Abstract
Neurosteroids regulate neuronal excitability and are expressed at particularly high levels in the CNS during the perinatal period. Further, neurosteroid levels are increased by a variety of stressors including hypoxia, asphyxia, parturition, ethanol exposure and infection. One mechanism by which neurosteroids regulate neuronal activity is by negative or positive modulation of GABAA receptor function. Perinatal respiration is strongly modulated by GABAergic synaptic drive, and GABA release is increased during hypoxia to contribute to hypoxia-induced depression of neonatal ventilation. Here, we use in vitro and in vivo rat models to test the hypothesis that GABAA receptor-mediated modulation of perinatal respiration is markedly influenced by the presence of neurosteroids. The principal finding of this study was that the efficacy of GABAA receptor-mediated modulation of respiratory membrane potential and rhythmogenesis is markedly enhanced by allopregnanolone and depressed by dehydroepiandrosterone sulphate. These data demonstrate that the modulation of breathing via GABAA receptor activation will be determined by the overall balance of negative and positive neurosteroid modulators within respiratory nuclei. This adds a level of complexity that must be considered when examining the depression of breathing in mammals associated with various behavioural states and pathogenic conditions such as apnoea and sudden death suspected to be associated with central respiratory dysfunction.
The frequency and amplitude of respiratory rhythmic drive is dynamically regulated to meet the varied demands for ventilation. In particular, rhythmogenesis generated within a key area of the ventrolateral medulla, the preBötzinger complex (preBötC), is modulated by synaptic drive from multiple neurotransmitter systems (reviewed in Rekling & Feldman, 1998). An important inhibitory input arises from GABAergic neurons (Johnson et al. 1996; Shao & Feldman, 1997; Brockhaus & Ballanyi, 1998; Ritter & Zhang, 2000). Further, the levels of GABA released are increased during hypoxia, and contribute to hypoxia-induced depression of neonatal ventilation (Huang et al. 1994). In a recent study (Ren & Greer, 2006), we demonstrated the age-dependent changes of GABAA receptor-mediated actions on respiratory rhythmogenesis during the perinatal period in the rat. Here, we extend upon that work by testing the hypothesis that GABAA receptor-mediated modulation of perinatal respiration can be profoundly influenced by the presence of neurosteroids.
Steroid hormones and their derivatives regulate neuronal excitability and function (Akwa et al. 1991; Majewska, 1992; Mellon, 1994; Baulieu & Robel, 1996, 1998; Baulieu, 1997; Joels, 1997; Jung-Testas & Baulieu, 1998). These actions are mediated by modulating neurotransmitter receptor function, ion channel kinetics, and directly via binding to steroid receptors located on neuronal membranes. Neurosteroids within the CNS arise from two general sources. There are neurosteroids that are synthesized within the CNS from cholesterol or steroid hormone precursors (e.g. pregnenolone, allopregnanolone, progesterone, dehydroepiandorosterone, oestradiol, 5α-dihydrotesterone), and those that arrive via the circulation (e.g. testosterone). The level of neurosteroid synthesis in the CNS is particularly high during the perinatal period (Brown & Papadopoulos, 2001; Mellon & Vaudry, 2001) and increases during periods of physiological stress (e.g. hypoxia, parturition, infection; Barbaccia et al. 2001). Neurosteroids, depending on their structure, can act as either negative or positive modulators of GABAA receptor function (Park-Chung et al. 1999). Thus, there is potential for a neurosteroid–GABAA receptor interaction, with important implications for the control of perinatal breathing. Specifically, the net effect of GABAergic synaptic input to respiratory neuronal populations could vary markedly depending on the type and concentration of neurosteroids during a given state. In this study, we examined the actions of allopregnanolone and dehydroepiandrosterone sulphate (DHEAS) on the spontaneous respiratory drive generated by in vitro models and in vivo. These two neurosteroids are prevalent in the perinatal CNS and have pronounced modulatory effects on GABAA receptor function (Compagnone et al. 1995; Nguyen et al. 2003).
Methods
Brainstem–spinal cord preparations
Fetal Sprague-Dawley rats (E17–21) were delivered from timed-pregnant dams anaesthetized with halothane (2.5% delivered in 95% O2 and 5% CO2) and maintained at 37°C by radiant heat, following procedures approved by the Animal Welfare Committee at the University of Alberta. The timing of pregnancies was determined from the appearance of sperm plugs in the breeding cages. The ages of fetuses were confirmed by comparison of their crown–rump length measurements with those published by Angulo Y González, 1932). Newborn rats were anaesthetized by inhalation of metofane (2–3%). Embryos and newborns were decerebrated, and the medulla–spinal cord dissected following procedures similar to those established (Smith et al. 1990; Greer et al. 1992). The neuraxis was continuously perfused at 27 ± 1°C (perfusion rate 5 ml min−1; chamber volume 1.5 ml) with modified Krebs solution that contained the following (mm): 128 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 23.5 NaHCO3, 0.5 NaH2PO4, and 30 d-glucose (equilibrated with 95%O2–5%CO2).
Medullary slice preparations
Details of the preparation have been previously described (Smith et al. 1991). Briefly, the brainstem–spinal cords isolated from perinatal rats as described above were pinned down, ventral surface upward, on a paraffin-coated block. The block was mounted in the vise of a vibratome bath (VT1000S; Leica, Nussloch, Germany). The brainstem was sectioned serially in the transverse plane starting from the rostral medulla, to within approximately 150 μm of the rostral boundary of the preBötC, as judged by the appearance of the inferior olive. A single transverse slice containing the preBötC and more caudal reticular formation regions was then cut (500–700 μm thick), transferred to a recording chamber, and pinned down onto a Sylgard elastomer. The medullary slice was continuously perfused in physiological solution similar to that used for the brainstem–spinal cord preparation except for [K+]o, which was increased to 9 mm to stimulate the spontaneous rhythmic respiratory motor discharge in the medullary slice (Smith et al. 1991).
Extracellular recording and analysis
Recordings of hypoglossal (XII) nerve roots and cervical (C4) ventral roots were made with suction electrodes. Suction electrodes were placed into the XII nuclei and preBötC region of the ventrolateral medulla to record extracellular neuronal population discharge from medullary slice preparations. Signals were amplified, rectified, low-pass filtered, and recorded on a computer using an analog-to-digital converter (Digidata 1322A; Axon Instruments, Union City, CA, USA) and data acquisition software (Clampfit, Axon Instruments). Mean values of respiratory frequency relative to control were calculated pre-, during and post-drug delivery using Matlab software (Mathworks, Inc.). Values of EC50 and IC50 are defined as the concentration of drug necessary to produce 50% of the maximum mean measured response. Results were expressed as mean ± standard deviation (s.d.). Statistical significance was tested using paired/unpaired difference Student's t test (two groups) or one-way RM ANOVA (multiple groups); significance was accepted at P values < 0.05.
Intracellular recordings from medullary slice preparations
Patch electrodes were fabricated from thin-walled borosilicate glass (1.5 mm external and 1.12 mm internal diameter; A-M Systems, Everett, WA, USA). The pipette resistances were between 3 and 5 MΩ. All whole-cell patch recordings were obtained from the somata of small neurons within the region of the preBötC, localized ventrolaterally to the pars compacta of the ambiguous nucleus. Neurons were approached under visual control using a microscope equipped with an infrared contrast enhancement system (Axioskop, Zeiss, Oberkochen, Germany). To establish whole-cell recording, additional suction was applied to rupture the underlying plasma membrane. The standard pipette solution contained the following (mm): 140 K-gluconate, 4 NaCl, 1 CaCl2, 10 EGTA or BAPTA (10), 10 Hepes, 5 MgATP, and 0.3 Na3GTP, pH adjusted to 7.3 with KOH. Whole-cell current-clamp recordings were initially established in modified Krebs solution and performed with an NPI Electronics SEC05LX amplifier (NPI Electronics, Tamm, Germany). All liquid junction potentials were corrected before seal formation with the compensation circuitry of the patch-clamp amplifier. Neurons with resting membrane potentials (Vrest) more negative than −40 mV and overshooting action potentials were analysed. Data were digitized with an analog-to-digital interface (Digidata 1322A; Axon Instruments) and analysed with the use of pClamp 9.2 (Axon Instruments).
Pharmacological agents
The following drugs were used: allopregnanolone (5α-pregnan-3α-ol-20-one), pregnanolone (5β-pregnan-3α-ol-20-one), alfaxalone (5α-pregnan-3α-ol-11,20-dione), allotetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one), isopregnanolone (5α-pregnan-3β-ol-20-one), pregnenolone (5-pregnen-3β-ol-20-one), 5α-pregnane-3β,20β-diol, 17β-oestradiol, and progesterone, dehydroisoandrosterone (DHEA), DHEAS, bicuculline (free base; soluble in DMSO), muscimol, tetrodotoxin (TTX), ethylene glycol-bis(b-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 1,2-bis(2-amino-phenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrapotassium salt (BAPTA). The doses of neurosteroids used were based on similar in vitro studies and pharmacological profiles of the EC50 and IC50 (Majewska, 1990; Spivak, 1994; Sousa & Ticku, 1997; Rupprecht & Holsboer, 2000). Drugs were purchased from Sigma (St Louis, MO, USA) or RBI (Oakville, ON, USA). Stock solutions of drugs were prepared as concentrates. All drugs used in vitro were dissolved in modified Krebs solution, and the pH adjusted to 7.4. Muscimol (soluble in physiological 0.9% NaCl saline; 0.3–0.5 mg kg−1), bicuculline (free base, soluble in DMSO, 0.6–1 mg kg−1), allopregnanolone (0.5–4 mg kg−1 dissolved in DMSO; Gizerian et al. 2004), and DHEAS 10 mg kg−1 dissolved in DMSO; Hoffman et al. 2003) were administered i.p.in vivo.
In vivo neonatal plethysmographic measurements
Whole-body plethysmographic measurements of frequency and depth of breathing were made from E20–P4 unanaesthetized rats of either sex. Pressure changes associated with perinatal rat breathing were measured using a 27 ml whole-body plethysmograph chamber, a pressure transduced (model DP103, Validyne, Northridge, CA, USA) and signal conditioner (CD-15). The plethysmograph was contained within an infant incubator (model C-86, Isolette, Warminster, PA, USA) in order to maintain the ambient temperature at the approximate nest temperature of 32°C.
Results
Modulation of respiratory frequency in vitro by allopregnanolone
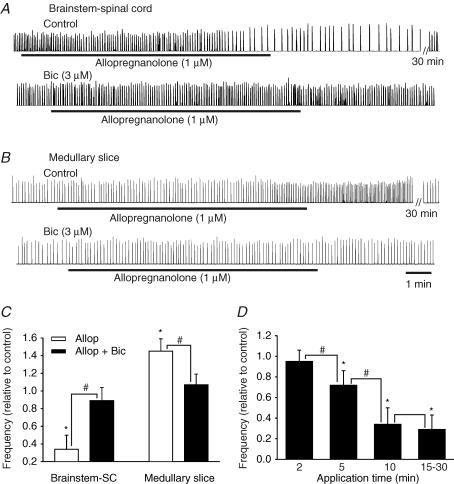
Allopregnanolone had marked effects on respiratory frequency on its own and greatly enhanced the actions of exogenously applied muscimol. Figure 1 illustrates the actions of allopregnanolone (1 μm) on respiratory rhythm generated by in vitro preparations isolated from P0 rats. The effects of allopregnanolone were time-dependent with a steady-state effect being reached at 10–20 min, and continuing for 30–60 min, which is consistent with previous in vitro studies of allopregnanolone actions (Fancsik et al. 2000). The respiratory frequency of brainstem–spinal cord preparations was markedly depressed (34 ± 16% of control, n = 6) by allopregnanolone. In contrast, allopregnanolone caused an increase (145 ± 14% of control, n = 5) of respiratory frequency generated by medullary slice preparations. The amplitude and duration of inspiratory bursts were not changed significantly. The effects of allopregnanolone were antagonized by the GABAA receptor antagonist bicuculline (3 μm). As demonstrated in Ren & Greer (2006), chloride-mediated conductances are influenced by [K+]o due to the role of potassium ions in the function of the KCC2 chloride-cotransporter (DeFazio et al. 2000). Thus, the contrasting actions of allopregnanolone in the two types of in vitro preparations can be explained by the fact that the brainstem–spinal cord and medullary slice preparations are bathed in media containing 3 mm and 9 mm [K+]o, respectively.
Figure 1. Modulation of respiratory frequency by allopregnanolone.
A, rectified and integrated suction electrode recordings of C4 ventral root activity in a P0 brainstem–spinal cord preparation bathed in 3 mm [K+]o showing allopregnanolone-induced depression of respiratory frequency. The effects of allopregnanolone (1 μ m applied for 10 min indicated by solid line) started at approximately 8 min, and reached a steady-state maximal level at 15 min, continuing for 30 min. The effect of allopregnanolone was antagonized by the presence of bicuculline (3 μm). B, rectified and integrated recordings of XII cranial root activity in a medullary slice preparation from a P0 rat, bathed in 9 mm [K+]o showing allopregnanolone-induced increase of respiratory frequency. Similar to that observed in the brainstem–spinal cord preparations, the effects of allopregnanolone (1 μm for 10 min) started at approximately 8 min, and reached a steady-state maximal level at 13 min, continuing for 35 min. The effects of allopregnanolone were antagonized by the presence of bicuculline (3 μm). C, population data from brainstem–spinal cord (SC) and medullary slice preparations showing changes in frequency of respiration relative to control in response to allopregnanolone (Allop, 1 μm) in the absence and presence of bicuculline (Bic, 3 μm). Each data point was from five to six P0 preparations. D, the depression of respiratory frequency by allopregnanolone (1 μm) was increased with the time of the drug application. The maximal effects were achieved with 10 min application of allopregnanolone, since there were no significant further depressing effects observed with 15–30 min. Each data point was from five to eight P0 brainstem–spinal cord preparations. * indicates significant difference relative to control (P < 0.05); # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
Our main focus was on the actions of allopregnanolone and DHEAS. However, we screened a variety of neurosteroids' actions in vitro and observed that respiratory frequency was modulated by steroid neuromodulators possessing a 3α-hydroxyl group (i.e. allopregnanolone/3α,5α-P, pregnanolone/3α,5β-P, alfaxolone/3α,5α-P, allotetrahydro-corticosterone/3α,5α-P), but not a 3β-hydroxyl group (isopregnanolone/3β,5β-P, pregnenolone/3β-P, 5α-pregnane-3β,20β-diol). Further, the neurosteroids 17β-oestradiol and progesterone had no effects on respiratory frequency at the concentration of 3 μm.
Developmental changes in the actions of allopregnanolone in vivo and in vitro
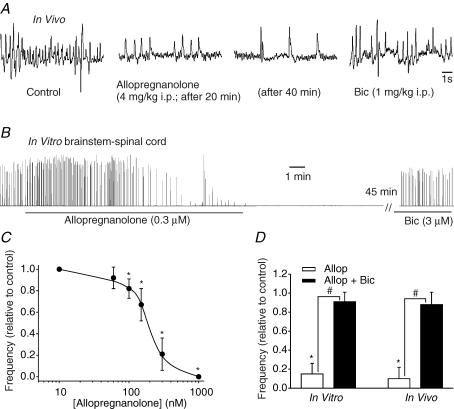
The frequency of breathing in unanaesthetized E20 rats in vivo was decreased after 10 min of allopregnanolone administration (4 mg kg−1i.p., Fig. 2A). The effects of allopregnanolone were prolonged with a steady-state effect being reached at ∼30 min, continuing for up to one hour. Suppression of breathing was reversed within minutes of the administration of bicuculline (1 mg kg−1, i.p., Fig. 2A). In vitro, respiratory frequency was markedly depressed by bath application of allopregnanolone (0.3 μm). The effects of allopregnanolone were prolonged, continuing for more than 2 h before recovery (n = 4). However, the suppression of respiratory frequency was rapidly reversed by administration of bicuculline (3 μm, Fig. 2B). The effects of allopregnanolone were dose dependent with an IC50 of 175 ± 47 nm (Fig. 2C). Figure 2D shows population data for the depression of respiratory frequency in vitro and in vivo in response to allopregnanolone administration, and the reversal of effects by bicuculline (1 mg kg−1i.p.in vivo; 3 μmin vitro).
Figure 2. Depression of respiratory frequency by allopregnanolone in vitro and in vivo.
A, whole-body plethysmograph recordings illustrating the allopregnanolone-induced depression of E20 rat breathing frequency. The depression reached a maximum level 40 min after i.p. injection of allopregnanolone and was antagonized by i.p. injection of bicuculline. B, rectified and integrated recordings of hypoglossal nerve root activity in the brainstem–spinal cord preparation from an E20 rat. The depression reached a maximum level 20 min after bath application of allopregnanolone (0.3 μm for 15 min). The respiratory rhythm stopped for approximately 1 h until administration of bicuculline (3 μm). C, dose–response curve for changes in respiratory frequency of brainstem spinal cord preparations in response to 15 min bath application of allopregnanolone (E20). Each data point was from four to eight preparations. D, comparison of effects of allopregnanolone (Allop) actions in vitro and in vivo at E20 in the absence and presence of bicuculline (Bic). Each data point was from four preparations. * indicates significant difference relative to control (P < 0.05); # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
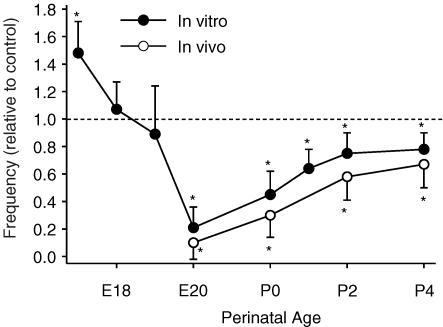
We next examined the age-dependent effects of allopregnanolone in vivo and in vitro (Fig. 3). Similar to what was observed for the age-dependent effects of chloride-mediated effects (Ren & Greer, 2006), allopregnanolone caused an increase in respiratory frequency in pre-E18 brainstem–spinal cord in vitro preparations. From E20 onwards, there was a marked depression of respiratory frequency by allopregnanolone. The depression of respiratory frequency was very pronounced at E20–P0. In vivo, there was also a marked depression of respiratory frequency during the perinatal period starting at the earliest age studied, E20. Note that due to lung immaturity, it was not feasible to examine fetal rats at earlier stages of development. Administration of 5–10 μl DMSO alone did not affect respiratory frequency.
Figure 3. Age-dependent effects of allopregnanolone.
Results are shown for in vitro preparations (300 nm for 15 min application, E17–P4, •) and in vivo (4 mg kg−1, i.p., E20–P4, ○). Each data point was from four to eight preparations. * indicates significant difference relative to control (P < 0.05).
Combined modulatory actions of allopregnanolone and muscimol
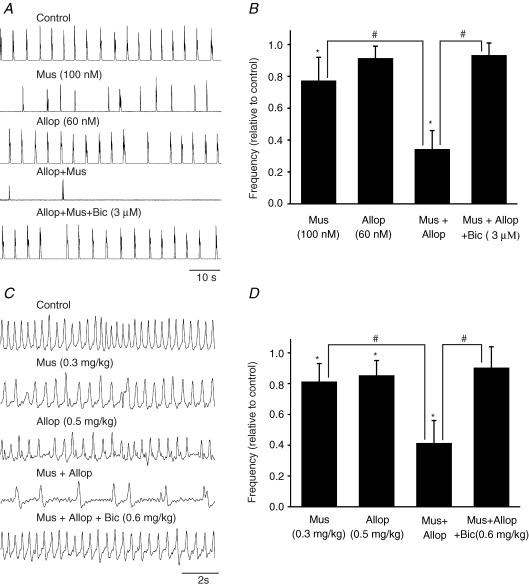
The combined actions of allopregnanolone and muscimol on respiratory frequency were tested using P0 brainstem–spinal cord preparations (Fig. 4A and B). The respiratory frequency was depressed to 77 ± 15% (n = 5) of control by 100 nm muscimol. The respiratory rhythm recovered within 5 min of muscimol washout. Allopregnanolone was then perfused for 20 min and, at a concentration of 60 nm, did not cause a significant depression (91 ± 8% of control, n = 5) of respiratory frequency. However, after a 20 min preapplication of 60 nm allopregnanolone, the administration of 100 nm muscimol caused a very marked depression of respiratory frequency (34 ± 12% of control, n = 5). The respiratory rhythm was not recovered within 20 min (n = 3), but immediately recovered with application of bicuculline (3 μm; returned to 93 ± 8% of control, n = 4).
Figure 4. The combined action of allopregnanolone and the GABAA receptor agonist muscimol in vitro and in vivo.
A, rectified and integrated suction electrode recordings of C4 ventral root activity in brainstem–spinal cord preparation from a P0 rat. Traces show the effects of muscimol (Mus) and allopregnanolone (Allop) compared to the combined application of both. The very marked depression of respiratory frequency caused by allopregnanolone and muscimol was blocked by bicuculline (Bic). B, population data showing changes in respiratory frequency relative to control for each of the drug protocol administrations shown in A. Each data point was from four to five P0 preparations. C, whole-body plethysmograph recordings from an unanaesthetized rat pup showing the effects of i.p. administration of muscimol (0.3 mg kg−1) or allopregnanolone (0.5 mg kg−1). Either agent caused a small depression of breathing. However, when muscimol (i.p., 0.3 mg kg−1) was administered 30 min after i.p. injection of allopregnanolone (0.5 mg kg−1) (trace 4), a period of apnoea occurred. The depression of respiratory frequency caused by muscimol and allopregnanolone was antagonized by bicuculline (0.6 mg kg−1). D, population data showing changes in respiratory frequency relative to control for each of the drug protocol administrations shown in C. Each data point was from four P0 preparations. * indicates significant difference relative to control (P < 0.05); # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
Plethysmographic recordings were performed to examine the interaction of muscimol and allopregnanolone on regulating respiratory frequency in vivo (Fig. 4C and D). The respiratory frequency was depressed to 81 ± 12% (n = 4) of control by administration of low doses of muscimol (i.p., 0.3 mg kg−1). The respiratory rhythm recovered within 30 min. The respiratory frequency was then depressed to steady-state level (85 ± 10% of control, n = 5) 30 min after i.p. injection of low doses of allopregnanolone (0.5 mg kg−1). The administration of muscimol (i.p., 0.3 mg kg−1) caused a further depression of respiratory frequency (41 ± 15% of control, n = 4) after 30 min i.p. injection of allopregnanolone (0.5 mg kg−1). The respiratory rhythm was not recovered within 1 h, however, administration of bicuculline (i.p., 0.6 mg kg−1) immediately returned the breathing frequency toward control value (90 ± 14% of control, n = 4).
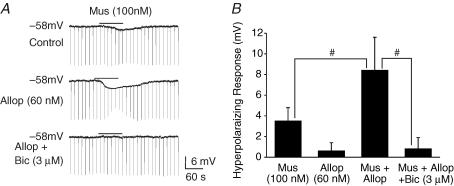
Recordings from inspiratory neurons in P0 medullary slices bathed in 3 mm [K+]o and TTX (1 μm) were performed to examine the interaction of allopregnanolone and muscimol at the single-cell level (Fig. 5). Note that in the past study (Ren & Greer, 2006) examining chloride-mediated conductances in respiratory neurons, perforated-patch recordings were used to avoid perturbation of normal intracellular [Cl−]i. However, whole-cell patch recordings sufficed for this study directed at examining the interactions of muscimol and neurosteroids. Application of muscimol (100 nm) caused an 3.5 ± 1.3 mV hyperpolarization from Vrest of −58 mV, and slight decrease (92 ± 7% of control) in membrane resistance (n = 7). Administration of 60 nm allopregnanolone on its own (20 min) did not cause significant changes in Vm(0.5 ± 1.1 mV hyperpolarization) and membrane resistance (98 ± 6% of control). After a 20 min preapplication of 60 nm allopregnanolone, application of the same dose of muscimol caused an 8.4 ± 3.2 mV hyperpolarization of Vm and a pronounced decrease (58 ± 11% of control, n = 6) in membrane resistance. Bicuculline (3 μm, n = 5), which did not affect Vrest or membrane resistance on its own, antagonized the effects of muscimol plus allopregnanolone.
Figure 5. Combined actions of allopregnanolone and muscimol.
A, whole-cell patch clamp recording from an inspiratory neuron in a P0 medullary slice preparation bathed in 3 mm [K+]o and TTX (1 μ m). The muscimol (Mus)-induced hyperpolarization and decrease in input resistance (determined by application of hyperpolarizing current pulses) were amplified in the presence of allopregnanolone (Allop). These effects were antagonized by bicuculline (bic). B, population data showing a hyperpolarizing response for each of the drug applications shown in A. Each data point was from five to seven P0 preparations. # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
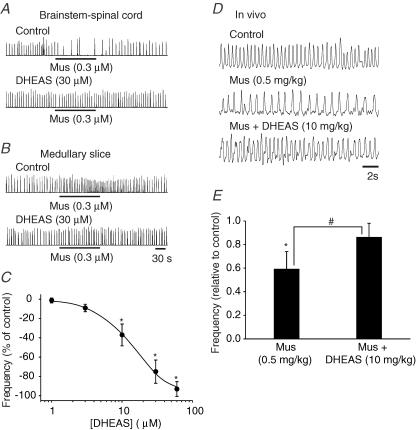
Modulation of respiratory rhythm by DHEAS
As shown in Fig. 6A, the inhibition of respiratory rhythm generated by the brainstem–spinal cord preparation caused by muscimol (0.3 μm) was antagonized by DHEAS (30 μm), a negative modulator of GABAA receptor activation. Similarly, DHEAS antagonized the muscimol-induced stimulation of respiratory rhythm generated by the medullary slice preparation bathed in elevated [K+]o (Fig. 6B). Administration of DHEAS (30 μm) on its own had no significant effects on the frequency of respiratory rhythm in both brainstem–spinal cord and medullary slice preparations. The overall depression of muscimol-induced actions by DHEAS in both types of in vitro preparations is plotted in Fig. 6C. The IC50 of 14 ± 3.8 μm is similar to that reported from other studies examining DHEAS-mediated inhibition of muscimol-mediated responses in vitro (Majewska, 1990; Spivak, 1994; Sousa & Ticku, 1997). Application of the related compound DHEA (10–60 μm, n = 6) did not modulate muscimol-mediated effects.
Figure 6. Effects of DHEAS on muscimol-induced changes of respiratory frequency in vitro and in vivo.
A, rectified and integrated suction electrode recordings of C4 ventral root activity in a P0 brainstem–spinal cord preparation. The inhibition of respiratory frequency by muscimol (Mus) was antagonized by DHEAS. B, rectified and integrated suction electrode recordings of XII nerve roots in a P0 medullary slice preparation. The muscimol-induced increase of respiratory frequency in 9 mm [K+]o bathing solution was antagonized by DHEAS. C, dose–response curve for the DHEAS blockade of muscimol-induced effects of respiratory frequency in brainstem–spinal cord and medullary slice preparations. Each data point was from five to eight P0 preparations. D, the effects of i.p. administration of muscimol (0.5 mg kg−1) to an unanaesthetized P0 rat pup. The muscimol-induced depression of breathing was antagonized by DHEAS (10 mg kg−1). E, population data showing changes in respiratory frequency relative to control for each of the drug protocol administrations shown in D. Each data point was from four P0 preparations. * indicates significant difference relative to control (P < 0.05); # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
Plethysmographic recordings were performed to examine the interaction of muscimol and DHEAS on regulating respiratory frequency in vivo (Fig. 6D and E). Muscimol (0.5 mg kg−1) decreased respiratory frequency to 59 ± 15% of control. The muscimol-induced depression of respiratory frequency was reduced to 86 ± 12% after administration of 10 mg kg−1 DHEAS (n = 4).
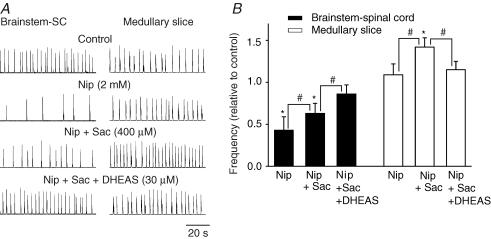
A further demonstration of the interaction of DHEAS and chloride-mediated conductances via GABAA receptors is illustrated in Fig. 7. The endogenous level of GABA in the brainstem–spinal cord preparation was elevated using the GABA-uptake inhibitor nipecotic acid (2 mm for 20 min). This caused a marked slowing of respiratory frequency (43 ± 16%) in the brainstem–spinal cord preparation. The effects were due to the combination of actions via GABAA and GABAB receptors. To minimize the actions via the GABAB receptor, an antagonist to that receptor subtype, saclofen (400 μm), was added to the superfusate. The remaining depression of respiratory frequency was primarily via GABAA receptor-mediated events (63 ± 12% of control). The further addition of DHEAS (30 μm) returned the respiratory frequency toward control values (86 ± 11% of control). In medullary slice preparations, there was a slight, but statistically insignificant, increase in respiratory frequency after 20 min of nipecotic acid application (109 ± 13% of control). However, there was a significant increase (142 ± 11% of control) in respiratory frequency after the administration of saclofen (400 μm). The GABAA receptor-mediated increase was diminished (115 ± 10% of control) by DHEAS (30 μm).
Figure 7. The effects of DHEAS on the changes of respiratory frequency caused by elevated levels of endogenous GABA.
A, rectified and integrated suction electrode recordings of C4 ventral root in P0 brainstem–spinal cord (SC) (left panel) and XII nerve root activity in P0 medullary slice (right panel) preparations. Endogenous levels of GABA were elevated by bath application of the GABA uptake inhibitor nipecotic acid (Nip). This resulted in a clear decrease in respiratory frequency of brainstem–SC preparations and a slight, but insignificant increase in medullary slice preparations after 20 min of nipecotic acid application. The antagonist to GABAB receptor, saclofen (Sac), was added to the bathing medium to minimize the component of the respiratory frequency depression resulting from the actions of GABAB receptors. The remaining GABAA receptor-mediated decrease and increase of respiratory frequency in brainstem–spinal cord and medullary slice preparations, respectively, was depressed by DHEAS. B, population data showing changes in respiratory frequency relative to control for in vitro preparations exposed to the drug paradigms shown in A. Each data point was from four P0 preparations. * indicates significant difference relative to control (P < 0.05); # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
Depression of muscimol-induced modulation of respiratory activity by DHEAS
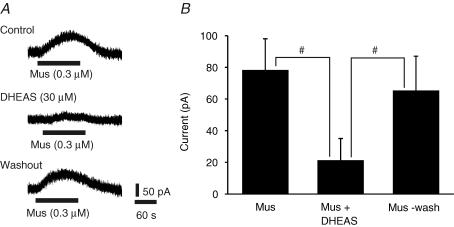
The effects of DHEAS on muscimol-induced outward currents were tested using whole-cell patch recordings of inspiratory neurons under voltage clamp in P1 medullary slices bathed in 3 mm[K+]o and TTX (1 μm) (Fig. 8). With a holding membrane potential (Vh) of −58 mV, bath application of muscimol (0.3 μm) caused a 78 ± 20 pA outward current (n = 5). The muscimol-induced outward current was reduced to 21 ± 14 pA in the presence of bath-applied DHEAS (30 μm). DHEAS (30 μm) on its own did not cause any significant current. Reapplication of muscimol after washout of DHEAS induced a 65 ± 22 pA. outward current.
Figure 8. Effects of DHEAS on muscimol-induced outward currents.
A, whole-cell patch clamp recording from an inspiratory neuron in a P1 medullary slice preparation bathed in 3 mm[K+]o and TTX (1 μm). The neuron was clamped at −58 mV in voltage-clamp mode, and the outward current caused by bath application of muscimol (Mus) recorded. The muscimol-induced current was depressed by bath application of DHEAS (30 μm) and recovered after 5 min washout of DHEAS. B, population data showing the amplitude of outward current measured in response to muscimol (Mus) and muscimol plus DHEAS. Each data point was from five P1 preparations. # indicates significant difference between groups (P < 0.05). Results are expressed as mean ± standard deviation (s.d.)
Discussion
Past work has demonstrated that circulating sex hormones progesterone and testosterone stimulate breathing (Behan et al. 2003). Here, we focus on respiratory modulation by. neurosteroids synthesized de novo in glial and neuronal cells, which begin to express the necessary enzymes for this biochemical process early in development (Mellon & Vaudry, 2001). Neurosteroids interact with the GABAA receptor and modulate GABAergic activity (Majewska et al. 1986). Data from this study demonstrate that the overall efficacy of GABAA-mediated modulation of respiratory frequency can be markedly regulated by their presence. Specifically, allopregnanolone and DHEAS are positive and negative modulators of the GABAA receptor function on respiratory neurons, respectively, during the perinatal period.
In a related study, we determined the actions of chloride-mediated conductances on respiratory rhythmogenesis in perinatal rats from the time of inception of fetal inspiratory drive through to the newborn period (Ren & Greer, 2006). The transition from excitatory to inhibitory effects on respiratory rhythmogenesis occurs at approximately E19. By birth, GABA induces a hyperpolarization of the membrane potential in respiratory medullary neurons, and a suppression of respiratory frequency. The age-dependent change in the actions of chloride-mediated conductances is regulated by the development of chloride cotransporters (KCC2 and NKCC1).
Allopregnanolone potentiates the actions of GABA and, at higher concentrations, directly gates the GABAA receptor chloride channel at a site distinct from the GABA-binding site (Callachan et al. 1987; Majewska, 1992; Lambert et al. 1996; Ueno et al. 1997; Rupprecht & Holsboer, 2000). The excitability of respiratory neurons in the ventrolateral medulla of perinatal rodents is clearly affected by allopregnanolone on its own, and at more physiological levels in the nanomolar range there was a clear accentuation of muscimol-induced currents. Data from brainstem–spinal cord preparations show a transition from excitatory to inhibitory action of allopregnanolone at E19. This is similar to the transition observed for the effects of the GABAA receptor agonist muscimol (Ren & Greer, 2006). The post-E19 suppression of respiratory frequency by allopregnanolone was particularly pronounced from E20–P0; also similar to what was observed for muscimol-induced actions. This may reflect developmental changes in GABAA receptor subunit composition, density and/or distribution within the somata and dendritic membranes. The suppression of respiratory frequency by allopregnanolone was also observed in vivo. In all experimental models, the allopregnanolone-mediated actions were countered by the GABAA receptor antagonist bicuculline. The seemingly paradoxical effects of increased respiratory rhythm by allopregnanolone in the medullary slice preparation can be accounted for by elevated level of [K+]o in the media used to bathe the preparation (see Ren & Greer, 2006). Specifically, [K+]o influences the function of cotransporters that determine transmembrane Cl− gradients, and thus the net effect of chloride-mediated conductances through the GABAA receptor.
The physiological relevance of allopregnanolone actions for the control of respiration during the perinatal period is significant. The levels of allopregnanolone and steroidogenic enzymes are particularly high in the brain during the perinatal period (Brown & Papadopoulos, 2001; Mellon & Vaudry, 2001). They are markedly increased further by a variety of stressors including hypoxia, asphyxia, parturition, ethanol exposure and infection; possibly as a neuroprotectant response to prevent excessive excitation (Morrow et al. 1999; Brown & Papadopoulos, 2001; Billiards et al. 2002; Nguyen et al. 2003, 2004). This raises the distinct possibility that neurosteroids depress, in a state-dependent manner, respiratory drive in perinatal mammals. For example, an elevation of allopregnanolone and GABA (Hoop et al. 1999) levels during hypoxia, and their combined actions could lead to a profound depression of respiratory drive. The precedent for neurosteroid modulation of respiration comes from a previous study demonstrating the suppression of fetal breathing methods (FBM) in sheep by infusion of pregnanolone (Nicol et al. 1999).
DHEAS belongs to the class of neurosteroids that acts to depress the effects of GABAA receptor ligands. Further it may play a role in neurodevelopment, due to a transient expression of its synthesizing enzyme (Compagnone et al. 1995) and the potential ability of DHEAS to aid in neuronal pathway formation (Compagnone & Mellon, 1998). Data from this study indicate that DHEAS suppressed the inhibition of respiratory frequency caused by GABAA receptor activation either by muscimol or by accentuating the endogenous levels of GABA. It should be noted that DHEAS can also act as a positive modulator of sigma1 and NMDA receptors (Monnet & Maurice, 2006); the implications for those actions on respiratory modulation were not investigated in this study.
In summary, these data should be considered in context with those from a recent study demonstrating the actions of chloride-mediated conductances, including those via GABAA receptors, on respiratory frequency during the perinatal period (Ren & Greer, 2006). Specifically, the net effect of GABA acting via GABAA receptors will be determined by the overall balance of negative and positive neurosteroid modulators within respiratory nuclei. This adds a level of complexity that must be considered when examining the depression of breathing in mammals associated with various behavioural states and pathogenic conditions such as apnoea and sudden death suspected to be associated with central respiratory dysfunction.
Acknowledgments
The research was funded by grants from the Toronto Hospital for Sick Children Foundation, Alberta Lung Association (ALA) and the Canadian Institutes of Health (CIHR). J.J.G. is a Scientist of the Alberta Heritage Foundation for Medical Research (AHFMR) and J.R. is a recipient of CIHR-SIDS, CIHR-CLA and AHFMR Fellowships.
References
- Akwa Y, Young J, Kabbadj K, Sancho MJ, Zucman D, Vourch C, Jung-Testas I, Hu ZY, Le Goascogne C, Jo DH. Neurosteroids: biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. J Steroid Biochem Mol Biol. 1991;40:71–81. doi: 10.1016/0960-0760(91)90169-6. [DOI] [PubMed] [Google Scholar]
- Angulo Y, González AW. The prenatal growth of the albino rat. Anat Record. 1932;52:117–138. [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrasterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids. J Endocrinol. 1996;150:S221–239. [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Walker DW, Canny BJ, Hirst JJ. Endotoxin increases sleep and brain allopregnanolone concentrations in newborn lambs. Pediatr Res. 2002;52:892–899. doi: 10.1203/00006450-200212000-00014. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Papadopoulos V. Role of the peripheral-type benzodiazepine receptor in adrenal and brain steroidogenesis. Int Rev Neurobiol. 2001;46:117–143. doi: 10.1016/s0074-7742(01)46061-2. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Steroidogenic enzyme P450c17 is expressed in the embryonic central nervous system. Endocrinology. 1995;136:5212–5223. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neo cortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizerian SS, Morrow AL, Lieberman JA, Grobin AC. Neonatal neurosteroid administration alters parvalbumin expression and neuron number in medial dorsal thalamus of adult rats. Brain Res. 2004;1012:66–74. doi: 10.1016/j.brainres.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Generation of respiratory and locomotor patterns by an in vitro brainstem–spinal cord fetal rat preparation. J Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Hoffman SW, Virmani S, Simkins RM, Stein DG. The delayed administration of dehydroepiandrosterone sulfate improves recovery of function after traumatic brain injury in rats. J Neurotrauma. 2003;20:859–870. doi: 10.1089/089771503322385791. [DOI] [PubMed] [Google Scholar]
- Hoop B, Beagle JL, Maher TJ, Kazemi H. Brainstem acid neurotransmitters and hypoxic ventilatory response. Respir Physiol. 1999;118:117–129. doi: 10.1016/s0034-5687(99)00072-9. [DOI] [PubMed] [Google Scholar]
- Huang J, Suguihara C, Hehre D, Lin J, Bancalari E. Effects of GABA receptor blockage on the respiratory response to hypoxia in sedated newborn piglets. J Appl Physiol. 1994;77:1006–1010. doi: 10.1152/jappl.1994.77.2.1006. [DOI] [PubMed] [Google Scholar]
- Joels M. Steroid hormones and excitability in the mammalian brain. Frontiers Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1998;65:243–251. doi: 10.1016/s0960-0760(97)00191-x. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Callachan H, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. Steroid regulation of the GABAA receptor: ligand binding, chloride transport and behaviour. Ciba Found Symp. 1990;153:83–106. doi: 10.1002/9780470513989.ch5. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroids: biochemistry, models of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int Rev Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active) steroids: molecular, physiological, and behavioral aspects. J Pharmacol Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- Morrow Al, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action. Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Gilliares SS, Wlaker DW, Hirst JJ. Changes in 5α-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003;53:956–964. doi: 10.1203/01.PDR.0000064905.64688.10. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Yan EB, Castillo-Melendez M, Walker DW, Hirst JJ. Increased allopregnanolone levels in the fetal sheep brain following umbilical cord occlusion. J Physiol. 2004;560:593–602. doi: 10.1113/jphysiol.2004.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MB, Hirst JJ, Walker D. Effects of pregnanolone on behavioural parameters and the responses to GABA(A) receptor antagonists in the late gestation fetal sheep. Neuropharmacol. 1999;38:49–63. doi: 10.1016/s0028-3908(98)00166-x. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate GABA-A receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. Pre-Botzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride mediated conductances during the perinatal period. J Neurosci. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 2000;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellengerger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–728. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brainstem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Sousa A, Ticku MK. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABAA receptor complex reveals that it may act via the picrotoxin site. J Pharmacol Exp Ther. 1997;282:827–833. [PubMed] [Google Scholar]
- Spivak CF. Desensitization and noncompetitive blockade of GABAA receptors in ventral midbrain neurons by a neurosteroid dehydroepiandrosterone sulfate. Synapse. 1994;16:113–122. doi: 10.1002/syn.890160205. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]