesthetic was supplied when needed. Cardiac arrest was induced under anaesthesia with an intracardiac or intravenous injection of saturated potassium chloride (KCl). All experiments were performed with the approval of and in the accordance with the Norwegian State Commission for Laboratory Animals.
Measurement of Pif
Pif was measured with a micropuncture technique (Wiig et al. 1981) in thigh skin and muscle and in the fore paw, hind paw and back skin of anaesthetized Chy and wt mice. In brief, Pif measurements were obtained with sharpened glass capillaries (tip diameter 4–7 μm) connected to a servo-controlled counterpressure system. Measurements in skin were performed through intact skin at a depth of 0.1–0.2 mm determined as described in a previous publication (Wiig, 1985), while thigh muscle was exposed by a skin incision previous to measurements and covered with a drop of paraffin to prevent drying of the exposed muscle (Wiig et al. 1981). Zero reference pressure measurements were performed within a cup of isotonic saline placed at the level of the actual puncture site. A measurement was accepted after fulfilment of the following three criteria (Wiig et al. 1981): (1) the measurement was unaltered with increased feedback gain; (2) applying suction to the pipette by the servo-controlled pump resulted in a resistance change. This was to verify that the capillary was open after the insertion through skin/muscle; (3) there was no change in zero reference pressure before and after the pressure measurement in the tissue.
Measurements of tissue fluid volumes
To quantify the effect of reduced number of lymphatics on tissue fluid volumes we determined extracellular fluid volume (Vx), intravascular plasma volume (Vp) and interstitial fluid volume (Vi). In the anaesthetized Chy and wt mice both kidney pedicles were ligated to prevent escape of extracellular tracer and fluid through the kidneys during the experiment. One catheter (P-25) was placed in the jugular vein for intravenous (i.v.) injections, and another catheter (P-25) in the carotid artery for measurements of blood pressure and for blood sampling. Body temperature was kept at 37°C throughout the experiment with the aid of a servo-controlled heating pad. 51Cr-EDTA (∼200 000 cpm in 0.1 ml saline) was injected i.v. and allowed to distribute for 115 min before 125I-HSA (∼200 000 cpm, in 0.1 ml saline) was injected i.v. A final blood sample was collected through the arterial catheter 5 min after 125I-HSA injection, and the experiment ended with an i.v. injection of saturated KCl. The blood samples coagulated for 30 min before they were spun for collection of serum at 10 000 r.p.m. for 10 min. Tissue samples were excised from fore paw, hind paw, thigh and back skin and from thigh muscle. The samples were put in preweighed vials, and the vials weighed once more for the calculation of tissue wet weight. Radioactivity of the samples was determined using a LKB gamma counter (model 1282 Compugamma). After counting, the samples were dried in a heating chamber keeping a temperature of 60°C until constant weight for calculation of total tissue water (Vw), i.e. the difference between tissue wet and dry weight.
Vx and Vp were found as the plasma equivalent spaces of 51Cr-EDTA and 125I-HSA, respectively. Vi was calculated as the difference between Vx and Vp.
Experimentally induced overhydration
To study the response in Pif with increased volume in a tissue with reduced lymphatic drainage capacity, we induced overhydration by i.v. injection of saline. Surgical procedures were similar to those described above. Pif was measured in the hind paw of anaesthetized mice in the control situation before i.v. injection of 51Cr-EDTA (∼200 000 cpm in 0.1 ml saline). After a 90-min equilibration period, control Pif was measured again, and a tissue sample excised from one hind paw (control paw) for measurement of radioactivity and calculation of control Vi. Before excision the circulation was shut off by tightening an elastic band around the wrist of the control paw to prevent inflammatory substances from entering the general circulation during the remaining part of the experiment. A blood sample (∼100 μl) was collected from the carotid artery catheter and spun for 10 min at 14000 r.p.m. for collection of serum.
Overhydration was induced by injecting isotonic saline corresponding to 15% of body weight over a period of 60 min using an infusion pump. The infused saline was preheated to body temperature. Pif was measured in the contralateral hind paw (experimental paw) 85 min after the start of the infusion period, and followed by i.v. injection of 125I-HSA (∼200 000 cpm, in 0.1 ml saline). The 125I-HSA circulated for 5 min before a final blood sample was collected, and cardiac arrest induced with saturated KCl. A tissue sample was excised from the experimental hind paw. The tissue samples were weighed, counted and dried, and volumes were determined as described above.
Sampling of interstitial fluid by centrifugation
The composition of the interstitial fluid in lymphoedema is of major importance for understanding the pathophysiology of this condition. In order to sample such fluid we used a centrifugation method recently described by Wiig et al. (2003). In brief, skin samples from the fore paw, hind paw, thigh and back were excised and placed on a circular piece of nylon mesh (15–20 μm pore size) with the subcutis facing down. The mesh was mounted in an Eppendorf tube and centrifuged 10 min at 424 g; a g-force shown to give representative samples of interstitial fluid in rat skin (Wiig et al. 2003). After centrifugation, 1–7 μl of interstitial fluid could be sampled from the bottom of the tube. To avoid evaporation from the skin and the collected interstitial fluid, all procedures and sample handling were performed in humidity chamber, keeping the humidity at 100% at all times. The sampled interstitial fluid was either used directly for measurement of COPif or prepared for either HPLC or multiplex cytokine analysis according to respective protocols (see below).
Colloid osmotic pressure (COP)
COP was measured on a colloid osmometer designed for submicrolitre samples described in detail by Wiig et al. (1988). Interstitial fluid samples (1–7 μl) and serum samples (5 μl) were used for COP measurements directly after centrifugation and applied with a pipette onto the osmometer membrane (cut-off 30 kDa).
HPLC analysis
To investigate whether the lymphoedematous condition affected the size selectivity of the capillary membrane and thus the relative protein distribution and pattern in interstitial fluid and serum, such fluid was subjected to HPLC analysis. The macromolecules were separated by a TSK-Gel Super SW3000 size-exclusion HPLC column (TOSOH bioscience, Germany) and quantified by UV detection at 280 nm (Spectraseries UV10; San Jose CA, USA). The UV signal was digitized, sampled at 2 Hz and analysed on a computer using ChromQuest (version 2.51, Thermoquest, USA). Interstitial fluid and serum samples were diluted 1 : 50 with HPLC buffer (m): 0.1 Na2SO4, 0.72 NaH2PO4, 0.28 K2HPO4, pH 6.7) and injected on the HPLC system (20 μl loop, run time 20 min, perfusion rate 0.35 μl min−1), using a Gilson 234 autosampler (Gilson, Mildred, USA).
Multiplex cytokine assay analysis
Since inflammation has been ascribed a pathogenic role in lymphoedema we determined a panel of cytokines that are involved in inflammation in interstitial fluid and serum. A multiplex fluorescent bead immunoassay kit (Linco Research Inc, MO, USA) was used for the simultaneous quantification of the following cytokines: tumour necrosis factor (TNF)-α, IL-1β, IL-2, IL-4, IL-6, IL-10 and interferon (IFN)-γ. Interstitial fluid samples were collected as described in the previous section, except that prior to centrifugation, protease inhibitor cocktail (Sigma-Aldrich Norway AS) at a final concentration of 5% was added to the Eppendorf tube to avoid the immediate proteolysis of cytokines. For serum samples, the protease mixture was added to the blood sample prior to centrifugation. Samples were frozen at −20°C prior to analysis.
The samples were prepared for analysis by dilution with serum matrix diluent as specified by the manufacturer. Serum and interstitial fluid samples were reacted with a mixture of fluorescent beads bound with specific anticytokine primary antibodies, resulting in binding of the cytokines in the sample to the beads with the corresponding antibody. Then biotin anticytokine secondary antibodies were added and allowed to bind to the cytokine–bead complex, followed by addition of fluorescent phycoerythrin-conjugated streptavidin. Total surface fluorescence was then measured with a BioPlex flow-based fluorescence detection system (BioPlex multiplex suspension system (CH), Biorad).
Immunohistochemistry (IHC)
After killing anaesthetized Chy and wt mice with an intracardiac injection of saturated KCl, tissue samples were excised from the thigh muscle, back, thigh, hind paw and fore paw skin. The tissue samples were fixed in 4% paraformaldehyde and dehydrated in sucrose. The dehydrated tissue samples were mounted in Tissue-Tek (O.C.T. Compound 4583, Tamro MedLab), snap-frozen in liquid nitrogen and stored at −80°C prior to sectioning. In order to study whether any lymph vessels were located deeper in the dermis and subcutis than could be visualized when skin was removed for sectioning, we made tissue sections of intact paw in wt and Chy mice. After removal, paws were decalcified at 4°C as described by Jonsson et al. (1986), using an EDTA buffer (Tris 12.11 g, KOH 10 g, EDTA 100 g polyvinylpyrrolidone 75 g, and adding distilled H2O until the volume was 1 l). PH was adjusted to 6.95 using HCl. The buffer was replaced every third day until decalcified at ∼14 days. The decalcified paws were then snap-frozen in CO2 and mounted in Tissue Tek.
Skin sections (5 μm) and paw sections (10 μm) were cut in a freezing slide microtome and mounted onto poly-l-lysine-coated glass slides. To visualize lymphatic endothelium, the sections were stained with an antibody against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) with the avidin-biotin complex (ABC) method using a commercially available kit (Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc.). Briefly, the sections were blocked for endogenous peroxidase activity by incubating for 30 min in 0.3% H2O2 in methanol. Triton X-100 in phosphate-buffered saline (PBS-TX) was used throughout the IHC procedure to increase membrane permeability and hence facilitate binding. To prevent unspecific binding, the sections were incubated for 1 h in 1.35% goat serum (Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc.) in PBS-TX with 1% BSA. The sections were then incubated for 3 days at 4°C with mouse LYVE-1 (1 : 1000 dilution) polyclonal antibody raised in rabbit. After PBS rinses, the sections were incubated with secondary antibody (1 : 50 dilution, Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc.) for 1 h. Following PBS rinses, the sections were reacted with the avidin-biotin complex for 1 h. After repeated PBS washings, sections were incubated with 3,3′-diaminobenzidine (DAB) for 10 min to visualize bound antigen. Nickel was added to the DAB solution to facilitate binding. The sections were counterstained with Richardson's staining.
Statistics
Results were analysed statistically with unpaired t tests comparing the results from the two groups (Chy and wt) within the same skin area. Paired t tests were used when comparing results within the same group of animals before and after an experimental challenge. A value of P < 0.05 was considered statistically significant. Values are given as means ± s.d.
Results
Animals
Most Chy mice seemed healthy and developed normally except for visible swelling of the limbs. As described above, ∼10% Chy mice developed gross swelling of the abdominal region and were killed immediately. There were no apparent size or weight differences between the Chy mice and their wt littermates.
Tissue fluid volume
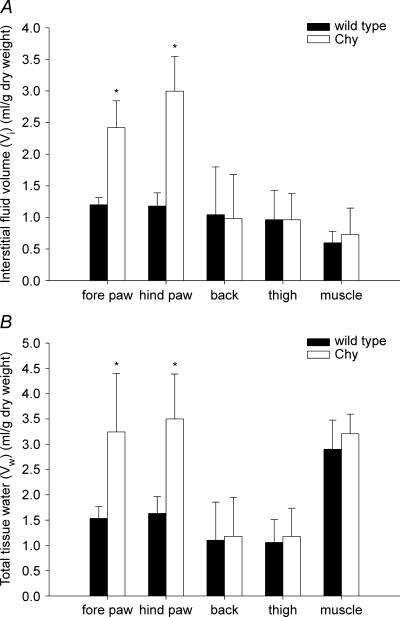
We measured fluid volumes in skin areas that had visible oedema as well as in organs that appeared normal. Vi and total Vw were measured in the fore paw, hind paw, thigh and back skin together with thigh muscle (Fig. 1). Dramatic differences in Vi and Vw were found when comparing areas in Chy with visible swelling, i.e. fore and hind paw, with corresponding areas in wt mice. Vi in hind paw averaged 3.0 ± 0.6 ml (g dry weight)−1 (n = 13) in Chy mice and 1.2 ± 0.2 ml (g dry weight)−1 in wt mice (n = 13) (P < 0.001), while the corresponding Vi values in fore paw were 2.4 ± 0.4 (n = 5) and 1.2 ± 0.1 ml (g dry weight)−1 (n = 5) (P < 0.001) as shown in Fig. 1A. The difference in Vw between Chy and wt mice followed the same pattern as for Vi, with a 114% (P < 0.001) and 112% (P < 0.05) increase in Chy hind and fore paw Vw when compared with wt (Fig. 1B). Vi and Vw for the other areas measured were similar in Chy and wt mice.
Figure 1. Fluid volumes in wt and Chy mice.
Interstitial fluid volume (A) and total tissue water (B) in fore paw, hind paw, thigh and back skin together with thigh muscle in Chy and wild type (wt) mice. The values are mean ± s.d. *P < 0.05, significant difference (unpaired t test) between Chy and wt mice within the same area of measurement.
Interstitial fluid pressure (Pif)
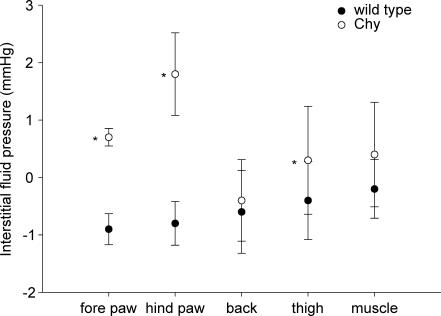
Pif was measured in vivo in the areas corresponding to the fluid volume determinations. In the hind and fore paw of Chy mice, Pif averaged 1.8 ± 0.7 (n = 17) and 0.7 ± 0.2 mmHg (n = 8), respectively (Fig. 2). Pif was significantly higher in hind paw than fore paw skin of Chy mice (P < 0.05). These pressures were also significantly elevated relative to the slightly subathmospheric Pif measured in wt hind and fore paw skin, of −0.8 ± 0.4 mmHg (n = 13) (P < 0.001) and −0.9 ± 0.3 mmHg (n = 5) (P < 0.001), respectively. An elevated Pif was also found in thigh skin of Chy as compared to wt mice (0.3 ± 0.9 mmHg, n = 17, and −0.4 ± 0.7 mmHg, n = 13; P < 0.05), while no significant difference between the two strains was observed either in back skin or thigh muscle.
Figure 2. Interstitial fluid pressure in wt and Chy mice.
Interstitial fluid pressure in fore paw, hind paw, thigh and back skin together with thigh muscle in Chy and wt mice. The values are mean ± s.d.*P < 0.05, significant difference (unpaired t test) between Chy and wt mice within the same area of measurement.
Pif and Vi response to fluid volume overload in hind paw skin
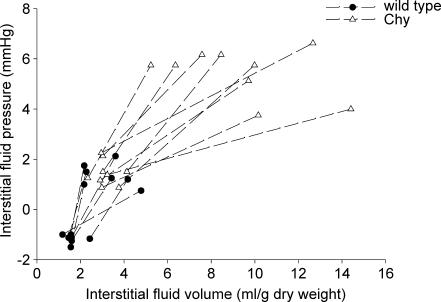
In euvolemia, Pif and Vi in wt mice in the volume expansion group averaged −1.2 ± 0.2 mmHg and 1.6 ± 0.4 ml (g dry weight)−1 (n = 7), respectively (Fig. 3). The infusion of a saline volume load corresponding to 15% of body weight in the same mice led to an increase in Pif to 1.4 ± 0.5 mmHg and Vi to 3.2 ± 1.1 ml (g dry weight)−1 (P < 0.05, paired t test). In Chy mice the control situation was quite different from that of wt mice, with pressures and volumes corresponding to the hypervolaemic situation of wt mice; Pif averaging 1.4 ± 0.5 mmHg and Vi averaging 3.2 ± 0.5 ml (mg dry weight)−1 (n = 9). Infusion of the saline volume load in Chy mice lead to a further dramatic increase in Vi to 9.4 ± 2.9 ml (mg dry weight)−1 (P < 0.001, paired t test), while Pif, which could be considered very high compared with wt already at the starting point, increased substantially to 5.5 ± 1.0 mmHg (P < 0.001, paired t test).
Figure 3. Response in interstitial fluid pressure to volume loading.
Corresponding values for interstitial fluid pressure and volume in hind paw in wt (•) and Chy (▵) mice before and after infusion of saline corresponding to 15% body weight. Values in the same animal have been connected.
There was no significant difference in systemic arterial blood pressure at the time of Pif measurement before and after volume infusion in either wt (79 ± 13 mmHg and 64 ± 18 mmHg, respectively) or Chy mice (74 ± 9 mmHg and 71 ± 13 mmHg, respectively) as well as between wt and Chy mice.
HPLC analysis
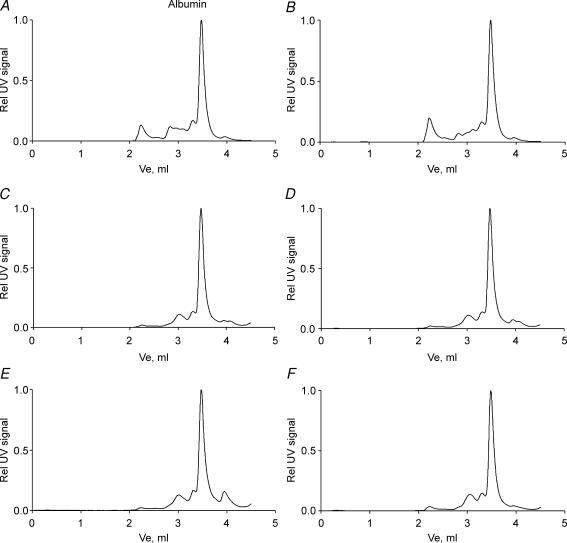
HPLC analysis of serum and interstitial fluid from fore paw, hind paw, back and thigh skin revealed no difference in the distribution pattern of macromolecules between Chy and wt mice. Representative chromatograms from Chy and wt serum, thigh and hind paw skin are presented in Fig. 4A–F. We noted a larger fraction of globulins (eluting before albumin in the chromatogram) in serum than in interstitial fluid in wt and Chy mice suggesting no difference in capillary size selectivity between the strains, and again indicating that the lymphoedematous condition did not affect this parameter.
Figure 4. Size distribution of macromolecules in serum and interstitial fluid.
HPLC chromatograms of the macromolecular distribution pattern in serum and interstitial fluid (IF) from Chy and wt mice. The left panel shows representative chromatograms from wt serum (A), thigh skin IF (C) and hind paw skin IF (E), while the right panel shows Chy serum (B), thigh skin IF (D) and hind paw skin IF (F). The areas under the peaks are normalized as relative to the albumin peak for each chromatogram.
COP of serum and interstitial fluid
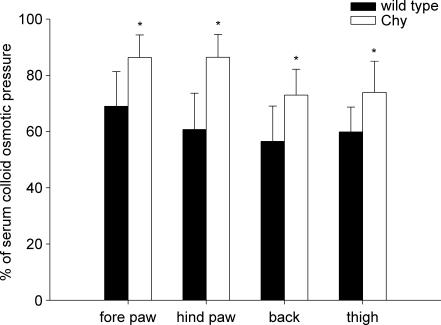
COP in serum averaged 17.3 ± 1.9 mmHg (n = 9) in Chy mice and 19.9 ± 4.6 mmHg (n = 7) in wt mice. In interstitial fluid from fore paw, hind paw, thigh and back skin of Chy mice, COP averaged 15.2 ± 1.2, 15.0 ± 2.4, 12.7 ± 2.7 and 12.7 ± 2.0 mmHg, respectively. In the corresponding areas in wt, COP was measured as 14.6 ± 3.0, 11.7 ± 1.3, 11.2 ± 1.8 and 11.8 ± 2.2 mmHg. Because of the difference in control COP of serum in the two strains, all COPif values were calculated as relative to the corresponding serum COP for each mouse, in order to be able to compare the pressures measured in interstitial fluid. COPif was found to be significantly (P < 0.05) elevated in Chy mice compared to wt in all areas studied: fore paw, hind paw, back and thigh skin (Fig. 5). The increase was most pronounced in the hind paw with a 26% increase compared to wt. In fore paw, back and thigh skin COPif increased by 17%, 16% and 14%, respectively.
Figure 5. Colloid osmotic pressure in interstitial fluid relative to serum.
Colloid osmotic pressure in interstitial fluid isolated from fore paw, hind paw, thigh and back skin in Chy and wt mice. The pressures were calculated as relative to the corresponding serum COP for each mouse. The values are then given as mean ± s.d. *P < 0.05, significant difference (unpaired t test) between Chy and wt mice within the same area of measurement.
Cytokines in interstitial fluid and serum
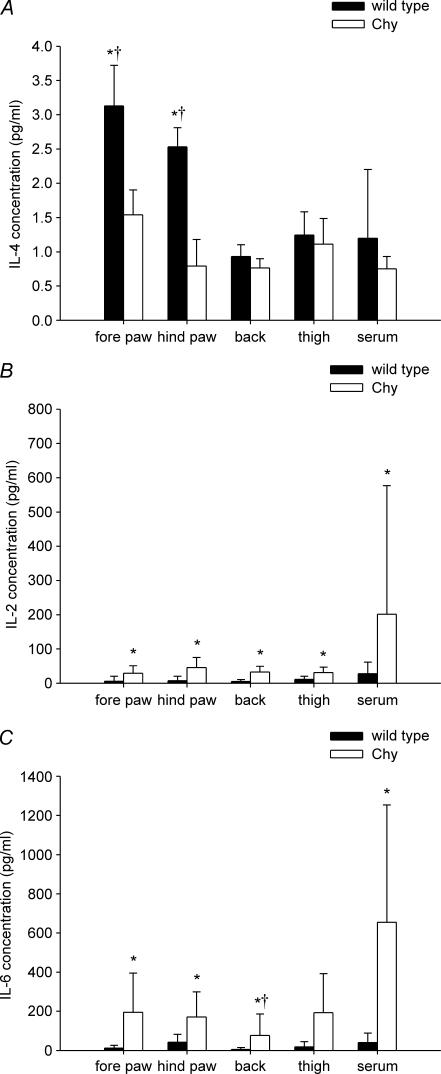
No significant difference in the levels of the inflammatory cytokines TNF-α, IL-1β, IL-2, IL-6, IL-10 and IFN-γ were observed between young adult (3–4 months) Chy and wt mice, either in serum or in interstitial fluid (data not shown). The anti-inflammatory cytokine IL-4, however, was found in low concentrations in general (<4 pg ml−1), but was significantly decreased (P < 0.05) in both hind paw and fore paw interstitial fluid of young adult Chy mice compared with wt mice (Fig. 6A). In these Chy mice, IL-4 averaged 1.5 ± 0.4 pg ml−1 and 0.8 ± 0.4 pg ml−1 in the fore paw and hind paw, respectively, while corresponding IL-4 values in wt mice averaged 3.1 ± 0.6 pg ml−1 and 2.5 ± 0.3 pg ml−1. Furthermore, the interstitial fluid IL-4 concentration in fore and hind paw in wt mice differed significantly (P < 0.05) from that of the corresponding serum, suggesting local production of this cytokine, whereas this was not the case for Chy mice. There was no difference between Chy and wt mice in the expression of IL-4 in thigh and back skin or in serum.
Figure 6. Interleukins in serum and interstitial fluid.
Interleukins in serum and interstitial fluid from fore paw, hind paw, thigh and back skin of young (3–4 months) and old (11–13 months) adult Chy and wt mice. A, interleukin-4 levels in young adult mice; B, interleukin-2 levels in old adult mice; C, interleukin-6 levels in old adult mice. Values are presented as mean ± s.d. *P < 0.05, significant difference (unpaired t test) between Chy and wt mice within the same area of measurement. †P < 0.05 when compared with corresponding serum.
Our finding of a reduced level of the immunomodulatory cytokine IL-4 (Paul, 1991) in young adult mice led us to ask whether this reduction influenced the level of mediators at a more developed stage of lymphoedema. In old adult (11–13 months) Chy mice, levels of pro-inflammatory IL-2 were significantly (P < 0.05) increased in interstitial fluid from the fore paw, hind paw, thigh and back skin as well as serum when compared with wt (Fig. 6B). None of these levels differed from the corresponding level in serum. The IL-6 levels of Chy mice were also significantly (P < 0.05) increased in serum and all skin areas except thigh skin (Fig. 6C) when compared with wt. Except for back skin, none of the IL-6 levels differed significantly from that in serum in the corresponding strain of mice. There was no significant difference between old adult Chy and wt mice in the interstitial fluid and serum levels or between the same strain tissue and serum level for TNF-α, IL-1β, IL-4, IL-10 and IFN-γ (data not shown).
Immunohistochemical analysis
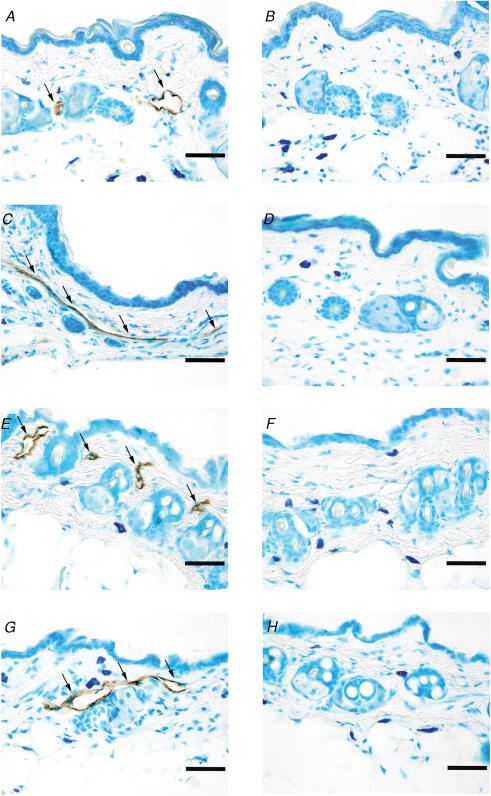
Results from the immunohistochemical analysis of removed skin are displayed in Fig. 7. In wt mice, lymphatics stained with LYVE-1 could be detected in all the skin areas studied: fore paw, hind paw, back and thigh (Fig. 7A, C, E and G). In contrast, no LYVE-1-stained lymphatics could be found in sections from the same areas in Chy mice (Fig. 7B, D, F and H).
Figure 7. Initial (LYVE-1)-positive lymphatics in sections from skin.
Skin sections (∼5 μm) from Chy and wt mice stained with the lymphatic marker lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1). The arrows show LYVE-1 staining in the endothelium of lymph vessels. The left panel displays skin sections from wt hind paw (A), fore paw (C), back (E) and thigh (G) while the right panel displays skin sections from Chy hind paw (B), fore paw (D), back (F) and thigh (H). Bar = 50 μm.
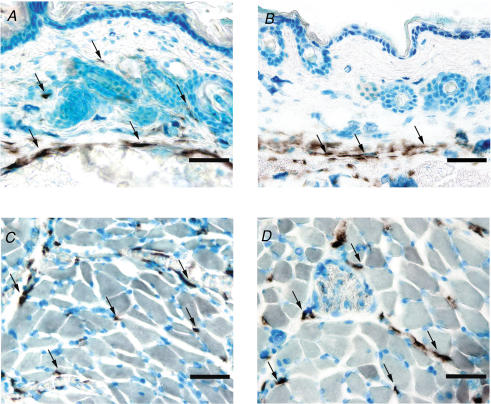
Whereas sections of removed skin showed no lymphatics in the dermis, sections made from intact paw demonstrated that Chy mice had lymph vessels more deeply located in skin, i.e. in the subcutis (Fig. 8B) similar to wt mice (Fig. 8A). The LYVE-1 staining of lymphatics in hindlimb skeletal muscle appeared similar in wt and Chy mice (Fig. 8C and D), showing that the Chy phenotype is not pronounced in this organ.
Figure 8. Initial (LYVE-1)-positive lymphatics in sections from intact paw.
Sections (∼10 μm) from wt (left panel) and Chy (right panel) mice made from intact paw after decalcification, and stained with the lymphatic marker lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1). The arrows show LYVE-1 staining in the endothelium of lymph vessels. Whereas full-thickness skin in wt mice (A) had lymphatics in the dermis as well as the subcutis, initial lymphatics were located in subcutis only in Chy mice (B). In hindlimb muscle, no difference in staining could be demonstrated between wt (C) and Chy mice (D). Bar = 50 μm.
Discussion
The main focus of our study was to use one of the newly developed mouse models of primary lymphoedema (Saaristo et al. 2002; Oliver & Alitalo, 2005) in an attempt to determine the functional consequences of missing lymphatics in relation to transcapillary fluid filtration, and thus try to answer some unresolved questions related to the pathophysiology of this disease. In our heterozygous model, the Chy mouse, we were not able to identify any initial lymphatics in dermis in any of the various areas of the body studied: the fore paw, hind paw, thigh and back, in contrast to the abundant vessels found in wt mice. Lymphatics were, however, localized deeper in the subcutis in Chy as well as wt mice, and lymph drainage from these vessels may explain why the phenotype is not as severe as the data from dermis may indicate. In spite of the general lack of initial lymphatics in the dermis, oedema and thus tissue fluid volume and Pif were found to be significantly increased in the areas with visible swelling only, whereas a general increase in COPif was observed in all skin areas studied. The lack of lymphatics had dramatic consequences for transcapillary fluid balance when challenged with a volume load, resulting in a much exaggerated increase in Pif and Vi in Chy mice as compared to wt. Based on our finding of similar levels of proinflammatory cytokines in young wt and Chy mice in interstitial fluid as well as serum, we suggest that accumulation of protein-rich lymph is not sufficient to induce an inflammatory reaction and thus contribute to further fluid accumulation in the initial stages of primary lymphoedema, whereas data from older mice show that this may be the case at later stages.
Choice of experimental model and methodological issues
Although secondary lymphoedema due to parasitic infection or trauma induced by surgery and/or radiation therapy are quantitatively more important, we chose one of the recently described mouse models of primary lymphoedema to be able to explore the functional consequences due to lack of initial lymphatics. A common feature for most of these models is that the homozygous mutation is embryonally lethal (Baluk et al. 2005), and that the heterozygous mutation has a barely detectable or inconspicuous peripheral lymphoedema. As an example, Foxc2 haploinsufficient mice, a model for lymphoedema distichiasis (Kriederman et al. 2003), rarely exhibit hind limb swelling even with clearly dysfunctional lymphatics (Petrova et al. 2004). As one of the few lymphoedema models with a clear peripheral phenotype and with a mutation in the gene encoding for VEGFR-3, similar to that of humans with Milroy's disease, the Chy mouse model was suitable to study the role of initial lymphatics in hereditary lymphoedema generation (and prevention) by the use of techniques and experimental procedures that cannot be used in humans. Furthermore, there was a need for further characterization of this model with respect to transcapillary fluid balance parameters.
In the present study we used methods that have been validated in other studies. Access to native interstitial fluid was crucial here. Since the major changes in transcapillary fluid parameters were found in body areas where use of other methods like wicks would have been difficult (Markhus & Wiig, 2004), we used a newly developed centrifugation method (Wiig et al. 2003) to isolate skin interstitial fluid. In our method evaluation study we found that less than 2% of the fluid isolated by centrifugation of skin at g < 424 derived from plasma, showing that the contamination from plasma is negligible. Accordingly the high COPif found in our study is not a consequence of a high content of plasma in the isolated fluid. Aside from our study on lymphoedema mice, this method has also been applied in studies on tumours (Wiig et al. 2003), skin inflammation (Nedrebo et al. 2004) and bone marrow (Iversen & Wiig, 2005), and has shown to be able to reflect pathological responses reliably.
Transcapillary fluid balance parameters
As all oedemas result from an imbalance between lymph drainage and fluid filtration, the pathogenesis of lymphoedema is not necessarily explained by the deficient lymphatics alone, but is also mutually dependent on the factors governing capillary filtration. The significance of Pif is its important role in tissue fluid homeostasis both by regulating fluid filtration across the capillary and by serving as the filling pressure for the lymphatics (Aukland & Reed, 1993). Here we found Pif to be increased with 1.6 mmHg in the fore paw and 2.6 mmHg in the hind paw in Chy mice in relation to wt in control conditions, corresponding to an increase in fluid volume of approximately 100% and 150% in these areas. Previously an increase in Pif of the same magnitude has been observed in rats with oedemas of other origins (Wiig & Reed, 1981). Our findings in lymphoedema mice are also comparable with the findings of Bates et al. (1992, 1994) where Pif was found to increase by 4.5 cmH2O (3.4 mmHg) in the human lymphoedematous arm as measured by the wick-in-needle technique. Christenson et al. (1985) reported a much higher Pif of 17.6 mmHg measured with the split catheter technique in the leg of lymphoedema patients. Measurements with a micropuncture technique revealed pressures in the same range (Zaugg-Vesti et al. 1993) as the latter in leg lymphatic capillaries in human primary lymphoedema (onset after puberty); capillary lymphatic pressure was 15.0 mmHg compared to 7.9 mmHg in healthy control subjects. Although some of the variation in the recorded Pif may result from methodological differences related to the technique used for Pif measurement, the main explanation for the more modest increase in Pif observed in our study is probably the more pronounced effect of gravity as a force enhancing the oedema formation, especially in the limbs of humans. Primary lymphoedema patients usually present a gross oedema in the limbs while in our Chy mice such severe oedemas were never observed. Nevertheless, the increased Pif observed in our study would have the potential to both reduce the net filtration pressure across the capillaries and lead to a more efficient drainage of fluid through more rapid filling of the initial lymphatics (Schmid-Schonbein, 1990; Aukland & Reed, 1993). The importance of the latter effect is difficult to ascertain in primary lymphoedema, considering the lack of lymphatics in patients (Bollinger et al. 1983; Pfister et al. 1990) as well as in our mouse model. Actually, considering our and previous (Karkkainen et al. 2001) findings of a dermis practically devoid of initial lymphatics not only in the areas with visible swelling but also in thigh and back skin, suggest that a new steady state is reached using other oedema-preventive mechanisms than increased lymph flow. In this context it is interesting to note that the Chy phenotype is especially pronounced in skin and that lymphatics in skeletal muscle appear normal, in agreement with previous data for the Chy mouse (Karkkainen et al. 2001). That the phenotype differs strongly depending on the organ has also been shown for other mouse models with lymphatic pathology (Alitalo et al. 2005).
Another determinant of capillary filtration, COPif, was significantly increased in Chy mice in all the skin areas studied, although it was most pronounced in the hind paw (26%). This finding is in agreement with the increased protein content of oedema fluid observed in humans with primary lymphoedema by Taylor et al. (1958) and in experimentally induced obstructive lymphoedema in dogs (Knight et al. 1994). In contrast, Olszewski (2003) and Bates et al. (1993) found unaltered and decreased COPif in human lymphoedematous arm. The observations of the latter two studies were explained by the possibility of a vascular contribution, i.e. increased capillary filtration, adding to the oedema and hence diluting the interstitial proteins and reducing COPif. Vascular abnormalities could be due to the external obstructive origin of the oedema in these studies which differs from the situation in primary lymphoedema. Our data support the traditional view of an increased COPif as a result of the oedema's lymphatic origin (Crockett, 1956; Taylor et al. 1958). A reduction in capillary filtration in response to reduced lymph transport and the concomitant increase in Pif would allow the proteins to accumulate in the tissue due to lack of protein transport away from the interstitium. There is also a possibility that excessive amounts of proteins could be filtered across the capillaries due to a decrease in the reflection coefficient for proteins in response to an ongoing inflammatory process in the tissue (see below), but the HPLC data suggesting intact size-selectivity in Chy mice do not support such a mechanism.
A novel observation in our study was the finding of a pronounced effect on Vi and Pif when exposed to a systemic volume load. Whereas infusion of saline amounting to 15% of body weight increased Vi by 1.6 ml (g dry weight)−1 in wt mice, the corresponding increase in Chy mice was close to four times higher, i.e. 6.2 ml (g dry weight)−1. The resulting increases in Pif were 2.4 mmHg and 3.8 mmHg in wt and Chy mice, respectively. These data suggest an altered interstitial compliance of the lymphoedematous skin and enable us to estimate the role of the lymphatics in disposing of an acutely filtered fluid load.
Interstitial compliance (C) defines the response in Pif to a given change in Vi, i.e. C = ΔVi/Pif. In normally hydrated skin the Pif–Vi relationship curve is steep, hence small fluid volume changes cause marked rises in Pif (low compliance). With increased volumes (overhydration/oedema) the curve typically levels off and no further volume increments affect Pif (high or infinite compliance) as described by Wiig & Reed (1981) in a compliance study on rat hind limb skin covering a wide range of tissue hydrations. Their curve reached a Pif plateau at +1 mmHg, an increase of 2 mmHg, at ∼50% increase in Vi, and did not exceed this level even if Vi increased 600%. Even if the present measurements were in the hind paw, which may be expected to have a slightly lower compliance due to tissue anatomy, Pif in Chy mice rose by up to +6 mmHg upon fluid expansion in most experiments, thus deviating strongly from the rat data discussed above. A likely explanation for this dramatic rise in pressure is that the interstitial compliance is altered, again a result of structural changes in Chy mouse hind paw skin. Our data need to be supported by a more extensive compliance study also covering other skin areas as well as a wider range of hydration, but this current interesting finding gives reason to pursue studies on composition and structure of the Chy mice skin.
A ‘local’ difference in interstitial compliance may explain the somewhat puzzling observation of fluid accumulation in the subcutis of Chy-mice, i.e. in a layer containing lymphatics, and not in the dermis, that are practically devoid of lymphatic vessels. Under normal conditions there is a small net filtration from the capillaries (Aukland & Reed, 1993). It is likely that for the same amount of fluid filtered, the increase in Pif will be higher in dermis with its high content of collagen than in the loose connective tissue in subcutis. Thus, a Pif gradient from dermis to subcutis is created, driving fluid in the same direction. The fact that fluid accumulates in the subcutis shows that although there are lymphatics in that layer, their number and/or function is insufficient to prevent the oedema generation.
Our finding of a fourfold fluid accumulation in a similar post-infusion period in Chy mice relative to wt, in response to an identical volume load is dramatic and most interesting. This occurred in spite of a significantly higher counterpressure (ΔPif) generated in Chy mice. In an attempt to understand the mechanism behind this exaggerated accumulation, all the factors of the Starling equation and their possible role as oedema-preventing factors have to be considered. One of these factors is the hydraulic conductivity of the capillaries in the region studied. Although possible, no such increase was found using plethysmography in lymphoedema patients (Gretener et al. 2000), suggesting that this factor was not of major importance. We have no data on vascular resistance and capillary pressure, but the similar systemic arterial blood pressure in the two strains in the control situation as well as during overhydration may indicate that capillary pressure was not central in this context. The high Pif in Chy mice will counteract filtration more in Chy than in wt, and not contribute to the observed finding. Furthermore, even though the role of tissue COPif has recently been questioned as a factor determining capillary filtration (Adamson et al. 2004), it is likely that a reduction in the high tissue COPif in Chy will act as an oedema-preventing factor and counteract filtration in this situation. The remaining factor is lymph flow per se. Previous studies in secondary lymphoedema patients have shown a modest reduction in lymph flow as monitored using isotope washout techniques (e.g. Stanton et al. 2001) that is not sufficient to explain the observed Chy swelling rate. The fairly moderate swelling in Chy compared with wt suggests that compensatory mechanisms discussed above are sufficient to compensate for the relative lack of lymphatics during conditions of low net filtration, but are easily overwhelmed in situations with high filtration. These experiments give an indication of the quantitative importance of lymphatic system fluid removal capacity, but also call for studies where lymph flow is quantified in mice with lymphatic system derangements such as those in the Chy strain.
Role of inflammation in the pathogenesis of lymphoedema
A widely held view is that a high interstitial protein concentration induces an inflammatory reaction, again resulting in fibrosis and tissue degeneration (e.g. Rockson, 2001; Alitalo et al. 2005). Having demonstrated that Chy mice have a high-protein oedema, we would expect an inflammatory reaction that would induce a response in inflammatory cytokines if this was the case, but no such response was observed in young adult mice. On the other hand, Olszewski (2003) found proinflammatory cytokines to be elevated in lymph from humans. This observation, however, may be a result of the postsurgical and postinflammatory condition that preceded the lymphoedema prevailing in these patients and/or that the patients were studied at a more developed stage of the condition. To our knowledge there are currently no other data on cytokines in primary lymphoedema interstitial fluid. An implication of our observations is that a high interstitial protein concentration per se is not sufficient to induce inflammation in lymphoedematous skin.
Interestingly, we found a significantly reduced level of IL-4 in Chy mice. IL-4 is an immunoregulatory cytokine (Paul, 1991). Of particular importance for our study is that this cytokine has anti-inflammatory properties (Li-Weber & Krammer, 2003). A reduced level of IL-4 may thus indicate that the immunological defence capacity of the skin in Chy is suppressed relative to wt mice. This phenomenon may lead to a reduced defence against invaders and contribute to the inflammation observed in Chy mice of more advanced age.
To summarize, the Chy mouse model has enabled us to study pathophysiological consequences of missing lymphatics per se. We have verified that primary lymphoedema is a high-protein oedema, and that a significant increase in Pif will be generated to counteract further filtration. A fourfold increase in fluid accumulation during acute overhydration in spite of the generation of a significant interstitial fluid hydrostatic counterpressure shows the quantitative importance of lymphatics for fluid homeostasis. Although our data do not support the assumption of a major role of inflammation in the initial pathogenesis of lymphoedema, a reduced level of the anti-inflammatory cytokine IL-4 may suggest an increased propensity for infections in lymphoedematous tissue and contribute to the increase in interstitial fluid cytokines observed at a later stage. Apart from the importance for our understanding of the pathophysiology of lymphoedema, our study may also have relevance for gene therapy effect evaluation, a type of therapy that has been proposed for this debilitating condition (Karkkainen et al. 2001; Saaristo et al. 2002; Oliver & Alitalo, 2005).
Acknowledgments
Financial support was received from Locus on Circulatory Research at University of Bergen, The Norwegian Council on Cardiovascular Diseases, The Research Council of Norway and EU 6th Framework Program Integrated Project ‘Angiotargeting’. We thank Bengt Age S. Borge for assistance with the cytokine assays, Ase R. Eriksen for help with immunohistochemical analysis and Gry Bernes for assistance in animal handling. The European Union (Lymphangiogenomics LSHG-CT-2004-503573), NIH (5 R01 HL075183-02) and Western Norway Regional Health Authority.
References
- Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Levick JR, Mortimer PS. Subcutaneous interstitial fluid pressure and arm volume in lymphoedema. Int J Microcirc Clin Exp. 1992;11:359–373. [PubMed] [Google Scholar]
- Bates DO, Levick JR, Mortimer PS. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin Sci. 1993;85:737–746. doi: 10.1042/cs0850737. [DOI] [PubMed] [Google Scholar]
- Bates DO, Levick JR, Mortimer PS. Starling pressures in the human arm and their alteration in postmastectomy oedema. J Physiol. 1994;477:355–363. doi: 10.1113/jphysiol.1994.sp020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger A, Isenring G, Franzeck UK, Brunner U. Aplasia of superficial lymphatic capillaries in hereditary and connatal lymphedema (Milroy's disease) Lymphology. 1983;16:27–30. [PubMed] [Google Scholar]
- Chen HC, Pribaz JJ, O'Brien BM, Knight KR, Morrison WA. Creation of distal canine limb lymphedema. Plast Reconstr Surg. 1989;83:1022–1026. doi: 10.1097/00006534-198906000-00016. [DOI] [PubMed] [Google Scholar]
- Christenson JT, Shawa NJ, Hamad MM, Al-Hassan HK. The relationship between subcutaneous tissue pressures and intramuscular pressures in normal and edematous legs. Microcirc Endothelium Lymphatics. 1985;2:367–384. [PubMed] [Google Scholar]
- Crockett DJ. The protein levels of oedema fluids. Lancet. 1956;271:1179–1182. doi: 10.1016/s0140-6736(56)90053-8. [DOI] [PubMed] [Google Scholar]
- Gretener SB, Lauchli S, Leu AJ, Koppensteiner R, Franzeck UK. Effect of venous and lymphatic congestion on lymph capillary pressure of the skin in healthy volunteers and patients with lymphedema. J Vasc Res. 2000;37:61–67. doi: 10.1159/000025714. [DOI] [PubMed] [Google Scholar]
- Iversen PO, Wiig H. Tumor necrosis factor alpha and adiponectin in bone marrow interstitial fluid from patients with acute myeloid leukemia inhibit normal hematopoiesis. Clin Cancer Res. 2005;11:6793–6799. doi: 10.1158/1078-0432.CCR-05-1033. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Tarkowski A, Klareskog L. A demineralization procedure for immunohistopathological use. Edta treatment preserves lymphoid cell surface antigens. J Immunol Meth. 1986;88:109–114. doi: 10.1016/0022-1759(86)90058-x. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. Missense mutations interfere with vegfr-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001b;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KR, Collopy PA, McCann JJ, Vanderkolk CA, Coe SA, Barton RM, Chen HC, O'Brien BM. Protein metabolism and fibrosis in experimental canine obstructive lymphedema. J Laboratory Clin Med. 1987;110:558–566. [PubMed] [Google Scholar]
- Knight KR, Ritz M, Lepore DA, Booth R, Octigan K, O'Brien BM. Autologous lymphocyte therapy for experimental canine lymphoedema: a pilot study. Aust N Z J Surg. 1994;64:332–337. doi: 10.1111/j.1445-2197.1994.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. Foxc2 haploinsufficient mice are a model for human autosomal dominant lymphedema–distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- Li-Weber M, Krammer PH. Regulation of IL4 gene expression by t cells and therapeutic perspectives. Nat Rev Immunol. 2003;3:534–543. doi: 10.1038/nri1128. [DOI] [PubMed] [Google Scholar]
- Markhus CE, Wiig H. Isolation of interstitial fluid from skeletal muscle and subcutis in mice using a wick method. Am J Physiol Heart Circ Physiol. 2004;287:H2085–H2090. doi: 10.1152/ajpheart.00379.2004. [DOI] [PubMed] [Google Scholar]
- Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998;83:2798–2802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nedrebo T, Reed RK, Jonsson R, Berg A, Wiig H. Differential cytokine response in interstitial fluid in skin and serum during experimental inflammation in rats. J Physiol. 2004;556:193–202. doi: 10.1113/jphysiol.2003.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- Olszewski WL. Pathophysiological aspects of lymphedema of human limbs. I. Lymph protein composition. Lymphat Res Biol. 2003;1:235–243. doi: 10.1089/153968503768330265. [DOI] [PubMed] [Google Scholar]
- Olszewski W, Machowski Z, Sokolowski J, Nielubowicz J. Experimental lymphedema in dogs. J Cardiovasc Surg (Torino) 1968;9:178–183. [PubMed] [Google Scholar]
- Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Pfister G, Saesseli B, Hoffmann U, Geiger M, Bollinger A. Diameters of lymphatic capillaries in patients with different forms of primary lymphedema. Lymphology. 1990;23:140–144. [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Saaristo A, Karkkainen MJ, Alitalo K. Insights into the molecular pathogenesis and targeted treatment of lymphedema. Ann N Y Acad Sci. 2002;979:94–110. doi: 10.1111/j.1749-6632.2002.tb04871.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Stanton AW, Svensson WE, Mellor RH, Peters AM, Levick JR, Mortimer PS. Differences in lymph drainage between swollen and non-swollen regions in arms with breast-cancer-related lymphoedema. Clin Sci. 2001;101:131–140. [PubMed] [Google Scholar]
- Taylor GW, Kinmonth JB, Dangerfield WG. Protein content of oedema fluid in lymphoedema. BMJ. 1958;14:1159–1160. doi: 10.1136/bmj.1.5080.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H. Comparison of methods for measurement of interstitial fluid pressure in cat skin/subcutis and muscle. Am J Physiol. 1985;249:H929–H944. doi: 10.1152/ajpheart.1985.249.5.H929. [DOI] [PubMed] [Google Scholar]
- Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol Heart Circ Physiol. 2003;284:H416–H424. doi: 10.1152/ajpheart.00327.2002. [DOI] [PubMed] [Google Scholar]
- Wiig H, Halleland EG, Fjaertoft M, Aukland K. Measurement of colloid osmotic pressure in submicrolitre samples. Acta Physiol Scand. 1988;132:445–452. doi: 10.1111/j.1748-1716.1988.tb08351.x. [DOI] [PubMed] [Google Scholar]
- Wiig H, Reed RK. Compliance of the interstitial space in rats. Ii. Studies on skin. Acta Physiol Scand. 1981;113:307–315. doi: 10.1111/j.1748-1716.1981.tb06901.x. [DOI] [PubMed] [Google Scholar]
- Wiig H, Reed RK, Aukland K. Micropuncture measurement of interstitial fluid pressure in rat subcutis and skeletal muscle: Comparison to wick-in-needle technique. Microvasc Res. 1981;21:308–319. doi: 10.1016/0026-2862(81)90014-5. [DOI] [PubMed] [Google Scholar]
- Zaugg-Vesti B, Dorffler-Melly J, Spiegel M, Wen S, Franzeck UK, Bollinger A. Lymphatic capillary pressure in patients with primary lymphedema. Microvasc Res. 1993;46:128–134. doi: 10.1006/mvre.1993.1041. [DOI] [PubMed] [Google Scholar]