Abstract
Baroreflex function is impaired in patients with obstructive sleep apnoea. We tested the hypothesis that short-term exposure to repetitive hypoxic apnoeas (RHA) produces prolonged impairment in baroreflex function. Baroreflex function was determined using the modified Oxford technique in 14 subjects (26 ± 1 years). Baroreflex sensitivity (BRS) was quantified from the R-R interval–systolic blood pressure (BP; cardiovagal BRS), heart rate–systolic BP (HR BRS) and muscle sympathetic nerve activity (MSNA)–diastolic BP (sympathetic BRS) relations. RHA involved subjects performing repetitive end-expiratory apnoeas (20 s) every minute for 30 min during intermittent hypoxia to accentuate oxygen desaturation. After RHA, BP and MSNA at rest were elevated. BRS was measured ∼7 (Post 1), ∼30 (Post 2) and ∼50 min (Post 3) after RHA to provide insight into the temporal pattern of responses. Cardiovagal BRS (16.8 ± 1.3, 16.5 ± 1.6, 17.6 ± 2.0 and 17.4 ± 1.5 ms mmHg−1 for Pre, Post 1, Post 2 and Post 3, respectively), HR BRS (−1.1 ± 0.1, −1.1 ± 0.1, −1.3 ± 0.1 and −1.4 ± 0.1 beats min−1 mmHg−1) and sympathetic BRS (−4.5 ± 0.6, −4.4 ± 0.7, −3.7 ± 0.5 and −4.7 ± 1.0 arbitrary units (au) beat−1 mmHg−1) were unchanged by RHA. In contrast, the operating points of the baroreflexes were shifted rightward (to higher levels of BP) and upward (to higher levels of heart rate and MSNA) after RHA (P < 0.05). Time control studies performed in five additional subjects showed no change in any of the measured variables over time. Collectively, these data indicate that short-term exposure to RHA shifts (‘resets’) the baroreflex stimulus–response curve to higher levels of BP without influencing BRS for extended periods of time.
Obstructive sleep apnoea (OSA) is a prevalent form of sleep-disordered breathing estimated to afflict between 2 and 4% of middle-aged men and women (Young et al. 1993). OSA is characterized by the partial or complete obstruction of the upper airway during sleep resulting in intermittent periods of increased respiratory effort and periods of apnoea and/or hypopnoea. These periods of apnoea/hypopnoea are associated with arterial oxygen desaturation and hypercapnia, which are in turn associated with sympathoexcitation and increases in arterial blood pressure (BP) (Imadojemu et al. 2002). Individuals with OSA are at elevated risk of developing cardiovascular disease (Hung et al. 1990; Peker et al. 1999; Leung & Bradley, 2001), particularly hypertension (Guilleminault & Robinson, 1997; Nieto et al. 2000) and have increased rates of all-cause mortality (Yaggi et al. 2005). Therefore, identifying mechanisms underlying or contributing to the negative consequences associated with OSA are of biomedical significance. In this context the autonomic nervous system may be important (Shamsuzzaman et al. 2003).
Previous studies suggest that both basal and reflexive measures of autonomic nervous system function, such as directly recorded muscle sympathetic nerve activity (MSNA) at rest (Carlson et al. 1993) and peripheral chemoreflex sensitivity (Narkiewicz et al. 1999) are elevated in OSA. Moreover, baroreflex control of cardiovagal and sympathetic outflow appears to be impaired in OSA (Carlson et al. 1996; Bonsignore et al. 2002). Treatment of OSA with night-time continuous positive airway pressure increases cardiovagal baroreflex function during sleep and wakefulness (Bonsignore et al. 2002). These data strongly suggest that periods of sleep-disordered breathing, associated with cyclical exposure to asphyxia (hypoxia and hypercapnia), inspiratory resistance, and apnoeas, may be a critical factor underlying changes in autonomic control in OSA. In healthy humans baroreflex function is impaired acutely by increased inspiratory resistance and/or hypoxia and hypercapnia exposure (Cooper et al. 2004). These data support the contention that breathing alterations, which mimic those experienced by OSA patients during sleep, acutely induce baroreflex dysfunction. However, an important limitation of these previous data is that detrimental effects of inspiratory resistance and/or hypoxia and hypercapnia were only assessed during application of the stressors. The fact that baroreflex function is depressed in OSA patients, even in the wakeful hours, suggests that the consequences of stress imposed during sleep persist for extended periods of time after resumption of regular breathing.
Accordingly, in the present study we tested the hypothesis that short-term exposure to intermittent hypoxia and apnoeas impairs cardiac and sympathetic baroreflex function. Moreover, we hypothesized that impairment in baroreflex function persists for an extended period of time after resuming normal breathing. To test these hypotheses, baroreflex function was assessed before and for ∼1 h after completing a 30 min period of repetitive apnoeas performed during intermittent hypoxia (RHA).
Methods
Subjects
Nineteen young (18–35 years of age) subjects were studied. All were non-obese (body mass index < 30 kg m−2), normotensive (resting BP < 140/90 mmHg), and non-smokers. Subjects were further screened by medical history and physical examination. The experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine. Written informed consent was obtained from all subjects.
Measurements
Subjects were studied supine after an overnight fast (12 h).
MSNA
Recordings of MSNA were obtained using a tungsten microelectrode inserted in the peroneal nerve. This electrode was adjusted until a site with clear spontaneously occurring MSNA bursts was established using standard criteria (Vallbo et al. 1979). Raw nerve recordings were amplified (20 000–70 000 times), filtered (700–2000 Hz), full-wave rectified, and integrated (0.1 s time constant) to obtain mean voltage neurograms.
Cardiovagal and sympathetic BRS
Cardiovagal and sympathetic BRS were assessed using the modified Oxford technique (Ebert & Cowley, 1992). Briefly, nitroprusside was injected (50–100 μg) intravenously followed 60 s later by phenylephrine (100–150 μg). Drugs were administered at doses that decreased and increased systolic BP ∼15 mmHg from baseline levels (Monahan et al. 2004; Monahan & Ray, 2005). For nitroprusside these doses were 50 μg in 5 subjects, 75 μg in 5 subjects, and 100 μg in 4 subjects. For phenylephrine, doses were 100 μg in 2 subjects, 125 μg in 8 subjects, and 150 μg in 4 subjects. Drug doses were the same within a given subject before and after the intervention (see below). Data acquisition began 3 min before nitroprusside infusion and continued for 2 min after phenylephrine infusion. At least 15 min separated consecutive BRS trials. Previously we have established that this interval of time is sufficient to allow for reproducible values of BRS as well as to allow for BP and heart rate (HR) at rest to return to pre-infusion levels (Monahan & Ray, 2005). Cardiovagal BRS was assessed in all subjects. However, due to difficulties in obtaining or maintaining satisfactory MSNA recording sites sympathetic BRS was only measured in 10 experimental and 4 control subjects (see below).
Respiration and oxygen saturation
Partial end-tidal CO2, arterial oxygen saturation (SaO2; earlobe pulse oximetry) and minute ventilation were measured with a respiratory gas monitor (Ohmeda RGM 5200).
Arterial blood pressure and heart rate
Resting BP was determined using a semi-automated device (Welch Allyn). Continuous measurements of BP were made using a Finapres photoplethysmograph (Ohmeda). HR was determined from the ECG.
Experimental protocol
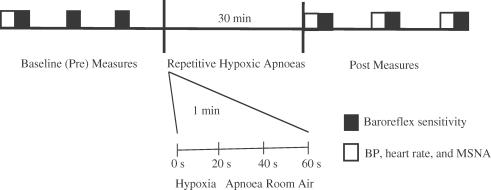
A schematic diagram of the experimental protocol is provided in Fig. 1. Three BRS trials were performed at baseline (Pre) and three trials were performed after the intervention (Post). Post-intervention measures were made ∼7 (Post 1), ∼30 (Post 2) and ∼50 min (Post 3) after completing the intervention to gain insight into possible temporal patterns of changes in physiological function. BP and HR were determined in triplicate before the first baseline (Pre) trial and each trial after the intervention (Post 1, Post 2 and Post 3). The intervention consisted of a 30 min period in which subjects performed repetitive hypoxic apnoeas (RHA) (n = 14) (Leuenberger et al. 2005). RHA involved subjects performing repetitive voluntary end-expiratory apnoeas (20 s duration) every minute for 30 min (30 total apnoeas) while breathing through a 2-way non-rebreathing facemask. To enhance oxygen desaturation during apnoeas hypoxic gas (10.5% O2) was administered during the period of free breathing. The period of time hypoxic gas was inspired during the 40 s free breathing period was individually adjusted in an attempt to achieve post-apnoeic nadirs in SaO2 in the mid to low 80s (%). At the end of each apnoea subjects were instructed to briefly (1 s) exhale to allow measurement of end-tidal CO2.
Figure 1. Schematic diagram of the protocol in experimental subjects.
Baroreflex sensitivity (BRS) was measured in triplicate at baseline (Pre) and after (Post) the intervention. The intervention consisted of 30 consecutive apnoeas performed during intermittent exposure to hypoxia (10.5% oxygen). The length of intermittent hypoxia exposure before each apnoea was adjusted so that arterial oxygen saturations decreased into the mid to low 80s (%). Apnoeas were voluntarily performed at end-expiration and lasted 20 s. At least 15 min separated each BRS trial. Post measurements of BRS were made at time points corresponding to ∼7, ∼30 and ∼50 min after completion of repetitive hypoxic apnoeas (RHA). Blood pressure (BP), heart rate and muscle sympathetic nerve activity (MSNA) at rest were measured before the first baseline (Pre) trial and before each of the 3 trials performed after RHA. In 5 control subjects the above protocol was followed identically except subjects were not exposed to RHA. The intervention in this group was a 45 min period of rest.
Five additional subjects served as time controls. The experimental protocol in these subjects was identical to those exposed to RHA (Experimental) except that during the period of the intervention subjects were asked to rest quietly for 45 min.
Data analysis
All data were digitally stored on a computer (MacLab 8e, ADInstruments) at a sampling frequency of 400 Hz.
MSNA at rest was quantified as bursts min−1 and as the sum of the area under individual bursts per minute (au min−1). The largest burst at rest was assigned an arbitrary amplitude of 1000 and a portion of the neurogram in which neural silence (i.e. no efferent discharges) occurred was used to set the baseline to zero.
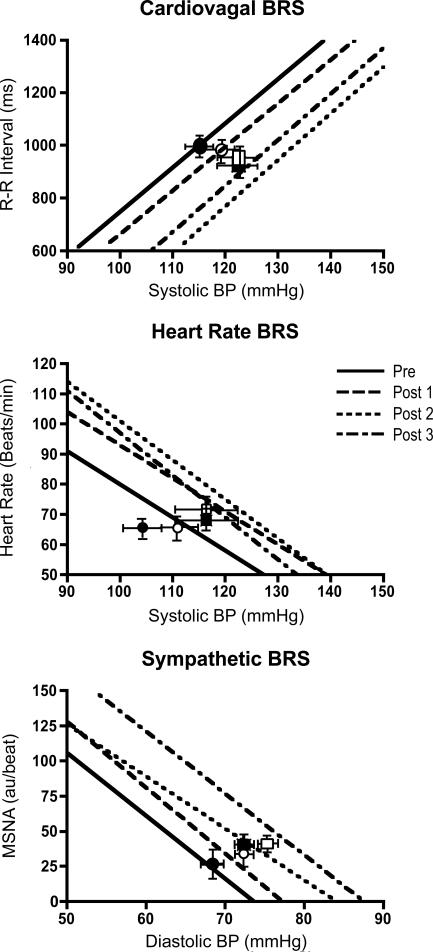
Cardiovagal BRS was quantified as the slope of the linear portion of the R-R interval–systolic BP relation (over 2 mmHg pressure ranges or ‘bins’) from the nadir to peak systolic BP response during each BRS trial (Ebert & Cowley, 1992; Rudas et al. 1999). Additionally, a measure of HR BRS was obtained from the HR–systolic BP relation. Points clearly falling in either the threshold or saturation region were removed from the analysis (Halliwill & Minson, 2002). Both measures were made to avoid any potential concerns that may be present if resting HR changes. The operating point of the cardiac arm of the baroreflex in relation to brachial systolic BP, HR and R-R interval was determined as the mean values of these variables measured before each BRS trial (Fig. 5).
Figure 5. Group average regressions between R-R interval and heart rate versus systolic BP and between MSNA and diastolic BP in Experimental group subjects.
Operating points are indicated by symbols (•, Pre; ○, Post 1; ▪, Post 2; and □, Post 3) and error bars. Note the similar responses, as evidenced by the slope of the respective regressions that were observed before (Pre) and after (Post 1, Post 2, and Post 3) the 30 min period of RHA for each expression of BRS (see also Fig. 4). In contrast the regression lines and operating points are shifted rightward and upward (for heart rate and MSNA) towards higher pressure.
Sympathetic BRS was quantified as the slope of the linear portion of the MSNA–diastolic BP relation (over 3 mmHg bins) from the start of the nitroprusside-induced decrease to the peak diastolic BP response during the trial (Ebert & Cowley, 1992; Rudas et al. 1999). MSNA was quantified as the integrated area underlying a sympathetic burst. Bursts were visually identified after accounting for conduction latency. Any heartbeat not associated with a sympathetic burst was assigned a value of zero and was included in the analysis (Ebert & Cowley, 1992; Rudas et al. 1999). Diastolic BP bins with no sympathetic activity were excluded from the analyses (Rudas et al. 1999). The operating point of the sympathetic baroreflex in relation to resting brachial diastolic BP and MSNA was determined as the mean values of these variables measured before each BRS trial.
To determine if baroreflex resetting (shift in the stimulus–response relation) occurred (Pre to Post) we calculated predicted HR, R-R interval and MSNA levels relative to an arbitrary pressure from the respective stimulus–response relation (Fig. 5). The arbitrary pressure used was the resting systolic (for HR and R-R interval) and diastolic BP (for MSNA) determined in the baseline period (Pre).
Cardiovagal BRS trials with linear correlation coefficients (r-value) > 0.70 (Smyth et al. 1969; Rudas et al. 1999) during the Pre measures were averaged (up to 3) and a single value is reported. Individual trials after the intervention (Post 1, Post 2 and Post 3) were compared on a trial-to-trial basis to baseline levels (Pre) to gain insight into possible temporal patterns of change. The same process occurred for sympathetic BRS except that the criterion for retaining a sympathetic BRS value was an r value > 0.50 (Carlson et al. 1996; Rudas et al. 1999). An investigator blinded to the experimental condition performed all analyses.
Statistical analysis
Differences in baseline subject characteristics were determined by t test. Responses to the intervention were determined using a linear mixed effects model (one-way repeated measures ANOVA). When significant main effects were identified, specific contrasts were then made using Dunnett's post hoc tests. Corrections for multiple comparisons were made. Statistical significance was established at P < 0.05.
Results
Subject characteristics
Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics
| Variable | Control (n = 5) | Experimental (n = 14) |
|---|---|---|
| Sex | 3 male/2 female | 7 male/7 female |
| Age (years) | 25 ± 2 | 26 ± 1 |
| Height (cm) | 177.8 ± 4.3 | 172.8 ± 2.6 |
| Body mass (kg) | 77.3 ± 5.8 | 71.9 ± 4.7 |
| BMI (kg m−2) | 24.3 ± 1.4 | 23.8 ± 1.1 |
| Systolic BP (mmHg) | 117 ± 3 | 114 ± 3 |
| Diastolic BP (mmHg) | 68 ± 3 | 69 ± 1 |
| Heart rate (beats min−1) | 62 ± 3 | 62 ± 2 |
Values are mean ±s.e.m. BMI, body mass index; BP, blood pressure.
Effect of apnoeas performed during intermittent hypoxia on SaO2 and end-tidal CO2
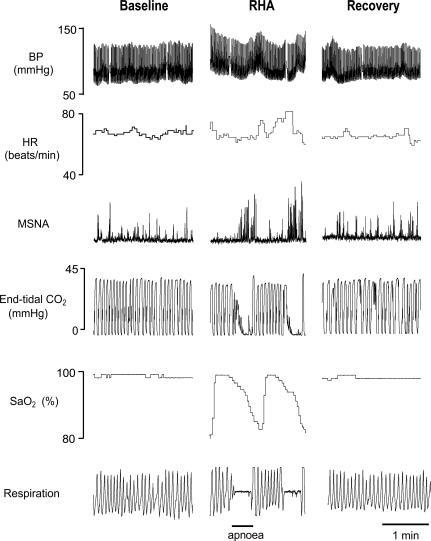
Repetitive apnoeas performed during intermittent hypoxia induced cyclical changes in SaO2 from immediately before apnoea to shortly after the end of apnoea (98.0 ± 0.2 to 83.0 ± 1.0%; P < 0.05) (Fig. 2). Additionally, apnoeas increased end-tidal CO2 from immediately before apnoea to the peak increase after the end of apnoea (35.0 ± 0.4 to 41.0 ± 0.4 mmHg; P < 0.05) (Fig. 2). Minute ventilations did not differ in the ∼5 min period before (7.3 ± 0.4 l min−1) and after (7.3 ± 0.3 l min−1) RHA.
Figure 2. Representative responses to the repetitive hypoxic apnoea (RHA) protocol.
Representative recording of BP, heart rate (HR), MSNA, end-tidal CO2, arterial oxygen saturation (SaO2) and respiration before (Baseline), during, and after (Recovery) a 30 min period of RHA in a subject in the Experimental group. Segments presented during each phase of the study (Baseline, RHA and Recovery) are 2 min in duration. Performing individual apnoeas (20 s each) during intermittent hypoxia exposure resulted in cyclical changes in SaO2 (from ∼98% to ∼83%) and surges in BP and MSNA.
Effect of RHA on BP, HR and MSNA at rest
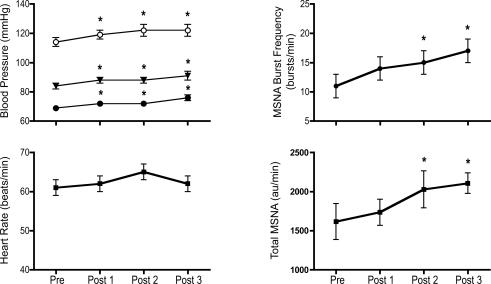
Systolic, diastolic and mean BP increased from baseline (Pre) levels after RHA. These increases persisted throughout the Post period (∼1 h) (Fig. 3). Similarly MSNA measured at rest increased from baseline (Pre) levels after RHA. These increases persisted throughout the Post period (Fig. 3). HR was unchanged from baseline (Pre) levels after RHA (Fig. 3).
Figure 3. Effect of the intervention on hemodynamics and muscle sympathetic nerve activity (MSNA) at rest.
Blood pressure, heart rate, MSNA burst frequency (bursts min−1) and total MSNA (sum of area under individuals' MSNA bursts expressed in au min−1) measured at rest at baseline (Pre) and before each of the 3 baroreflex trials measured after completion of the repetitive hypoxic apnoea protocol (Post 1, Post 2 and Post 3). Systolic (○), diastolic (•) and mean BP (▾), and MSNA, increased after RHA (* P < 0.05).
Effect of RHA on baroreflex function
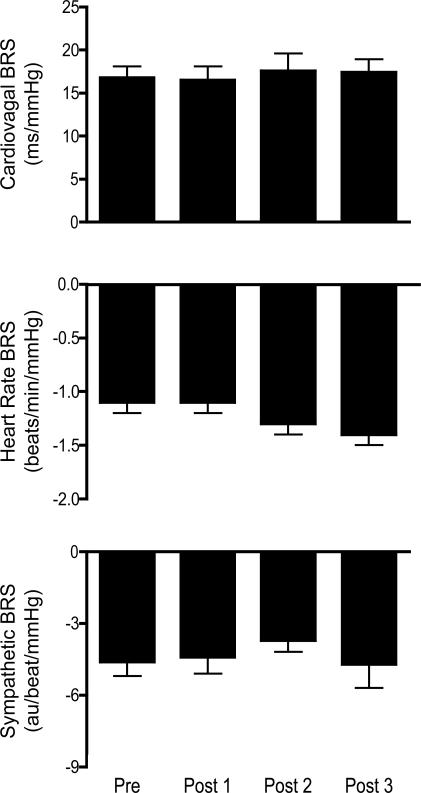
The effects of RHA on indices of BRS are presented in Fig. 4. Cardiovagal BRS was unchanged from baseline (Pre) levels after RHA (16.8 ± 1.3, 16.5 ± 1.6, 17.6 ± 2.0 and 17.4 ± 1.5 ms mmHg−1 for Pre, Post 1, Post 2, and Post 3, respectively; P = 0.92). Similar responses were noted for both HR BRS (−1.1 ± 0.1, −1.1 ± 0.1, −1.3 ± 0.1 and −1.4 ± 0.1 beats min−1 mmHg−1; P = 0.34) as well as sympathetic BRS (−4.5 ± 0.6, −4.4 ± 0.7, −3.7 ± 0.5 and −4.7 ± 1.0 au beat−1 mmHg−1; P = 0.20). In contrast the stimulus–response relations for all measures of baroreflex function were shifted rightward and upward (HR and sympathetic BRS) to higher levels of BP by RHA suggesting that RHA reset the baroreflexes. These shifts are visually apparent in the group-averaged stimulus–response relations (Fig. 5). To quantify the magnitude of resetting that occurred we calculated HR, R-R interval and MSNA levels at identical levels of systolic (HR and R-R interval) and diastolic (MSNA) BP Pre and Post. The BP level was the systolic or diastolic BP measured at baseline (Pre). These analyses indicated that HR (64 ± 4, 75 ± 5, 79 ± 5* and 78 ± 4* beats min−1 for Pre, Post 1, Post 2 and Post 3, respectively; *P < 0.05 compared to Pre) and MSNA (1783 ± 378, 2701 ± 544, 4470 ± 810* and 4153 ± 1025* au min−1) increased (main effect P < 0.05) and R-R interval (959 ± 61, 848 ± 56, 763 ± 42* and 784 ± 59* ms) decreased (main effect P < 0.05) at a given pressure after RHA.
Figure 4. Effects on baroreflex sensitivity (BRS).
Cardiovagal, heart rate and sympathetic BRS at baseline (Pre) and after (Post 1, Post 2, and Post 3) completion of 30 consecutive 20 s apnoeas performed during intermittent hypoxia (RHA). After RHA there were no changes in any of the respective expressions of BRS.
The ranges over which baroreflex function were determined Pre and Post were slightly greater after RHA (Δ39 ± 3 compared to Δ44 ± 3 mmHg for Pre and Post, respectively; P < 0.05). This difference appears to be the result of a greater decrease in BP after nitroprusside (Δ14 ± 1 compared to Δ21 ± 2 mmHg; P < 0.05) rather than a greater increase in BP after phenylephrine infusion (Δ26 ± 3 compared to Δ24 ± 3 mmHg; P = 0.35). In contrast the rate of change in BP from the nadir to peak change in BP during the BRS trials was identical Pre and Post (0.66 ± 0.06 compared to 0.66 ± 0.09 mmHg s−1; P = 0.96).
Time control studies
Haemodynamics, MSNA and BRS (cardiovagal, HR and sympathetic) were stable over the duration of the study in control subjects (Table 2).
Table 2.
Response to intervention
| Pre | Post | |||
|---|---|---|---|---|
| Variable | Baseline | Post 1 | Post 2 | Post 3 |
| Control subjects (n = 5) | ||||
| Systolic BP (mmHg) | 118 ± 3 | 119 ± 2 | 119 ± 2 | 119 ± 3 |
| Diastolic BP (mmHg) | 68 ± 3 | 69 ± 3 | 68 ± 3 | 70 ± 3 |
| Mean BP (mmHg) | 85 ± 3 | 86 ± 2 | 85 ± 3 | 86 ± 3 |
| Heart rate (beats min−1) | 62 ± 3 | 62 ± 2 | 61 ± 2 | 62 ± 3 |
| MSNA (bursts min−1) | 17 ± 3 | 15 ± 3 | 15 ± 2 | 14 ± 3 |
| Cardiovagal BRS (ms mmHg−1) | 15.6 ± 1.3 | 16.7 ± 4.0 | 17.9 ± 2.6 | 15.0 ± 0.09 |
| Sympathetic BRS (au beat−1 mmHg−1) | −3.7 ± 0.2 | −3.8 ± 0.3 | −3.6 ± 0.4 | −4.0 ± 0.3 |
Values are mean ±s.e.m. BP, blood pressure; MSNA, muscle sympathetic nerve activity; BRS, baroreflex sensitivity; au, arbitrary units.
Discussion
The primary new finding from this study is that short-term exposure to repetitive apnoeas performed during intermittent hypoxia does not impair cardiovagal or sympathetic BRS, but appears to reset the baroreflex stimulus–response curve rightward (to higher levels of BP) and upward (for HR and MSNA). This resetting may help explain why the body does not appear to resist RHA-induced increases in BP by inhibiting sympathetic outflow or decreasing HR.
The RHA protocol used in the present study provides a complex and variable stimulus to subjects, which acutely mimics the breathing patterns that occur during sleep in patients with OSA. These stimuli variably include periods of asphyxia, hyperpnoea, hypopnoea, and apnoea. Previous studies in healthy humans have used a similar RHA protocol to acutely study changes in cardiovascular/autonomic function in humans. These studies have reported that RHA increases MSNA (Cutler et al. 2004b; Leuenberger et al. 2005), BP (Leuenberger et al. 2005) and chemoreflex sensitivity (Cutler et al. 2004a) at rest for extended periods of time (up to 2 h) after RHA. These data establish that RHA is a powerful model to: (1) acutely induce changes in autonomic/cardiovascular function, (2) acutely study the effects of altered breathing on autonomic control in humans, and (3) gain insight into the temporal pattern of changes in physiological function by obtaining multiple measurements after resumption of regular breathing.
Our finding of no change in cardiovagal or sympathetic BRS, but a rightward and upward shift in the operating point of the baroreflexes (for HR and MSNA) after RHA is similar to those effects observed during acute continuous systemic hypoxia. Specifically, it has been reported that cardiovagal and sympathetic BRS are unaltered by short-term (18 min) continuous systemic hypoxia, but that the baroreflexes are reset to operate around a higher baseline level of BP (Halliwill & Minson, 2002; Halliwill et al. 2003). Thus, for a given level of BP both HR and MSNA are elevated by hypoxia. In the present study in addition to exposing individuals to systemic hypoxia (intermittently), repetitive apnoeas (30 in total) were performed. This protocol unlike continuous systemic hypoxia induces cyclical changes in arterial blood oxygenation (cycling saturation/desaturation). Thus, RHA (30 min) may be a more physiologically stressful stimulus than a shorter period of continuous systemic hypoxia (achieving similar decreases in SaO2). This factor may help explain how RHA induced prolonged periods of baroreflex resetting that persisted beyond the period of RHA. However, we cannot exclude the possibility that hypoxia exposure alone does not elicit prolonged periods of baroreflex resetting as Halliwill et al. did not make any measurements after removal of the hypoxic stimulus (Halliwill & Minson, 2002; Halliwill et al. 2003). However, as MSNA may remain elevated for extended periods of time after continuous systemic hypoxia exposure (20 min) (Morgan et al. 1995; Tamisier et al. 2004) we cannot rule out that the resetting persisted.
Previous studies in humans have examined the possible influences that inspiratory resistance, hypoxia, hypercapnia and apnoea acutely exert on baroreflex function. Recently, Cooper and colleagues reported that inspiratory resistance decreased the gain of baroreflex control of vascular resistance and that hypoxia exposure shifted the stimulus–response curve rightward suggesting resetting (Cooper et al. 2004). When both physiological stressors were applied simultaneously, baroreflex gain was reduced and the stimulus–response curve was displaced rightward to higher pressures. These data strongly suggest that increased inspiratory resistance and hypoxia alter baroreflex function (impaired BRS as well as resetting the carotid baroreflex). The effects of apnoea on baroreflex function are less clear. A single apnoea has been shown not to alter baroreflex control of MSNA during application of neck suction (Muenter Swift et al. 2003). However, after completion of the apnoea there was a very transient (< 1 min) impairment in baroreflex control of sympathoexcitation during neck pressure. Collectively, our present findings importantly extend these prior findings by showing that changes in baroreflex function that occur during stressors designed to mimic OSA can induce persistent changes in baroreflex function that extend beyond the exposure to the stressors (intermittent hypoxia, hypercapnia and apnoea).
Most studies that have assessed baroreflex function in humans with OSA have concluded that baroreflex dysfunction occurs. Specifically, cardiovagal BRS has been shown in most (Carlson et al. 1996; Bonsignore et al. 2002), but not all studies (Narkiewicz et al. 1998) to be depressed in OSA. Additionally, baroreflex control of MSNA (i.e. sympathetic BRS) during decreases in BP are blunted in OSA (Carlson et al. 1996; Narkiewicz et al. 1998). Our present study does not support a role for short-term periods of altered breathing (repetitive apnoeas performed during intermittent hypoxia exposure) promoting decreases in cardiovagal or sympathetic BRS. However, our data do suggest that the operating points of both the cardiovagal and sympathetic baroreflex are reset to higher levels of BP, HR and MSNA. The resetting of the baroreflex to higher levels of BP is a characteristic feature of hypertension (McCubbin et al. 1956). It is unknown how long this resetting persisted in our study as we only acquired measurements for ∼1 h after RHA. However, it is possible that even short-term shifts to higher levels of BP may be of physiological relevance. Over time these repetitive exposures to acute hypertension may predispose even normotensive OSA patients to the development of hypertension. This suggestion is supported by data suggesting that OSA may precede the development of chronic hypertension (Peppard et al. 2000).
Several limitations are associated with the present study. First, our RHA intervention exposed individuals to only 30 apnoeas performed during intermittent hypoxia. Thus, the level of stress exerted on our subjects during RHA was probably less than that exerted on an OSA patient during an entire nights sleep. Therefore, we cannot exclude the possibility that longer periods of RHA would depress BRS. Second, in our experience the modified Oxford technique does not allow full characterization of the baroreflex stimulus–response curve. This has been reported by others (Halliwill & Minson, 2002). Thus, we cannot determine with certainty if the set point of the baroreflex stimulus–response curve was altered, although our data relating to changes in the operating point pressure, MSNA and HR do suggest that this is the case. This is an inherent limitation in the methods available to assess integrated baroreflex responses in intact humans safely. Lastly, measurements were made in healthy young adults. It is possible that different effects would be observed in other populations.
In conclusion, these data suggest that short-term (30 min) exposure to repetitive apnoeas (single 20 s apnoea every minute) performed during intermittent hypoxia does not impair cardiovagal or sympathetic BRS in healthy young humans, but does appear to reset the baroreflexes to operate around a new higher level of BP at rest. This resetting is evident by a rightward (to higher BP) and upward shift (for HR and MSNA) in the stimulus–response curve. Thus both HR and MSNA are increased at a given level of BP after RHA.
Acknowledgments
We would like to thank Damian Dyckman, Amy Fogelman, Erin Muldoon, Kris Gray, and Charity Sauder for technical assistance and Cheryl Blaha and Chanty Webb for excellent nursing support. Grants from the National Institutes of Health (HL68699, HL77670, and DC006459) and National Space and Biomedical Research Institute (CA00404) supported this research. Additional support was provided by a NIH sponsored General Clinical Research Center with National Center for Research Resources Grants M01 RR10732 and C06 RR016499.
References
- Bonsignore MR, Parati G, Insalaco G, Marrone O, Castiglioni P, Romano S, Di Rienzo M, Mancia G, Bonsignore G. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:279–286. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:1490–1496. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor–vascular resistance reflex. J Physiol. 2004;557:1055–1065. doi: 10.1113/jphysiol.2004.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2004a;287:H2054–H2060. doi: 10.1152/ajpheart.00377.2004. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004b;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Cowley AW., Jr Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol. 1992;262:H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Robinson A. Sleep-disordered breathing and hypertension: past lessons, future directions. Sleep. 1997;20:806–811. doi: 10.1093/sleep/20.9.806. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol. 2002;93:857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol. 2003;552:295–302. doi: 10.1113/jphysiol.2003.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med. 2002;165:61–66. doi: 10.1164/ajrccm.165.1.2009062. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- McCubbin JW, Green JH, Page IH. Baroreceptor function in chronic renal hypertension. Circ Res. 1956;4:205–210. doi: 10.1161/01.res.4.2.205. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol. 2004;286:H2113–H2117. doi: 10.1152/ajpheart.01054.2003. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Ray CA. Cyclooxygenase inhibition and baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol. 2005;288:H737–H743. doi: 10.1152/ajpheart.00357.2004. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- Muenter Swift N, Cutler MJ, Fadel PJ, Wasmund WL, Ogoh S, Keller DM, Raven PB, Smith ML. Carotid baroreflex function during and following voluntary apnea in humans. Am J Physiol Heart Circ Physiol. 2003;285:H2411–H2419. doi: 10.1152/ajpheart.00139.2003. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32:1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Jama. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Jama. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Nieto L, Anand A, Cunnington D, Weiss JW. Sustained muscle sympathetic activity after hypercapnic but not hypocapnic hypoxia in normal humans. Respir Physiol Neurobiol. 2004;141:145–155. doi: 10.1016/j.resp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]